Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. RT-qPCR Analysis
2.3. Detection of Volatile Compounds in Strawberry Fruits
2.4. Statistical Analysis
3. Results
3.1. Effect of Substrate Culture on Volatile Contents of Strawberry Fruits
3.2. Changes in Volatile Chemicals in ‘Amaou’ Strawberry Fruits under Substrate and Soil Culture Conditions
3.3. Changes in Contents of Furanone and Expression of Synthesis-Related Gene Expression Caused by Substrate Culture in ‘Amaou’ Strawberry
3.4. Changes in Content of Beta-Linalool and Trans-Nerolidol and Expression of FaNES1 Caused by Substrate Culture in ‘Amaou’ Strawberry
3.5. Effect of Substrate Culture on Volatile Esters and Expression of FaSAAT and FaAAT2 in ‘Amaou’ Strawberry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.-W.; Ban, Z.-J.; Lu, H.-Y.; Li, D.; Poverenov, E.; Luo, Z.-S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Jetti, R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M. Quantification of Selected Aroma-Active Compounds in Strawberries by Headspace Solid-Phase Microextraction Gas Chromatography and Correlation with Sensory Descriptive Analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. Strawberry flavour: Analysis and biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Zhang, Y.; Du, B.; Feng, C.; Zhou, Y.; Mei, X.; Jiang, Y.; Duan, X.; Yang, Z. Differential responses of four biosynthetic pathways of aroma compounds in postharvest strawberry (Fragaria×ananassa Duch.) under interaction of light and temperature. Food Chem. 2017, 221, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry Flavor: Diverse Chemical Compositions, a Seasonal Influence, and Effects on Sensory Perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cul-tivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Miszczak, A.; Forney, C.F.; Prange, R.K. Development of Aroma Volatiles and Color during Postharvest Ripening of ‘Kent’ Strawberries. J. Am. Soc. Hortic. Sci. 1995, 120, 650–655. [Google Scholar] [CrossRef]
