Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Treatments and Growth Conditions
2.2. Measurement of Fruit Length, Weight, Transverse Diameters and Flesh Firmness
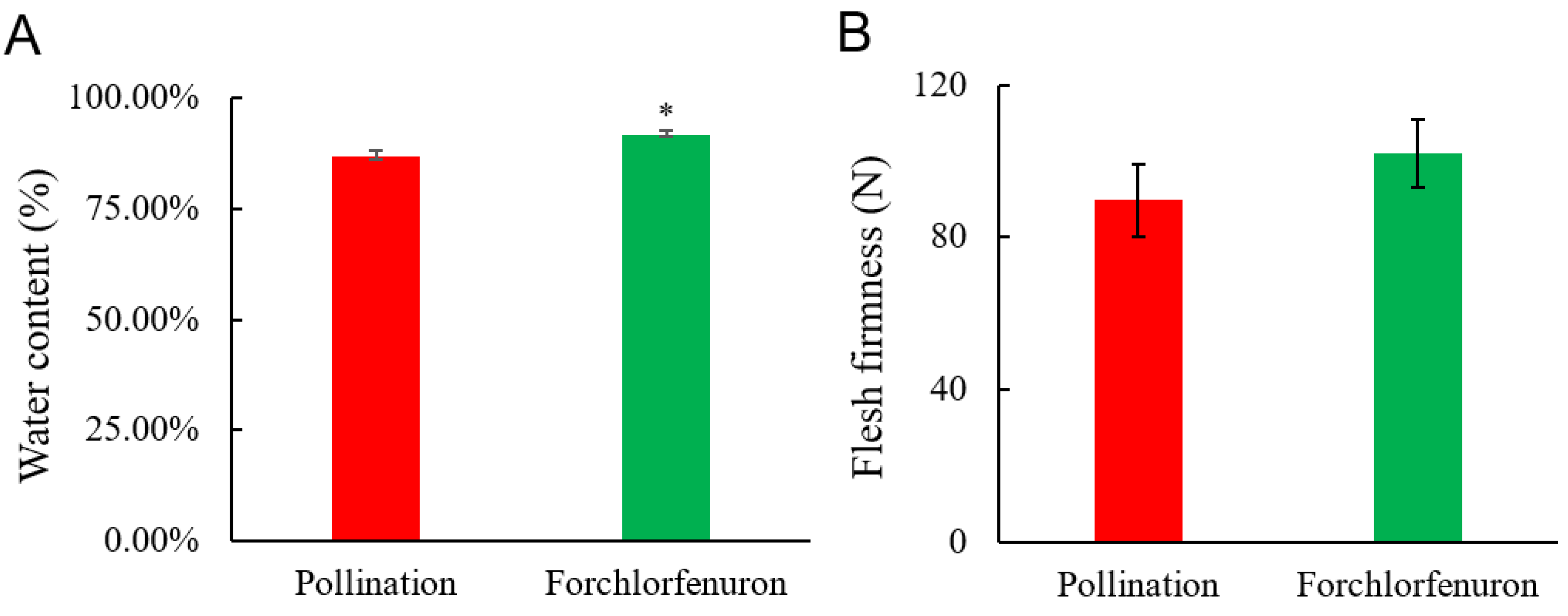
2.3. Measurement of Water Content
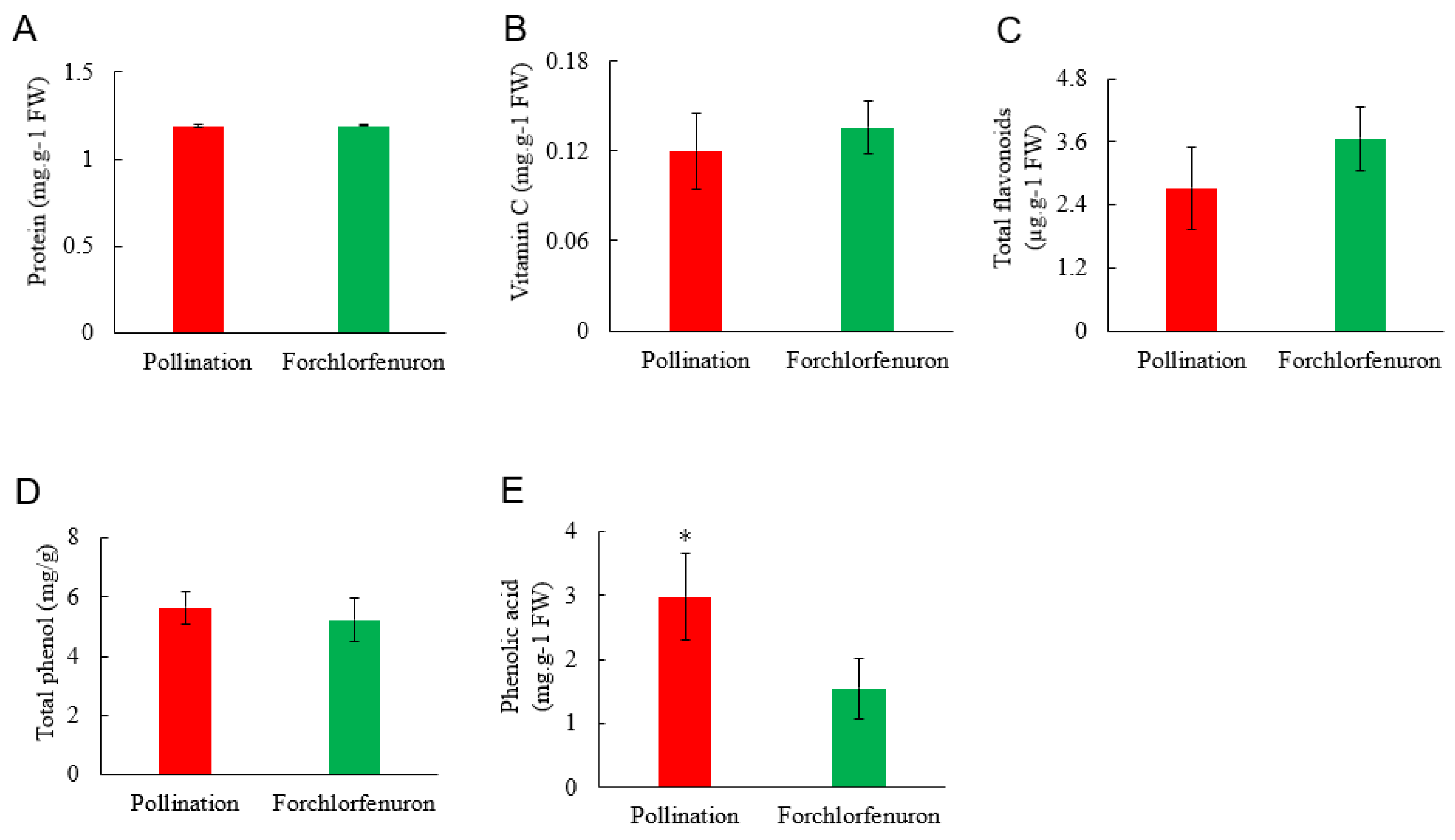
2.4. Measurement of Protein, Vitamin C, Total Flavonoids, Total Phenol and Phenolic Acid
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Effects of Forchlorfenuron on Cucumber Fruit Development
3.2. Effects of Forchlorfenuron on Fruit Texture
3.3. Effect of Forchlorfenuron on Nutrient Quality of Fruit
3.4. Expression of Cytokinin-Responsive Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kim, I.S.; Okubo, H.; Fujieda, K. Endogenous levels of IAA in relation to parthenocarpy in cucumber (Cucumis sativus L.). Sci. Hortic. 1992, 52, 1–8. [Google Scholar] [CrossRef]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 15, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Avenant, J.H. Effect of gibberellic acid, CPPU and harvest time on browning of Vitis vinifera L. ‘Regal Seedless’: Pre-cold storage and post-cold storage quality factors. Acta Hortic. 2017, 1157, 373–380. [Google Scholar] [CrossRef]
- Huitron, M.V.; Diaz, M.; Dianez, F.; Camacho, F.; Valverde, A. Effect of 2,4-D and CPPU on triploid watermelon production and quality. HortScience 2007, 42, 559–564. [Google Scholar] [CrossRef]
- Qian, C.L.; Ren, N.N.; Wang, J.Y.; Xu, Q.; Chen, X.H.; Qi, X.H. Effects of exogenous application of CPPU, NAA and GA4+7 on parthenocarpy and fruit quality in cucumber (Cucumis sativus L.). Food Chem. 2018, 243, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, T.; Arslan, A.; Naoum, F. Cucumber (Cucumis sativus L.) water use efficiency (WUE) under plastic mulch and drip irrigation. Agric. Water Mgt. 2013, 128, 149–157. [Google Scholar] [CrossRef]
