Effects of Plant Age and Root Damage on Internalization of Shiga Toxin-Producing Escherichia coli in Leafy Vegetables and Herbs
Abstract
1. Introduction
2. Materials and Methods
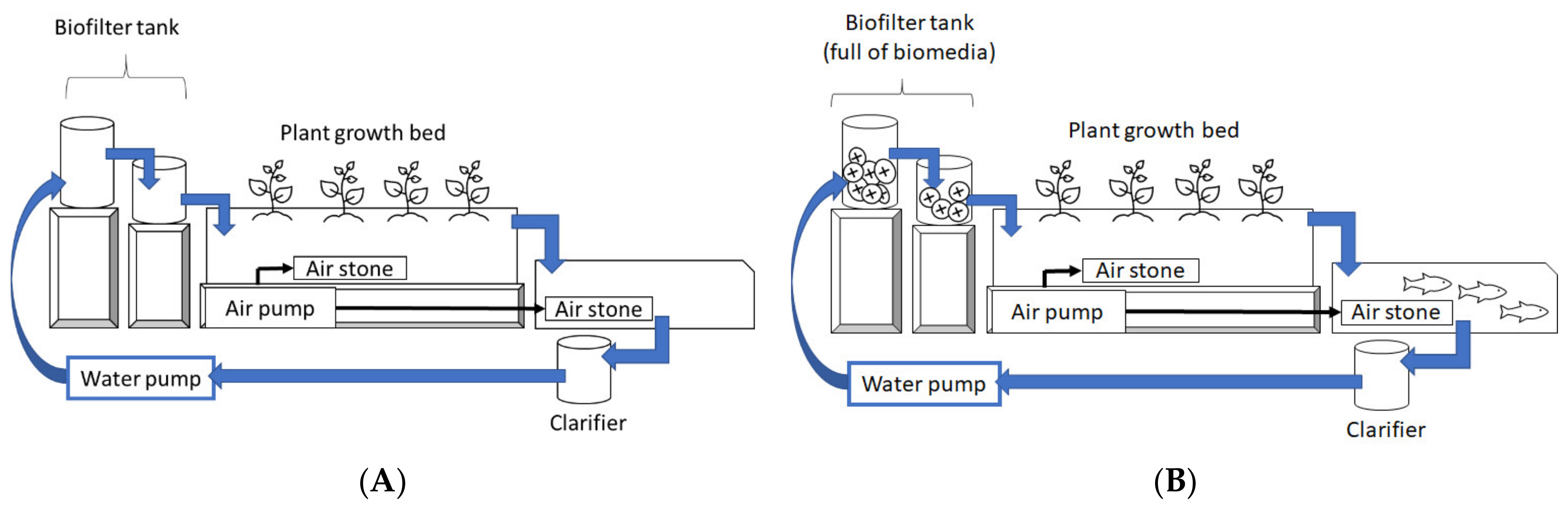
2.1. System Setup
2.2. Plant Materials and Growing Conditions
2.3. Root Damage Treatment
2.4. Plant Sample Collection and STEC E. coli Isolation
2.5. Microbial Detection in Water and Fish Feces Samples
2.6. PCR Assay for Detection of Virulence Genes
2.7. Experimental Design and Data Analysis
3. Results and Discussion
3.1. Water Conditions for Aquaponic and Hydroponic Systems
3.2. The Effects of Growing System and Plant Species on the Occurrence of STEC
3.3. The Effects of Plant Age and Root Damage on the Degree of Internalization of STEC in Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; Food and Agriculture Organization: Rome, Italy, 2012; 154p. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World: Safeguarding against Economic Slowdowns and Downturns; Food and Agriculture Organization: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- Rees, W.E. Why Urban Agriculture? Notes for the IDRC Development Forum on Cities Feeding People: A Growth Industry. Vancouver, BC; City Farmer; Canada’s Office of Urban Agriculture: Vancouver, BC, Canada, 1997. [Google Scholar]
- Garnett, T. Growing Food in Cities; Sustainable Agriculture, Food and Environment (SAFE) Alliance: London, UK, 1996; ISBN 1-900670-56-9. [Google Scholar]
- Hoornweg, D.; Munro-Faure, P. Urban Agriculture for Sustainable Poverty Alleviation and Food Security; Food and Agriculture Organization: Rome, Italy, 2008. [Google Scholar]
- Brook, R.; Dávila, J. The Peri-Urban Interface: A Tale of Two Cities; Brook, R., Dávila, J., Eds.; Development Planning Unit, UCL & University of Wales at Bangor: London, UK, 2000. [Google Scholar]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; de la Peña, C.M.; Silva, J.L.; Luna-Guevara, M.L. The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. Int. J. Microbiol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, L.; Ximenes, E.; Seockmo, K.; Ladisch, M. Foodborne pathogens in horticultural production systems: Ecology and mitigation. Sci. Hortic. 2018, 236, 192–206. [Google Scholar] [CrossRef]
- Dewey-Mattia, D.; Kisselburgh, H.; Manikonda, K.; Silver, R.; Subramhanya, S.; Sundararaman, P.; Whitham, H.; Crowe, S. Surveillance for Foodborne Disease Outbreaks, United States, 2017 Annual Report; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2018; p. 15.
