Nutrient Concentration of African Horned Cucumber (Cucumis metuliferus L) Fruit under Different Soil Types, Environments, and Varying Irrigation Water Levels
Abstract
1. Introduction
2. Material and Methods
2.1. Determination of β-Carotene
2.2. Determination of Total Soluble Sugars
2.3. Determination of Vitamin C and E
2.4. Determination of Total Flavonoids
2.5. Determination of Total Phenolic Content
2.6. Determination of Micro-Nutrients
2.7. Statistical Analysis
3. Results
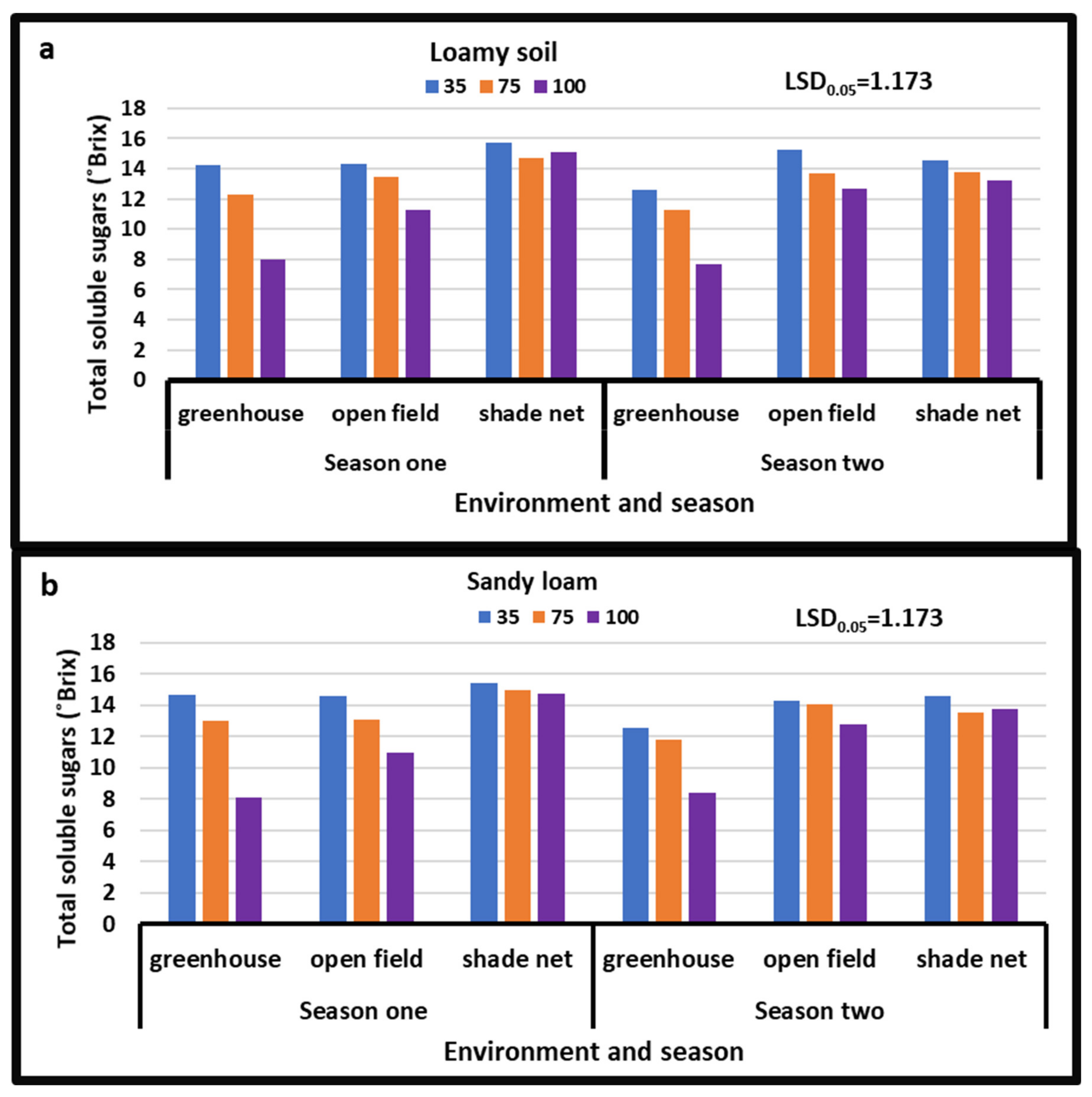
3.1. Total Soluble Sugars
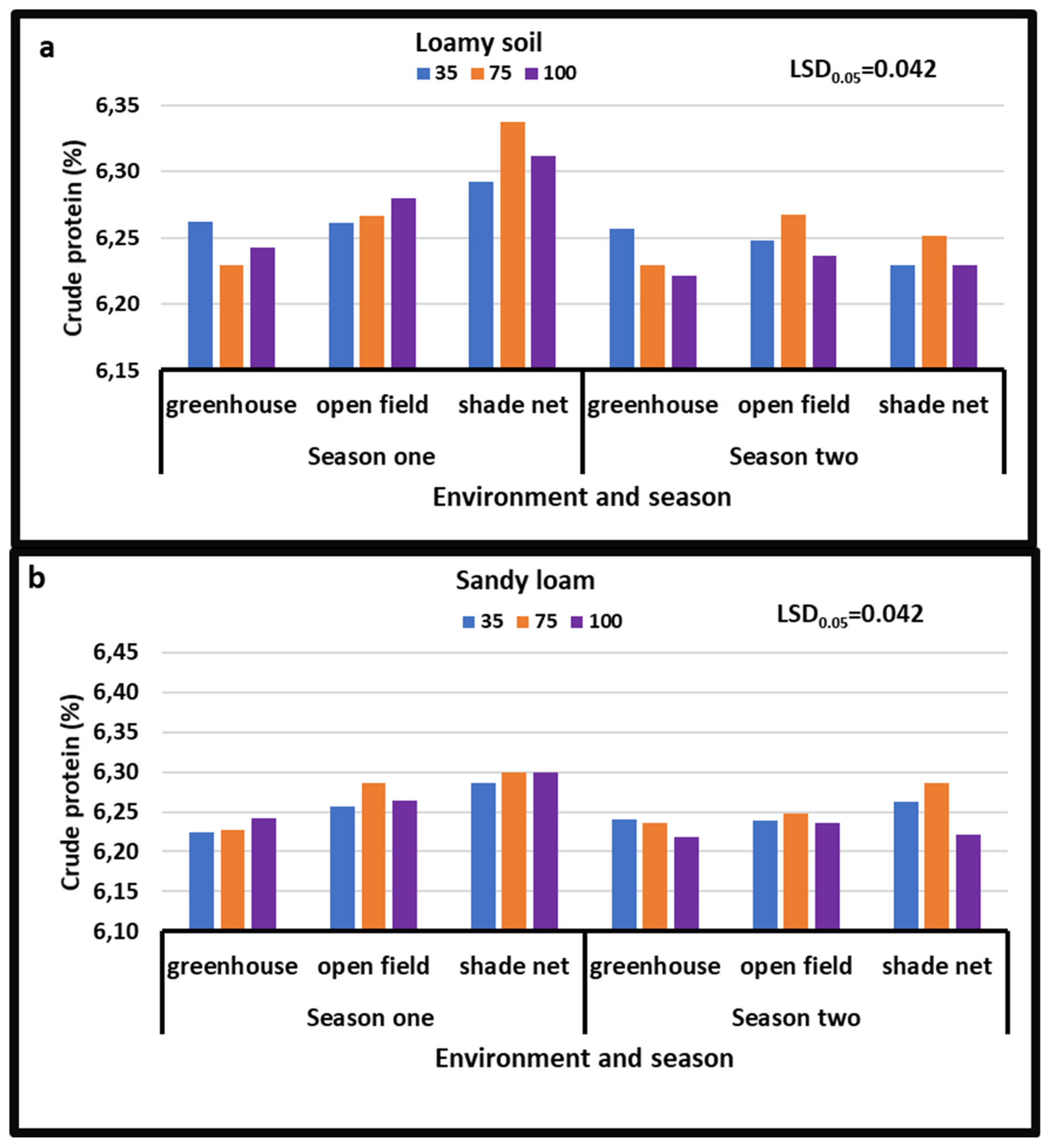
3.2. Crude Proteins
3.3. β-Carotene
3.4. Vitamin C
3.5. Vitamin E
3.6. Total Flavonoids
3.7. Total Phenols
3.8. Micro-Nutrients
4. Discussion
4.1. Bio-Chemical Constituents
4.2. Total Soluble Sugars
4.3. Crude Proteins
4.4. β-Carotene
4.5. Vitamin C
4.6. Vitamin E
4.7. Total Flavonoids
4.8. Total Phenols
4.9. Micro-Nutrients
5. Conclusions and Future Research
Author Contributions
Funding
Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, N.A.; Abdelatif, A.H. Chemical Measurement of Total Soluble Sugars as a parameter for cotton lint stickiness grading: Seminar of the project ‘Improvement of the marketability of cotton produced in zones affected by stickiness’. In Proceedings of the Seminar, Lille, France, 4–7 July 2001. [Google Scholar]
- Backeberg, G.R.; Water, A.J.S. Underutilised indigenous and traditional crops: Why is research on water use important for South Africa? South Afr. J. Plant Soil 2013, 1862, 291–292. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Bartova, V.; Barta, J.; Divis, J.; Svajner, J.; Peterka, J. Crude protein content in tubers of starch processing potato cultivars in dependence on different agroecological conditions. J. Cent. Eur. Agric. 2009, 10, 57–66. [Google Scholar]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar]
- Dold, A.P.; Cocks, M.L. The trade in medicinal plants in the Eastern Cape Province, South Africa. South Afr. J. Sci. 2002, 98, 589–597. [Google Scholar]