- Guo, L.J.; Fan, H.Y.; Deng, H.D.; Luo, Z.W.; He, S.; Hu, F.C.; Wang, X.H.; He, F.; Hua, M. Effect of forchlorfenuron (CPPU) and ethychlozate on fruit development and quality of mango cultivar ‘Tainong 1′. Plant Dis. Pests 2017, 8, 39–42. [Google Scholar]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Ann. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Castillo, J.G.; Baldicchi, A.; Frioni, T.; Marocchic, F.; Moscatellod, S.; Proiettid, S.; Battistellid, A.; Famiani, F. Pre-anthesis CPPU low dosage application increases ‘Hayward’ kiwifruit weight without affecting the other qualitative and nutritional characteristics. Food Chem. 2014, 158, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.F.; Wang, T.; Li, J.Z.; Yang, Q.Q.; Qian, M.J.; Teng, Y.W. Effects of exogenous application of GA4+7 and N-(2-chloro-4-pyridyl)-N′-phenylurea on induced parthenocarpy and fruit quality in Pyrus pyrifolia ‘Cuiguan’. Plant Growth Regul. 2014, 76, 251–258. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.L.; He, Z.P.; Zhao, Y.Y.; Mi, H.B.; Chen, X.H.; Mao, L.C. Maturity-dependent chilling tolerance regulated by the antioxidative capacity in postharvest cucumber (Cucumis sativus L.) fruits. J. Sci. Food Agric. 2013, 93, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Li, Q.Q.; Shen, J.T.; Qian, C.L.; Xu, X.W.; Xu, Q.; Chen, X.H. Sugar enhances waterlogging-induced adventitious root formation in cucumber by promoting auxin transport and signaling. Plant Cell Environ. 2020, 1–13. [Google Scholar] [CrossRef]