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. J. CDC 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hoc, A. Advisory Committee on the Microbiological Safety of Food; Food Standards Agency: London, UK, 2007.
- European Food Safety Authority. Urgent advice on the public health risk of Shiga-toxin producing Escherichia coli in fresh vegetables. EFSA J. 2011, 9, 2274. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Deering, A.J.; Mauer, L.J.; Pruitt, R.E. Internalization of E. coli O157:H7 and Salmonella spp. in plants: A review. Food Res. Int. 2012, 45, 567–575. [Google Scholar] [CrossRef]
- Jay-Russell, M.T.; Hake, A.F.; Bengson, Y.; Thiptara, A.; Nguyen, T. Prevalence and Characterization of Escherichia coli and Salmonella strains isolated from stray dog and coyote deces in a major leafy greens production region at the United States-Mexico Border. PLoS ONE 2014, 9, e113433. [Google Scholar] [CrossRef]
- Bhargaw, A.; Chauhan, P. Analysis of soilless farming in urban agriculture. J. Pharmacogn. Phytochem. 2020, 9, 239–242. [Google Scholar]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled environment food production for urban agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, G.; Kim, H.-J.; Brown, P.B.; Huang, J.-Y. Comparative life Cycle assessment of aquaponics and hydroponics in the midwestern United States. J. Clean. Prod. 2020, 275, 122888. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Nutrient management regime affects water quality, crop growth, and nitrogen use efficiency of aquaponic systems. Sci. Hortic. 2019, 256, 108619. [Google Scholar] [CrossRef]
- Azevedo, I.; Albano, H.; Silva, J.; Teixeira, P. Food safety in the domestic environment. Food Control 2014, 37, 272–276. [Google Scholar] [CrossRef]
- Oliver, L.P.; Coyle, S.D.; Bright, L.A.; Shultz, R.C.; Hager, J.V.; Tidwell, J.H. Comparison of four artificial light technologies for indoor aquaponic production of Swiss chard, Beta vulgaris, and kale, Brassica oleracea. J. World Aquac. Soc. 2018, 49, 837–844. [Google Scholar] [CrossRef]
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.-J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the U.S. midwest. J. Appl. Aquac. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Wirza, R.; Nazir, S. Urban aquaponics farming and cities—A systematic literature review. Rev. Environ. Health 2020, 36. [Google Scholar] [CrossRef]
- Chalmers, G.A. Aquaponics and Food Safety; Springer: Lethbridge, AB, Canada, 2004; p. 114. [Google Scholar]
- Wongkiew, S.; Popp, B.N.; Kim, H.-J.; Khanal, S.K. Fate of nitrogen in floating-raft aquaponic systems using natural abundance nitrogen isotopic compositions. Int. Biodeterior. Biodegrad. 2017, 125, 24–32. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing nutrient composition and concentration in tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Orozco, L.; Rico-Romero, L.; Escartín, E.F. Microbiological profile of greenhouses in a farm producing hydroponic tomatoes. J. Food Prot. 2008, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, G.J. Fertilizer management for greenhouse vegetables. Fla. Greenh. Veg. Prod. Handb. 2001, 3, 1–19. [Google Scholar]
- Wang, Y.-J.; Deering, A.J.; Kim, H.-J. The Occurrence of Shiga Toxin-Producing E. coli in Aquaponic and Hydroponic Systems. Horticulturae 2020, 6, 1. [Google Scholar] [CrossRef]
- Weller, D.L.; Saylor, L.; Turkon, P. Total coliform and generic E. coli levels, and Salmonella presence in eight experimental aquaponics and hydroponics systems: A brief report highlighting exploratory data. Horticulturae 2020, 6, 42. [Google Scholar] [CrossRef]