- Esch, J.R.; Friend, J.R.; Kariuki, J.K. Determination of the Vitamin C content of conventionally and organically grown fruits by cyclic voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1464–1474. [Google Scholar]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Hurr, B.M.; Huber, D.J.; Vallejos, C.E.; Talcott, S.T. Developmentally dependent responses of detached cucumber (Cucumis sativus L.) fruit to exogenous ethylene. Postharvest Biol. Technol. 2009, 52, 207–215. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B. A comparative study on the bioavailability of phenolic compounds from organic and nonorganic red grapes. Food Chem. 2019, 299, 125092. [Google Scholar] [CrossRef]
- Ity, Q.; Canino, O.F. Effect of spraying magnesium, boron, ascorbic acid and vitamin B complex on yield and fruit quality of “Canino” apricot. J. Agric. Sci. 2006, 14, 337–347. [Google Scholar]
- Khattab, M.M.; Shaban, A.E.; El-Shrief, A.H.; Mohamed, A.S.E. Growth and productivity of pomegranate trees under different irrigation levels II: Fruit quality. J. Hortic. Sci. Ornam. Plants 2011, 3, 259–264. [Google Scholar]
- Legwaila, G.M.; Mojeremane, W.; Madisa, M.E.; Mmolotsi, R.M.; Rampart, M. Potential of traditional food plants in rural household food security in Botswana. J. Hortic. For. 2011, 3, 171–177. [Google Scholar]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; D’Errico, G.; et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef]
- López, A.; Arazuri, S.; Jarén, C.; Mangado, J.; Arnal, P.; De Galarreta, J.I.R.; Riga, P.; López, R. Crude Protein Content Determination of Potatoes by NIRS Technology. Procedia Technol. 2013, 8, 488–492. [Google Scholar] [CrossRef]
- Manthey, J.A.; Perkins-Veasie, P. Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera Indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in Cucumber Seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maseko, I.; Mabhaudhi, T.; Tesfay, S.; Araya, H.T.; Fezzehazion, M.; Du Plooy, C.P. African Leafy Vegetables: A Review of Status, Production and Utilization in South Africa. Sustain. J. Rec. 2017, 10, 16. [Google Scholar] [CrossRef]