- Pérez, A.G.; Olías, R.; Sanz, C.; Olías, J.M. Furanones in Strawberries: Evolution during Ripening and Postharvest Shelf Life. J. Agric. Food Chem. 1996, 44, 3620–3624. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, R.; Perez, A. Effect of modified atmosphere on alcohol acyltransferase activity and volatile composition of strawberry. Acta Hortic. 2003, 600, 563–566. [Google Scholar] [CrossRef]
- Rohloff, J.; Nestby, R.; Folkestad, J.A.; Iversen, T.H. Influence of rain cover cultivation on taste and aroma quality of strawberries (Fragaria ananassa Duch.). J. Food Agric. Environ. 2004, 2, 74–82. [Google Scholar]
- Ojeda-Real, L.A.; Lobit, P.; Cárdenas-Navarro, R.; Grageda-Cabrera, O.; Farías-Rodríguez, F.; Valencia-Cantero, E.; Maías-Rodríguez, L. Effect of nitrogen fertilization on quality markers of strawberry (Fragaria × ananassa Duch. cv. Aromas). J. Sci. Food Agric. 2009, 89, 935–939. [Google Scholar] [CrossRef]
- Raab, T.; López-Ráez, J.A.; Klein, D.; Caballero, J.L.; Moyano, E.; Schwab, W.; Muñoz-Blanco, J. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. Plant Cell 2006, 18, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Fink, B.; Arold, B.; Eisenreich, W.; Schwab, W. Functional Characterization of Enone Oxidoreductases from Strawberry and Tomato Fruit. J. Agric. Food Chem. 2007, 55, 6705–6711. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.; Lavid, N.; Lunkenbein, S.; Lewinsohn, E.; Schwab, W.; Kaldenhoff, R. Isolation, cloning and expression of a multifunctional O-methyltransferase capable of forming 2,5-dimethyl-4-methoxy-3(2H)-furanone, one of the key aroma compounds in strawberry fruits. Plant J. 2002, 31, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Hong, X.; Zhao, S.; Liu, J.; Schulenburg, K.; Huang, F.-C.; Franz-Oberdorf, K.; Schwab, W. Glucosylation of 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone, the Key Strawberry Flavor Compound in Strawberry Fruit. Plant Physiol. 2016, 171, 139–151. [Google Scholar] [CrossRef]