- Moriarty, M.J.; Semmens, K.; Bissonnette, G.K.; Jaczynski, J. Internalization assessment of E. coli O157:H7 in hydroponically grown lettuce. LWT 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Hora, R.; Warriner, K.; Shelp, B.J.; Griffiths, M.W. Internalization of Escherichia coli O157:H7 following biological and mechanical disruption of growing spinach plants. J. Food Prot. 2005, 68, 2506–2509. [Google Scholar] [CrossRef]
- Aruscavage, D.; Miller, S.A.; Ivey, M.L.L.; Lee, K.; le Jeune, J.T. Survival and dissemination of Escherichia coli O157:H7 on physically and biologically damaged lettuce plants. J. Food Prot. 2008, 71, 2384–2388. [Google Scholar] [CrossRef]
- Jablasone, J.; Warriner, K.; Griffiths, M. Interactions of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system. Int. J. Food Microbiol. 2005, 99, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Mootian, G.; Wu, W.-H.; Matthews, K.R. Transfer of Escherichia coli O157:H7 from soil, water, and manure contaminated with low numbers of the pathogen to lettuce plants. J. Food Prot. 2009, 72, 2308–2312. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Effects of hydraulic loading rate on spatial and temporal water quality characteristics and crop growth and yield in aquaponic systems. Horticulturae 2020, 6, 9. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yang, T.; Lin, M.-Y.; Langenhoven, P. Plant propagation for successful hydroponic production©. Acta Hortic. 2018, 109–116. [Google Scholar] [CrossRef]
- Medina, V.F.; Jeffers, P.M.; Larson, S.L.; Perez, W. Sterilization of plants for phytoremediation studies by bleach treatment. Int. J. Phytoremed. 2000, 2, 287–295. [Google Scholar] [CrossRef]
- Guzel, M.; Moreira, R.G.; Omac, B.; Castell-Perez, M.E. Quantifying the effectiveness of washing treatments on the microbial quality of fresh-cut romaine lettuce and cantaloupe. LWT Food Sci. Technol. 2017, 86. [Google Scholar] [CrossRef]
- Andrews, W.H.; Wang, H.; Jacobson, A.; Hammack, T. Bacteriological Analytical Manual (BAM) Chapter 5: Salmonella; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2018.
- Feng, P.; Weagant, S.D.; Grant, M.A.; Burkhardt, W. BAM 4: Enumeration of Escherichia coli and the Coliform bacteria; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2002.
- Fode-Vaughan, K.A.; Maki, J.S.; Benson, J.A.; Collins, M.L.P. Direct PCR detection of Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 37, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Tirado, M.C.; Clarke, R.; Jaykus, L.A.; McQuatters-Gollop, A.; Frank, J.M. Climate change and food safety: A review. Food Res. Int. 2010, 43, 1745–1765. [Google Scholar] [CrossRef]
- Shadbolt, C.; Ross, T.; McMeekin, T.A. Differentiation of the effects of lethal pH and water activity: Food safety implications. Lett. Appl. Microbiol. 2001, 32, 99–102. [Google Scholar] [CrossRef]
- Guan, T.Y.; Holley, R.A. Pathogen survival in Swine manure environments and transmission of human enteric illness—A review. J. Environ. Qual. 2003, 32, 383–392. [Google Scholar] [CrossRef]
- Wang, G.; Doyle, M.P. Survival of Enterohemorrhagic Escherichia coli O157:H7 in water. J. Food Prot. 1998, 61, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Ellajosyula, K.R.; Doores, S.; Mills, E.W.; Wilson, R.A.; Anantheswaran, R.C.; Knabel, S.J. Destruction of Escherichia coli O157:H7 and Salmonella typhimurium in Lebanon bologna by interaction of fermentaron pH, heating temperature, and time. J. Food Prot. 1998, 61, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Patel, J.; Sharma, V.K. Role of curli and plant cultivation conditions on Escherichia coli O157:H7 internalization into spinach grown on hydroponics and in soil. Int. J. Food Microbiol. 2014, 173, 48–53. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Shaw, A.; Helterbran, K.; Evans, M.R.; Currey, C. Growth of Escherichia coli O157:H7, Non-O157 Shiga Toxin–Producing Escherichia coli, and Salmonella in water and hydroponic fertilizer solutions. J. Food Prot. 2016, 79, 2179–2183. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Deering, A.J.; Kim, H.-J. Reply to comment on “The occurrence of Shiga Toxin-Producing E. coli in aquaponic and hydroponic systems”. Horticulturae 2021, 7, 37. [Google Scholar] [CrossRef]