- Moyo, M.; Amoo, S.O.; Aremu, A.O.; Grúz, J.; Šubrtová, M.; Jarošová, M.; Tarkowski, P.; Dolezal, K. Determination of Mineral Constituents, Phytochemicals and Antioxidant Qualities of Cleome gynandra, Compared to Brassica oleracea and Beta vulgaris. Front. Chem. 2018, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Nyathi, M.; Mabhaudhi, T.; Van Halsema, G.; Annandale, J.; Struik, P. Benchmarking nutritional water productivity of twenty vegetables—A review. Agric. Water Manag. 2019, 221, 248–259. [Google Scholar] [CrossRef]
- Rahil, M.; Qanadillo, A. Effects of different irrigation regimes on yield and water use efficiency of cucumber crop. Agric. Water Manag. 2015, 148, 10–15. [Google Scholar] [CrossRef]
- Reinten, E.; Coetzee, J.; Van Wyk, B.-E. The potential of South African indigenous plants for the international cut flower trade. South Afr. J. Bot. 2011, 77, 934–946. [Google Scholar] [CrossRef]
- Rimpapa, Z.; Toromanovic, J.; Tahirovic, I.; Sofic, E. Total content of phenols and anthocyanins in edible fruits from Bosnia. J. Basic Med. Sci. 2007, 7, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Santos-Zea, L.; Guti, J.A.; Serna-Saldivar, S.O.; Monterrey, D.; Eugenio, A.; Sada, G. Comparative analyses of total phenols, antioxidant activity, and flavanol glycoside profile of cladode flours from different varieties of Opuntia spp. J. Agric. Food Chem. 2011, 59, 7054–7061. [Google Scholar] [CrossRef]
- Sezen, S.M.; Celikel, G.; Yazar, A.; Tekin, S.; Kapur, B. Effect of irrigation management on yield and quality of tomatoes grown in different soilless media in a glasshouse. Sci. Res. Essay 2010, 5, 41–48. [Google Scholar]