- Aharoni, A.; Giri, A.P.; Verstappen, F.W.; Bertea, C.M.; Sevenier, R.; Sun, Z.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Gain and Loss of Fruit Flavor Compounds Produced by Wild and Cultivated Strawberry Species. Plant Cell 2004, 16, 3110–3131. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa, and F. ves-ca, and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Aharoni, A.; Keizer, L.C.P.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Blaas, J.; Van Houwelingen, A.M.M.L.; De Vos, R.C.H.; Van Der Voet, H.; et al. Identification of the SAAT Gene Involved in Strawberry Flavor Biogenesis by Use of DNA Microarrays. Plant Cell 2000, 12, 647. [Google Scholar] [CrossRef]
- Cumplido-Laso, G.; Medina-Puche, L.; Moyano, E.; Hoffmann, T.; Sinz, Q.; Ring, L.; Studart-Wittkowski, C.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; et al. The fruit rip-ening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J. Exp. Bot. 2012, 63, 4275–4290. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.; Xiao, Y.; Zhang, Z.; Li, S.; Liu, X.; Zhang, B.; Yang, X.; Grierson, D.; Jiang, G.; et al. An ETHYLENE RESPONSE FACTOR-MYB transcription complex reg-ulates furaneol biosynthesis by activating QUINONE OXIDOREDUCTASE expression in strawberry. Plant Physiol. 2018, 178, 189–201. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Garrido-Gala, J.; Blanco-Portales, R.; Folta, K.M.; Muñoz-Blanco, J.; Caballero, J.L. Identification and validation of reference genes for transcript normalization in strawberry (Fragaria × Ananassa) defense re-sponses. PLoS ONE 2013, 8, e70603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, X.; Liu, Y.; Li, Y.; Luo, Y.; Wang, X.; Tang, H. Evaluation of suitable reference genes for qRT-PCR normalization in strawberry (Fragaria × Ananassa) under different experimental conditions. BMC Mol. Biol. 2018, 19, 8. [Google Scholar] [CrossRef]
