Opportunities of Reduced Nitrogen Supply for Productivity, Taste, Valuable Compounds and Storage Life of Cocktail Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Material
2.2. Cultivation and N Supply
2.3. Harvest
2.4. Storage
2.5. Spectroscopic Measurements
2.6. Firmness of the Fruit Pulp
2.7. Sampling and Chemical Analyses
2.8. Sensory Evaluation
2.9. Statistics
3. Results
3.1. Yield
3.2. External Quality Parameters
3.3. Chemical Composition of Tomato Fruits
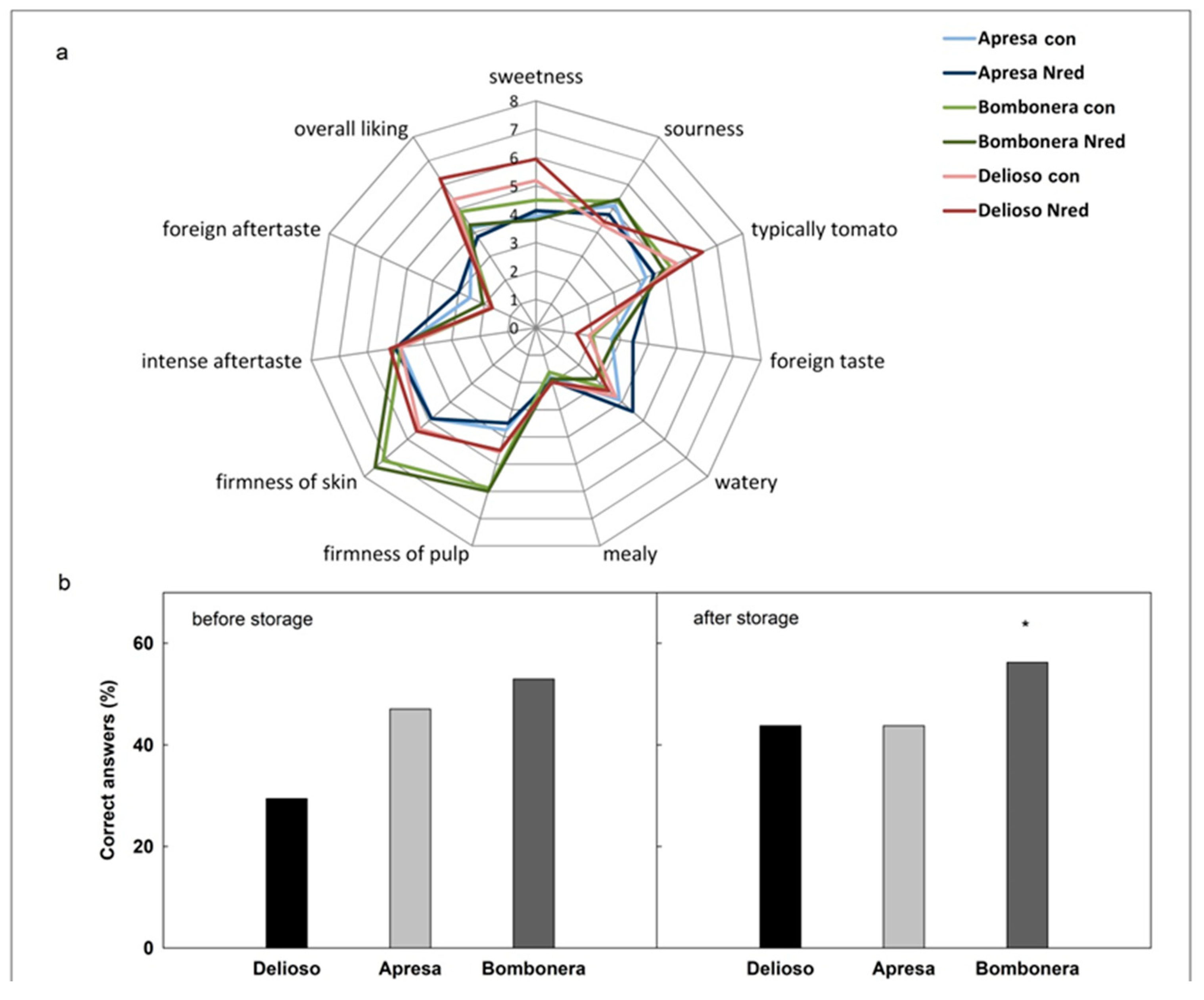
3.4. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Truffault, V.; Marlene, R.; Brajeul, E.; Vercambre, G.; Gautier, H. To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield. Agronomy 2019, 9, 80. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Bumgarner, N.R.; Scheerens, J.C.; Mullen, R.W.; Bennett, M.A.; Ling, P.P.; Kleinhenz, M.D. Root-zone temperature and nitrogen affect the yield and secondary metabolite concentration of fall- and spring-grown, high-density leaf lettuce. J. Sci. Food Agric. 2012, 92, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Bačkor, M. Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil 2007, 297, 255–265. [Google Scholar] [CrossRef]
- Statista. Erntemenge von Gemüse und Melonen weltweit nach Art im Jahr. 2014. Available online: www.statista.com/statistik/daten/studie/29305/umfrage/weltweite-erzeugung-von-gemuese-nach-arten/ (accessed on 28 September 2020).