- Sharma, S.; Rao, R. Nutritional quality characteristics of pumpkin fruit as revealed by its biochemical analysis. Int. Food Res. J. 2013, 20, 2309–2316. [Google Scholar]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Uusiku, N.P.; Oelofse, A.; Duodu, K.G.; Bester, M.J.; Faber, M. Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: A review. J. Food Compos. Anal. 2010, 23, 499–509. [Google Scholar] [CrossRef]
- Van Wyk, B.-E. Food Plants of the World; Timber Press: Portland, OR, USA, 2005. [Google Scholar]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z.; Yang, X.; Wang, X.; Pretorius, B. Systematic comparison of C3 and C4 plants based on metabolic network analysis. BMC Syst. Biol. 2017, 65, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragaria x ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2011, 62, 1103–1118. [Google Scholar] [CrossRef]
| Chemical Analysis (Micro-Minerals) | ||||||
|---|---|---|---|---|---|---|
| Fe | Mn | Cu | Zn | pH | ||
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||
| SL | 30.3 | 59.4 | 1.24 | 9.36 | 7.69 | |
| L | 33.2 | 59.8 | 1.27 | 8.96 | 7.74 | |
| P | Ca | Mg | K | Na | Total N | |
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | % | |
| SL | 35.16 | 1900 | 141 | 243 | 35.5 | 0.105 |
| L | 34.4 | 1810 | 133 | 217 | 28.7 | 0.113 |
| Treatment | β-carotene (mg 100 g−1 DW) | Vitamin C (mg 100 g−1 DW) | Vitamin E (mg 100 g−1 DW) | Total Flavonoids (CE g−1 DW) | Total Phenols (GAE g−1 DW) |
|---|---|---|---|---|---|
| Greenhouse | |||||
| W1S1 | 1.6(0.0) | 26.6(0.2) | 11.7(1.1) | 0.66(0.03) | 3.1(0.1) |
| W2S1 | 1.6(0.0) | 24.3(0.2) | 29.8(0.1) | 0.56(0.03) | 5.2(0.2) |
| W3S1 | 1.7(0.01) | 23.5(0.5) | 24.4(13.4) | 0.25(0.1) | 4.5(0.1) |
| W1S2 | 1.5(0.01) | 23.8(1.9) | 9.3(5.5) | 0.26(0.1) | 4.4(0.1) |
| W2S2 | 1.6(0.01) | 30.3(0.9) | 31.7(0.5) | 0.55(0.02) | 5.8(0.2) |
| W3S2 | 1.5(0.01) | 23.2(0.9) | 35.1(0.5) | 0.21(0.0) | 4.2(0.0) |
| Grand mean | 1.6 | 25.3(0.1) | 23.7(0.9) | 0.4 | 4.5 |
| LSD0.05 | 0.020 | 1.528 | 12.95 | 0.060 | 0.196 |
| p-value | 0.001 | 0.001 | 0.204 | 0.001 | 0.001 |
| Shade net | |||||
| W1S1 | 1.5(0.0) | 33.1(0.5) | 18.1(16.9) | 0.75(0.01) | 4.2(0.1) |