- Zorrilla-Fontanesi, Y.; Rambla, J.-L.; Cabeza, A.; Medina, J.J.; Sánchez-Sevilla, J.F.; Valpuesta, V.; Botella, M.A.; Granell, A.; Amaya, I. Genetic Analysis of Strawberry Fruit Aroma and Identification of O-Methyltransferase FaOMT as the Locus Controlling Natural Variation in Mesifurane Content. Plant Physiol. 2012, 159, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.; Rambla, J.L.; Alexiou, K.G.; Granell, A.; Monfort, A. Genetic analysis of the wild strawberry (Fragaria vesca) volatile composition. Plant Physiol. Biochem. 2017, 121, 99–117. [Google Scholar] [CrossRef]
- Ulrich, D.; Komes, D.; Olbricht, K.; Hoberg, E. Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet. Resour. Crop. Evol. 2006, 54, 1185–1196. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Vlasenko, E.; Kuznetsova, O. The Influence of Complex Additives on the Synthesis of Aroma Substances by Gray Oyster Culinary-Medicinal Mushroom, Pleurotus ostreatus (Agaricomycetes) during the Substrate Cultivation. Int. J. Med. Mushrooms 2020, 22, 305–311. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Huang, X.; Zhang, L.; Zhao, P.; Ma, H.; Li, X.; Ban, Z.; Liu, X. Label-free quantitative proteomics to investigate strawberry fruit proteome changes under controlled atmosphere and low temperature storage. J. Proteom. 2015, 120, 44–57. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Wu, L.; Liu, H.-R.; Zhang, B.; Yin, X.-R.; Ge, Y.-Q.; Chen, K.-S. Bagging Treatment Influences Production of C6 Aldehydes and Biosynthesis-Related Gene Expression in Peach Fruit Skin. Molecules 2014, 19, 13461–13472. [Google Scholar] [CrossRef]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fei, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.D.L.P.; Blanch, G.P.; Flores, G.; Del Castillo, M.L.R. Impact of postharvest methyl jasmonate treatment on the volatile composition and flavonol content of strawberries. J. Sci. Food Agric. 2010, 90, 989–994. [Google Scholar] [CrossRef]
- Kasperbauer, M.J.; Loughrin, J.H.; Wang, S.Y. Light Reflected from Red Mulch to Ripening Strawberries Affects Aroma, Sugar and Organic Acid Concentrations. Photochem. Photobiol. 2001, 74, 103–107. [Google Scholar] [CrossRef]