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2019, 55, 11–32. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Flores, P.; Hernández, V.; Hellín, P.; Fenoll, J.; Cava, J.; Mestre, T.; Martínez, V. Metabolite profile of the tomato dwarf cultivar Micro-Tom and comparative response to saline and nutritional stresses with regard to a commercial cultivar. J. Sci. Food Agric. 2016, 96, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Gkisakis, V.; Krumbein, A.; Livieratos, I.; Köpke, U. Old and endangered tomato cultivars under organic greenhouse production: Effect of harvest time on flavour profile and consumer acceptance. Int. J. Food Sci. Technol. 2010, 45, 2250–2257. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Huang, S.-W.; Liu, R.-L.; Jin, J.-Y. Effects of nitrogen application on flavor compounds of cherry tomato fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Sung, J.; Sonn, Y.; Lee, Y.; Kang, S.; Ha, S.; Krishnan, H.B.; Oh, T.-K. Compositional changes of selected amino acids, organic acids, and soluble sugars in the xylem sap of N, P, or K-deficient tomato plants. J. Plant Nutr. Soil Sci. 2015, 178, 792–797. [Google Scholar] [CrossRef]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Impact of nitrogen supply limitation on tomato fruit composition. Sci. Hortic. 2020, 264, 109173. [Google Scholar] [CrossRef]
- Schmidt, L.; Hey, M.; Kürbel, P.; Zinkernagel, J. Einfluss verringerter Stickstoffdüngung auf Ertrag und Produktqualität von zwei lycopinreichen Tomatensorten. DGG-Proceedings 2013, 3, 5. [Google Scholar] [CrossRef]
- Rather, K. Nährstoffversorgung von Tomaten in Bodenkultur unter dem Aspekt der Flüssigdüngung mit Tropfbewässerung. In Aktuelle Versuchsergebnisse und Informationen Aus Baden-Württemberg; LVG Heidelberg: Heidelberg, Germany, 2007; pp. 36–43. [Google Scholar]
- Skolik, P.; Morais, C.L.M.; Martin, F.L.; McAinsh, M.R. Determination of developmental and ripening stages of whole tomato fruit using portable infrared spectroscopy and Chemometrics. BMC Plant Biol. 2019, 19, 236. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- D’Souza, M.C.; Singha, S.; Ingle, M. Lycopene Concentration of Tomato Fruit can be Estimated from Chromaticity Values. HortScience 1992, 27, 465–466. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ronga, D.; Pentangelo, A.; Parisi, M. Optimizing N Fertilization to Improve Yield, Technological and Nutritional Quality of Tomato Grown in High Fertility Soil Conditions. Plants 2020, 9, 575. [Google Scholar] [CrossRef]
- Zotarelli, L.; Scholberg, J.M.; Dukes, M.D.; Muñoz-Carpena, R.; Icerman, J. Tomato yield, biomass accumulation, root distribution and irrigation water use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manag. 2009, 96, 23–34. [Google Scholar] [CrossRef]
- Hartz, T.K.; Bottoms, T.G. Nitrogen Requirements of Drip-irrigated Processing Tomatoes. HortScience 2009, 44, 1988–1993. [Google Scholar] [CrossRef]
- Duan, P.; Sun, Y.; Zhang, Y.; Fan, Q.; Yu, N.; Dang, X.; Zou, H. Assessing the Response of Tomato Yield, Fruit Composition, Nitrogen Absorption, and Soil Nitrogen Fractions to Different Fertilization Management Strategies in the Greenhouse. HortScience 2019, 54, 537–546. [Google Scholar] [CrossRef]
- Djidonou, D.; Lopiano, K.; Zhao, X.; Simonne, E.H.; Erickson, J.E.; Koch, K.E. Estimating nitrogen nutritional crop requirements of grafted tomatoes under field conditions. Sci. Hortic. 2015, 182, 18–26. [Google Scholar] [CrossRef]
- Qu, Z.; Qi, X.; Shi, R.; Zhao, Y.; Hu, Z.; Chen, Q.; Li, C. Reduced N Fertilizer Application with Optimal Blend of Controlled-Release Urea and Urea Improves Tomato Yield and Quality in Greenhouse Production System. J. Soil Sci. Plant Nutr. 2020, 20, 1741–1750. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agric. Water Manag. 2015, 161, 9–19. [Google Scholar] [CrossRef]
- Schmidt, L.; Zinkernagel, J. Is it possible to enhance the concentrations of valuable compounds by reduced nitrogen supply in several vegetable species? Acta Hort. 2014, 1106, 55–60. [Google Scholar] [CrossRef]