| W2S1 | 1.6(0.1) | 30.2(0.4) | 16.9(3.7) | 0.84(0.0) | 5.3(0.1) |
| W3S1 | 1.5(0.0) | 28.2(0.0) | 11.3(5.8) | 0.54(0.1) | 3.6(0.0) |
| W1S2 | 1.5(0.0) | 31.7(16.8) | 10(3.4) | 0.63(0.02) | 4.3(0.3) |
| W2S2 | 1.5(0.1) | 22.6(0.1) | 12.5(2.9) | 0.77(0.03) | 4.4(0.1) |
| W3S2 | 1.5(0.0) | 27.2(0.1) | 14.3(1.1) | 0.49(0.0) | 3.5(0.1) |
| Grand mean | 1.5 | 28.8 | 13.9 | 0.670 | 4.2 |
| LSD0.05 | 0.009 | 12.58 | 19.35 | 0.038 | 0.205 |
| p-value | 0.001 | 0.658 | 0.29 | 0.009 | 0.001 |
| Open field | |||||
| W1S1 | 1.6(0.0) | 17.0(0.7) | 10.7(0.5) | 0.73(0.03) | 6.4(0.01) |
| W2S1 | 1.5(0.0) | 19.0(0.4) | 11.8(2.7) | 0.47(0.02) | 4.1(0.1) |
| W3S1 | 1.5(0.01) | 16.6(0.6) | 8.3(3.0) | 0.85(0.02) | 4.8(0.1) |
| W1S2 | 1.6(0.01) | 18.7(0.6) | 13.4(3.2) | 0.42(0.02) | 5.4(0.1) |
| W2S2 | 1.5(0.0) | 27.5(0.9) | 13.5(0.4) | 0.41(0.02) | 5.1(0.0) |
| W3S1 | 1.5(0.01) | 15.5(0.7) | 9.7(0.3) | 0.65(0.04) | 3.1(0.2) |
| Grand mean | 1.5 | 19.03 | 11.2 | 0.59 | 4.8 |
| LSD0.05 | 0.009 | 1.231 | 6.079 | 0.057 | 0.207 |
| p-value | 0.001 | 0.001 | 0.809 | 0.001 | 0.001 |
| Treatment | Moisture (g) | Copper | Iron | Manganese | Zinc |
|---|---|---|---|---|---|
| Greenhouse | |||||
| W1S1 | 193(36) | 0.9(0.0) | 1.8(0.1) | 0.8(0.0) | 7.7(1.2) |
| W2S1 | 179(40) | 0.7(0.1) | 2.0(1.5) | 0.9(0.1) | 9.3(0.8) |
| W3S1 | 78(42) | 0.8(0.4) | 1.6(0.2) | 1.0(0.1) | 10.1(1.8) |
| W1S2 | 95(35) | 0.7(0.4) | 2.8(1.8 | 1.1(0.1) | 12.7(1.5) |
| W2S2 | 152(5) | 0.5(0.0) | 3.8(0.2) | 0.9(0.2) | 8.6(2.0) |
| W3S2 | 129(22) | 0.5(0.4) | 0.5(0.1) | 0.9(0.1) | 10.6(0.6) |
| Grand mean | 138 | 0.689 | 2.1 | 0.942 | 9.8 |
| LSD0.05 | 98.4 | 0.42 | 1.81 | 0.1854 | 2.228 |
| p-value | 0.15 | 0.99 | 0.06 | 0.01 | 0.01 |
| Shade net | |||||
| W1S1 | 162(24) | 0.7(0.2) | 0.9(0.1) | 0.8(0.0) | 7.2(1.1) |
| W2S1 | 140(30) | 0.8(0.3) | 2.7(0.3) | 0.9(0.1) | 7.1(2.2) |
| W3S1 | 83(4) | 0.6(0.1) | 2.7(0.9) | 0.9(0.1) | 7.2(0.5) |
| W1S2 | 157(5) | 0.6(0.1) | 1.4(0.6) | 0.8(0.0) | 8.8(0.0) |
| W2S2 | 146(5) | 0.8(0.3) | 1.8(0.2) | 0.8(0.1) | 12.7(0.6) |