- Hakala, M.A.; Lapveteläinen, A.T.; Kallio, H.P. Volatile Compounds of Selected Strawberry Varieties Analyzed by Purge-and-Trap Headspace GC-MS. J. Agric. Food Chem. 2002, 50, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Ross, C.F.; Davies, N.M.; Zhou, J. Fruit and soil quality of organic and conventional strawberry agroecosys-tems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
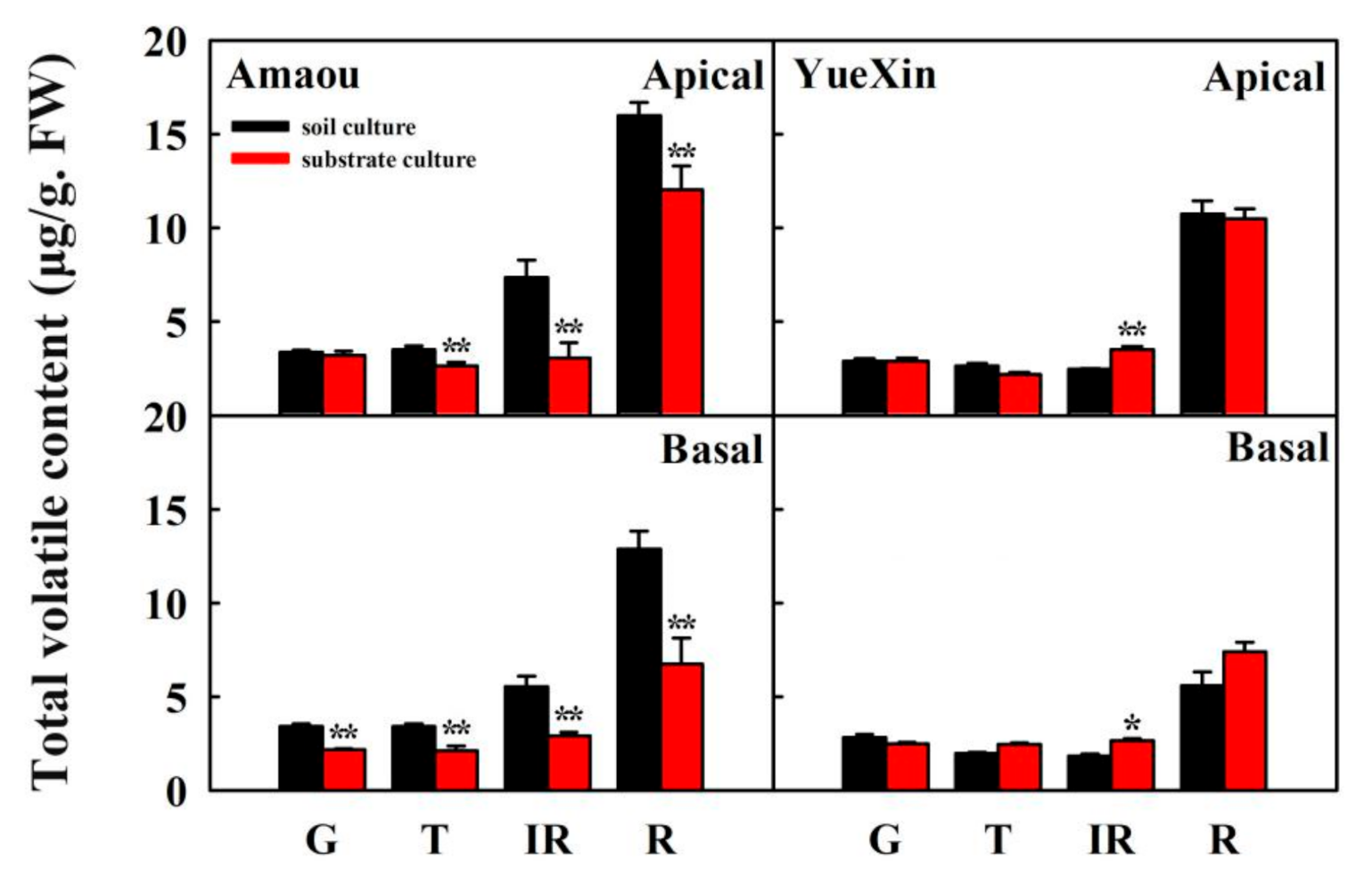
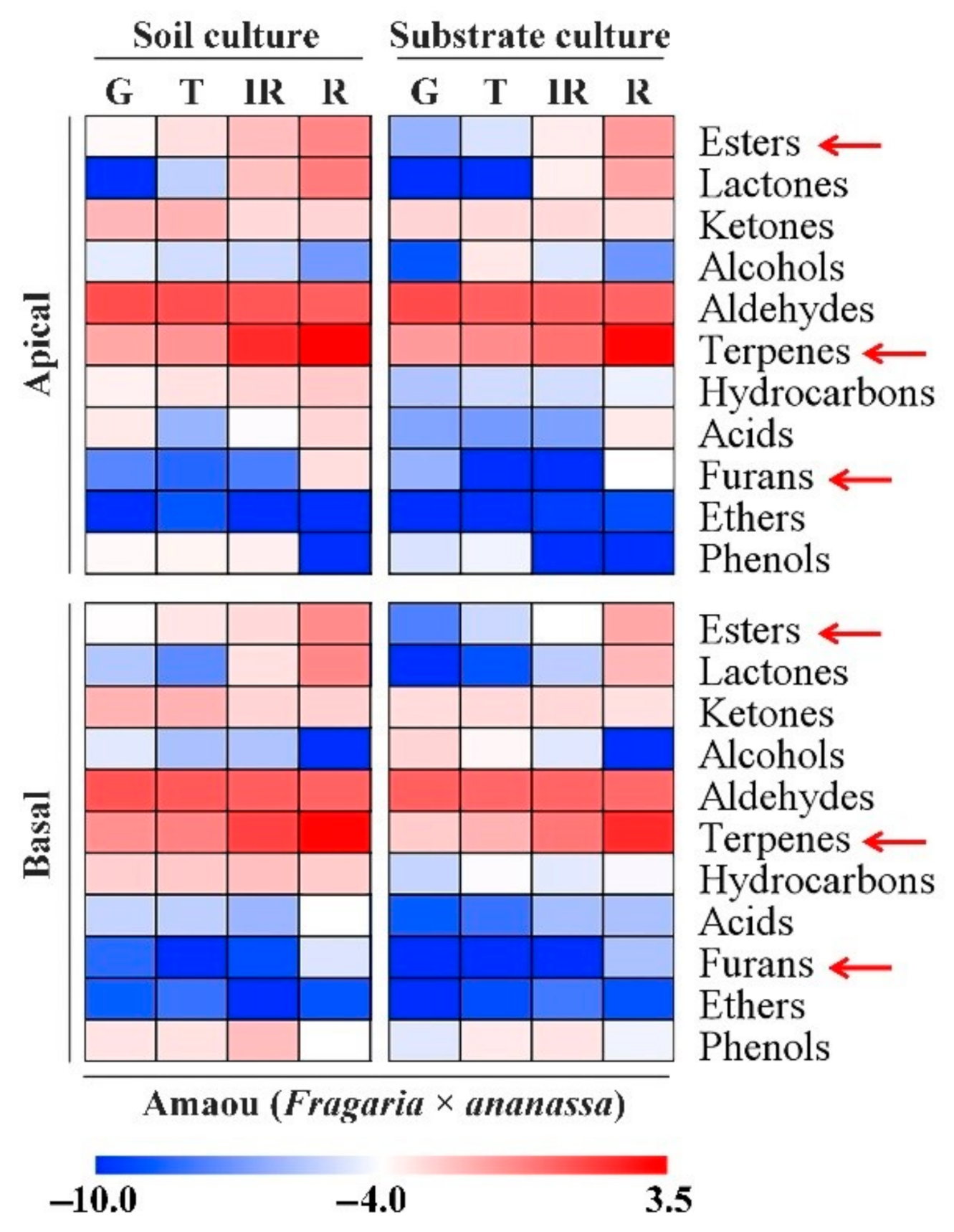

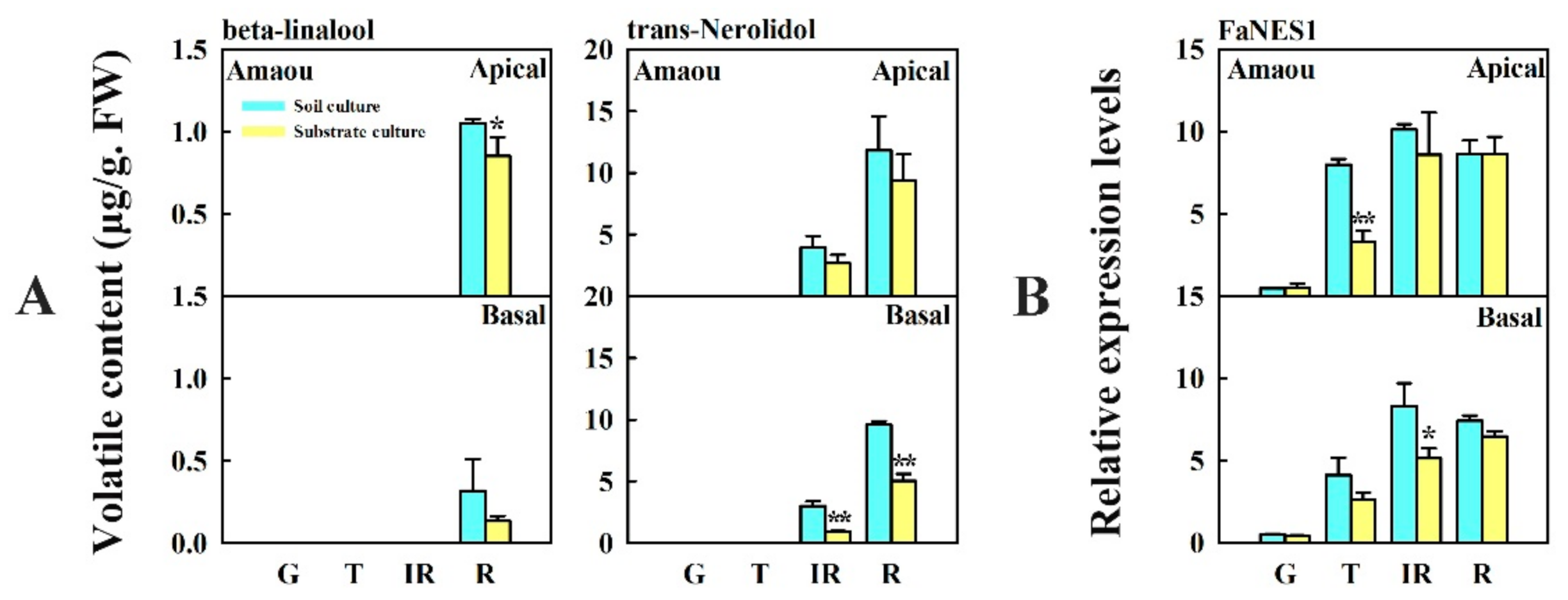

| Volatiles | Culture Conditions | G * | T | IR | R |
|---|---|---|---|---|---|
| Esters | Soil | 0.073 a | 0.124 a | 0.250 a | 0.764 a |
| Substrate | 0.013 b | 0.035 b | 0.095 b | 0.507 b | |
| Lactones | Soil | 0.000 a | 0.024 a | 0.231 a | 0.923 a |
| Substrate | 0.000 a | 0.000 b | 0.089 b | 0.415 b | |
| Ketones | Soil | 0.288 a | 0.305 a | 0.136 a | 0.158 a |
| Substrate | 0.159 b | 0.136 b | 0.138 a | 0.128 b | |
| Alcohols | Soil | 0.042 a | 0.031 a | 0.028 a | 0.008 a |
| Substrate | 0.002 b | 0.102 b | 0.037 a | 0.007 a | |
| Aldehydes | Soil | 2.340 a | 2.246 a | 1.954 a | 1.665 a |
| Substrate | 2.475 a | 1.606 b | 1.524 b | 1.480 a | |
| Terpenes | Soil | 0.388 a | 0.572 a | 4.439 a | 12.006 a |
| Substrate | 0.490 b | 0.658 a | 1.156 b | 9.280 b | |
| Hydrocarbons | Soil | 0.085 a | 0.117 a | 0.158 a | 0.189 a |
| Substrate | 0.018 b | 0.030 b | 0.031 b | 0.047 b | |
| Acids | Soil | 0.100 a | 0.013 a | 0.060 a | 0.150 a |
| Substrate | 0.010 b | 0.008 a | 0.009 b | 0.102 b | |
| Furans | Soil | 0.005 a | 0.003 a | 0.004 a | 0.126 a |
| Substrate | 0.013 a | ud b | ud b | 0.065 b | |
| Ethers | Soil | ud #,a | 0.002 a | ud a | ud a |
| Substrate | ud a | 0.001 a | 0.001 b | 0.002 b | |
| Phenols | Soil | 0.069 a | 0.078 a | 0.088 a | ud a |
| Substrate | 0.035 b | 0.050 b | ud b | ud a |
| Volatiles | Culture Conditions | G * | T | IR | R |
|---|---|---|---|---|---|
| Esters | Soil | 0.066 a | 0.109 a | 0.137 a | 0.690 a |
| Substrate | 0.005 b | 0.028 b | 0.064 b | 0.398 b | |
| Lactones | Soil | 0.020 a | 0.006 a | 0.118 a | 0.739 a |
| Substrate | 0.000 b | 0.002 a | 0.023 b | 0.273 b | |