- Popma, T.; Masser, M. Tilapia: Life History and Biology; SRAC Publication No. 283; Southern Regional Aquaculture Center: Stoneville, MS, USA, 1999. [Google Scholar]
- Suhalim, R.; Huang, Y.-W.; Burtle, G.J. Survival of Escherichia coli O157:H7 in channel catfish pond and holding tank water. LWT Food Sci. Technol. 2008, 41, 1116–1121. [Google Scholar] [CrossRef]
- Moriarty, M.J.; Semmens, K.; Bissonnette, G.K.; Jaczynski, J. Inactivation with UV-radiation and internalization assessment of coliforms and Escherichia coli in aquaponically grown lettuce. LWT 2018, 89, 624–630. [Google Scholar] [CrossRef]
- Wright, K.M.; Chapman, S.; McGeachy, K.; Humphris, S.; Campbell, E.; Toth, I.K.; Holden, N.J. The endophytic lifestyle of Escherichia coli O157:H7: Quantification and internal localization in roots. Phytopathology 2013, 103, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Merget, B.; Forbes, K.J.; Brennan, F.; McAteer, S.; Shepherd, T.; Strachan, N.J.C.; Holden, N.J. Influence of plant species, tissue type, and temperature on the capacity of Shiga-Toxigenic Escherichia coli to colonize, grow, and be internalized by plants. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Hou, Z.; Fink, R.C.; Radtke, C.; Sadowsky, M.J.; Diez-Gonzalez, F. Incidence of naturally internalized bacteria in lettuce leaves. Int. J. Food Microbiol. 2013, 162, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cardinale, M.; Grube, M.; Berg, G. Biotic stress shifted structure and abundance of Enterobacteriaceae in the lettuce microbiome. PLoS ONE 2015, 10, e0118068. [Google Scholar] [CrossRef]
- Wright, K.M.; Crozier, L.; Marshall, J.; Merget, B.; Holmes, A.; Holden, N.J. Differences in internalization and growth of Escherichia coli O157:H7 within the apoplast of edible plants, spinach and lettuce, compared with the model species Nicotiana benthamiana. Microb. Biotechnol. 2017, 10, 555–569. [Google Scholar] [CrossRef]
- Sharma, M.; Ingram, D.T.; Patel, J.; Millner, P.; Wang, X.; Hull, A.E.; Donnenber, M.S. A novel approach to investigate the uptake and internalization of Escherichia coli O157:H7 in spinach cultivated in soil and hydroponic medium. J. Food Prot. 2009, 72, 1513–1520. [Google Scholar] [CrossRef]
- Deering, A.J.; Pruitt, R.E.; Mauer, L.J.; Reuhs, B.L. Identification of the cellular location of internalized Escherichia coli O157:H7 in mung bean, Vigna radiata, by immunocytochemical techniques. J. Food Prot. 2011, 74, 1224–1230. [Google Scholar] [CrossRef]
- Bernstein, N.; Sela, S.; Neder-Lavon, S. Assessment of contamination potential of lettuce by Salmonella enterica Serovar Newport added to the plant growing medium. J. Food Prot. 2007, 70, 1717–1722. [Google Scholar] [CrossRef]
- Bernstein, N.; Sela, S.; Neder-Lavon, S. Effect of irrigation regimes on persistence of Salmonella enterica Serovar Newport in small experimental pots designed for plant cultivation. Irrig. Sci. 2007, 26, 1–8. [Google Scholar] [CrossRef]
- Ward, R.L.; Mahler, R.J. Uptake of bacteriophage F2 through plant roots. Appl. Environ. Microbiol. 1982, 43, 1098–1103. [Google Scholar] [CrossRef]
- Raftoyannis, Y.; Dick, M.W. Effects of inoculum density, plant age and temperature on disease severity caused by Pythiaceous fungi on several plants. Phytoparasitica 2002, 30, 67–76. [Google Scholar] [CrossRef]