| W3S2 | 79(25) | 0.6(0.1) | 1.7(0.5) | 0.8(0.1) | 6.4(0.8) |
| Grand mean | 127.7 | 0.7 | 1.9 | 0.811 | 8.23 |
| LSD0.05 | 35.9 | 0.4 | 0.7 | 0.1475 | 2.177 |
| p-value | 0.9 | 0.89 | 0.03 | 0.59 | 0.01 |
| Open field | |||||
| W1S1 | 146(50) | 0.5(0.0) | 2.4(0.8) | 0.5(0.1) | 5.1(0.9) |
| W2S1 | 220(21) | 0.8(0.1) | 2.6(0.3) | 0.7(0.2) | 7.9(0.4) |
| W3S1 | 29(16) | 0.6(0.2) | 1.8(1.3) | 0.7(0.1) | 7.7(0.2) |
| W1S2 | 162(6) | 0.7(0.5) | 1.3(0.2) | 0.8(0.1) | 6.8(0.4) |
| W2S2 | 155(4) | 0.6(0.1) | 2.1(0.8) | 0.7(0.0) | 6.9(0.1) |
| W3S1 | 80(19) | 0.7(0.1) | 0.6(0.2) | 0.6(0.1) | 7.5(1.4) |
| Grand mean | 137 | 0.7 | 1.8 | 0.7 | 6.98 |
| LSD0.05 | 40 | 0.341 | 1.211 | 0.231 | 1.153 |
| p-value | 0.03 | 0.20 | 0.61 | 0.181 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluleke, M.K.; Moja, S.J.; Nyathi, M.; Modise, D.M. Nutrient Concentration of African Horned Cucumber (Cucumis metuliferus L) Fruit under Different Soil Types, Environments, and Varying Irrigation Water Levels. Horticulturae 2021, 7, 76. https://doi.org/10.3390/horticulturae7040076
Maluleke MK, Moja SJ, Nyathi M, Modise DM. Nutrient Concentration of African Horned Cucumber (Cucumis metuliferus L) Fruit under Different Soil Types, Environments, and Varying Irrigation Water Levels. Horticulturae. 2021; 7(4):76. https://doi.org/10.3390/horticulturae7040076
Chicago/Turabian StyleMaluleke, Mdungazi K, Shadung J Moja, Melvin Nyathi, and David M Modise. 2021. "Nutrient Concentration of African Horned Cucumber (Cucumis metuliferus L) Fruit under Different Soil Types, Environments, and Varying Irrigation Water Levels" Horticulturae 7, no. 4: 76. https://doi.org/10.3390/horticulturae7040076
APA StyleMaluleke, M. K., Moja, S. J., Nyathi, M., & Modise, D. M. (2021). Nutrient Concentration of African Horned Cucumber (Cucumis metuliferus L) Fruit under Different Soil Types, Environments, and Varying Irrigation Water Levels. Horticulturae, 7(4), 76. https://doi.org/10.3390/horticulturae7040076