| Ketones | Soil | 0.308 a | 0.319 a | 0.154 a | 0.165 a |
| Substrate | 0.131 b | 0.136 b | 0.143 a | 0.120 a | |
| Alcohols | Soil | 0.040 a | 0.017 a | 0.018 a | 0.000 a |
| Substrate | 0.156 b | 0.075 b | 0.039 b | 0.000 a | |
| Aldehydes | Soil | 2.050 a | 1.808 a | 1.673 a | 1.536 a |
| Substrate | 1.636 b | 1.397 b | 1.445 a | 1.357 b | |
| Terpenes | Soil | 0.627 a | 0.830 a | 2.939 a | 9.397 a |
| Substrate | 0.182 b | 0.309 b | 1.025 b | 4.458 b | |
| Hydrocarbons | Soil | 0.174 a | 0.197 a | 0.239 a | 0.182 a |
| Substrate | 0.026 b | 0.060 b | 0.041 b | 0.055 b | |
| Acids | Soil | 0.026 a | 0.023 a | 0.014 a | 0.062 a |
| Substrate | 0.002 b | 0.004 b | 0.016 a | 0.017 b | |
| Furans | Soil | 0.003 a | ud #,a | 0.002 a | 0.037 a |
| Substrate | ud b | ud a | ud b | 0.018 b | |
| Ethers | Soil | 0.002 a | 0.004 a | ud a | 0.002 a |
| Substrate | ud b | 0.002 a | 0.004 b | 0.002 a | |
| Phenols | Soil | 0.111 a | 0.118 a | 0.258 a | 0.065 a |
| Substrate | 0.040 b | 0.098 a | 0.112 b | 0.049 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Liu, X.; Xiao, Y.; Zhang, Z.; Shi, Y.; Kong, W.; Yang, X.; Jiang, G.; Zhang, B.; et al. Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit. Horticulturae 2021, 7, 81. https://doi.org/10.3390/horticulturae7040081
Li Y, Zhang Y, Liu X, Xiao Y, Zhang Z, Shi Y, Kong W, Yang X, Jiang G, Zhang B, et al. Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit. Horticulturae. 2021; 7(4):81. https://doi.org/10.3390/horticulturae7040081
Chicago/Turabian StyleLi, Yunduan, Yuanyuan Zhang, Xincheng Liu, Yuwei Xiao, Zuying Zhang, Yanna Shi, Wenbing Kong, Xiaofang Yang, Guihua Jiang, Bo Zhang, and et al. 2021. "Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit" Horticulturae 7, no. 4: 81. https://doi.org/10.3390/horticulturae7040081
APA StyleLi, Y., Zhang, Y., Liu, X., Xiao, Y., Zhang, Z., Shi, Y., Kong, W., Yang, X., Jiang, G., Zhang, B., & Chen, K. (2021). Cultivation Conditions Change Aroma Volatiles of Strawberry Fruit. Horticulturae, 7(4), 81. https://doi.org/10.3390/horticulturae7040081