- Hayata, Y.; Niimi, Y.; Iwasaki, N. Synthetic cytokinin-1-(2=chloro=4= pyridyl)-3-phenylurea (CPPU)-promotes fruit set and induces parthenocarpy in watermelon. J. Am. Soc. Hortic. Sci. 1995, 120, 997–1000. [Google Scholar] [CrossRef]
- El-Shraiy, A.M.; Hegazi, A.M. Influence of JA and CPPU on growth, yield and α-Amylaseactivity in potato plant (Solanum tuberosum L.). Aust. J. Basic Appl. Sci. 2010, 4, 160–170. [Google Scholar]
- Su, L.; Rahat, S.; Ren, N.N.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Wang, M.Q.; Chen, X.H.; Qi, X.H. Cytokinin and auxin modulate cucumber parthenocarpy fruit development. Sci. Hortic. 2021, 282, 1–10. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Wang, M.; Wang, Y.; Sharif, R.; Ren, N.; Qian, C.; Xu, J.; Chen, X.; Qi, X. Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber. Horticulturae 2021, 7, 128. https://doi.org/10.3390/horticulturae7060128
Su L, Wang M, Wang Y, Sharif R, Ren N, Qian C, Xu J, Chen X, Qi X. Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber. Horticulturae. 2021; 7(6):128. https://doi.org/10.3390/horticulturae7060128
Chicago/Turabian StyleSu, Li, Miaoqing Wang, Yuean Wang, Rahat Sharif, Nannan Ren, Chunlu Qian, Jun Xu, Xuehao Chen, and Xiaohua Qi. 2021. "Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber" Horticulturae 7, no. 6: 128. https://doi.org/10.3390/horticulturae7060128
APA StyleSu, L., Wang, M., Wang, Y., Sharif, R., Ren, N., Qian, C., Xu, J., Chen, X., & Qi, X. (2021). Forchlorfenuron Application Induced Parthenocarpic Fruit Formation without Affecting Fruit Quality of Cucumber. Horticulturae, 7(6), 128. https://doi.org/10.3390/horticulturae7060128