- Islam, S.M.A.; Math, R.K.; Kim, J.M.; Yun, M.G.; Cho, J.J.; Kim, E.J.; Lee, Y.H.; Yun, H.D. Effect of plant age on endophytic bacterial diversity of balloon flower (Platycodon grandiflorum) root and their antimicrobial activities. Curr. Microbiol. 2010, 61, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Melotto, M. Human Pathogen colonization of lettuce dependent upon plant genotype and defense response activation. Front. Plant Sci. 2020, 10, 1769. [Google Scholar] [CrossRef] [PubMed]
| Sequence | PCR Program | Reference | |
|---|---|---|---|
| Shiga Toxin-Producing E. coli (STEC) | |||
| stx1-F | CAGTTAATGTGGTGGCGAAG | 95 °C for 3 min, 95 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min, repeat steps 2–4 30 times, 72 °C for 10 min | [43] |
| stx1-R | CACCAGACAATGTAACCGCTG |
| Production System | DO z (mg/L) | pH | Temperature (°C) | EC (dS/m) |
|---|---|---|---|---|
| Aquaponics | 7.81 ± 0.03 y (7.73–8.09) | 6.5 ± 0.0 x | 22.9 ± 0.1 (22.7–23.1) | 1.52 ± 0.04 (1.48–1.58) |
| Hydroponics | 7.78 ± 0.04 (7.70–7.84) | 6.5 ± 0.0 x | 23.0 ± 0.1 (22.7–23.3) | 1.54 ± 0.02 (1.50–1.56) |
| Significance | ns | ns | ns | ns |
| Vegetable | Tissue Type | Control | Root Damage at Transplanting (T1) | Root Damage at Preharvest (T2) | |||
|---|---|---|---|---|---|---|---|
| Aquaponics | Hydroponics | Aquaponics | Hydroponics | Aquaponics | Hydroponics | ||
| Basil | Internal leaf | – z | – | +(18/18) | – | – | – |
| Internal root | – | – | +(18/18) | – | +(18/18) | – | |
| Root surface | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Water | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Fish feces | +(18/18) | NA | +(18/18) | NA | +(18/18) | NA | |
| Cilantro | Internal leaf | – | – | +(18/18) | – | – | – |
| Internal root | – | – | +(18/18) | – | +(18/18) | – | |
| Root surface | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Water | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Fish feces | +(18/18) | NA | +(18/18) | NA | +(18/18) | NA | |
| Lettuce | Internal leaf | – | – | +(18/18) | – | – | – |
| Internal root | – | – | +(18/18) | – | +(18/18) | – | |
| Root surface | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Water | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Fish feces | +(18/18) | NA | +(18/18) | NA | +(18/18) | NA | |
| Fort Smith, Arkansas Kale | Internal leaf | – | – | +(18/18) | – | – | – |
| Internal root | – | – | +(18/18) | – | +(18/18) | – | |
| Root surface | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Water | +(18/18) | – | +(18/18) | – | +(18/18) | – | |
| Fish feces | +(18/18) | NA | +(18/18) | NA | +(18/18) | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; J. Deering, A.; Kim, H.-J. Effects of Plant Age and Root Damage on Internalization of Shiga Toxin-Producing Escherichia coli in Leafy Vegetables and Herbs. Horticulturae 2021, 7, 68. https://doi.org/10.3390/horticulturae7040068
Wang Y-J, J. Deering A, Kim H-J. Effects of Plant Age and Root Damage on Internalization of Shiga Toxin-Producing Escherichia coli in Leafy Vegetables and Herbs. Horticulturae. 2021; 7(4):68. https://doi.org/10.3390/horticulturae7040068
Chicago/Turabian StyleWang, Yi-Ju, Amanda J. Deering, and Hye-Ji Kim. 2021. "Effects of Plant Age and Root Damage on Internalization of Shiga Toxin-Producing Escherichia coli in Leafy Vegetables and Herbs" Horticulturae 7, no. 4: 68. https://doi.org/10.3390/horticulturae7040068
APA StyleWang, Y.-J., J. Deering, A., & Kim, H.-J. (2021). Effects of Plant Age and Root Damage on Internalization of Shiga Toxin-Producing Escherichia coli in Leafy Vegetables and Herbs. Horticulturae, 7(4), 68. https://doi.org/10.3390/horticulturae7040068