- Dadomo, M.; Macua, J.I.; Gainza, A.M.; Christou, M.; Dumas, Y.; Branthôme, X.; Bussières, P. Influence of water and nitrogen availability on yield components of processing tomato in the European Union countries. Acta Hort. 1994, 376, 271–274. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R. Role of internal atmosphere on fruit ripening and storability-A review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef]
- Verheul, M.J.; Slimestad, R.; Tjøstheim, I.H. From producer to consumer: Greenhouse tomato quality as affected by variety, maturity stage at harvest, transport conditions, and supermarket storage. J. Agric. Food Chem. 2015, 63, 5026–5034. [Google Scholar] [CrossRef]
- Xu, S.; Sun, X.; Lu, H.; Yang, H.; Ruan, Q.; Huang, H.; Chen, M. Detecting and Monitoring the Flavor of Tomato (Solanum lycopersicum) under the Impact of Postharvest Handlings by Physicochemical Parameters and Electronic Nose. Sensors 2018, 18, 1847. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Lara, I.; Ilahy, R.; Tlili, I.; Ali, A.; Homa, F.; Prasad, K.; Deshi, V.; Lenucci, M.S.; Hdider, C. Dynamic Changes in Health-Promoting Properties and Eating Quality During Off-Vine Ripening of Tomatoes. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1540–1560. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Assunção, N.S.; Silva, N.O.; Fernandes, F.L.; de Aquino, L.A.; de Sena Fernandes, M.E. Physico-chemical characteristics and productivity of tomato plants in function of nitrogen sources and doses. Biosci. J. 2020, 36. [Google Scholar] [CrossRef]
- Warner, J.; Zhang, T.Q.; Hao, X. Effects of nitrogen fertilization on fruit yield and quality of processing tomatoes. Can. J. Plant Sci. 2004, 84, 865–871. [Google Scholar] [CrossRef]
- Maboko, M.M.; Du Plooy, C.P. Response of Hydroponically Grown Cherry and Fresh Market Tomatoes to Reduced Nutrient Concentration and Foliar Fertilizer Application under Shadenet Conditions. HortScience 2017, 52, 572–578. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Rodríguez-Ruiz, M.; Mioto, P.T.; González-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Groher, T.; Schmittgen, S.; Noga, G.; Hunsche, M. Limitation of mineral supply as tool for the induction of secondary metabolites accumulation in tomato leaves. Plant Physiol. Biochem. 2018, 130, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Adamowicz, S.; Robin, C.; Han, P.; Desneux, N.; Le Bot, J. Interrelated responses of tomato plants and the leaf miner Tuta absoluta to nitrogen supply. Plant Biol. (Stuttg) 2016, 18, 495–504. [Google Scholar] [CrossRef]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Env. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Migliori, C.; Viscardi, D.; Parisi, M. Quality of tomato fertilized with nitrogen and phosphorus. Ital. J. Food Sci. 2010, 22, 186–191. [Google Scholar]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef]
- Kaniszewski, S.; Kosson, R.; Grzegorzewska, M.; Kowalski, A.; Badełek, E.; Szwejda-Grzybowska, J.; Tuccio, L.; Agati, G. Yield and Quality Traits of Field Grown Tomato as Affected by Cultivar and Nitrogen Application Rate. J. Agric. Sci. Technol. 2019, 21, 683–697. [Google Scholar]
- Frías-Moreno, M.N.; Espino-Díaz, M.; Dávila-Aviña, J.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F.; Molina-Corral, F.J.; Parra-Quezada, R.A.; Orozco, G.I.O. Preharvest nitrogen application affects quality and antioxidant status of two tomato cultivars. Bragantia 2020, 79, 134–144. [Google Scholar] [CrossRef]
- Kim, Y.X.; Kwon, M.C.; Lee, S.; Jung, E.S.; Lee, C.H.; Sung, J. Effects of Nutrient and Water Supply During Fruit Development on Metabolite Composition in Tomato Fruits (Solanum lycopersicum L.) Grown in Magnesium Excess Soils. Front. Plant Sci. 2020, 11, 562399. [Google Scholar] [CrossRef]
- Zotarelli, L.; Dukes, M.D.; Scholberg, J.; Muñoz-Carpena, R.; Icerman, J. Tomato nitrogen accumulation and fertilizer use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manag. 2009, 96, 1247–1258. [Google Scholar] [CrossRef]
- Geisseler, D.; Aegerter, B.J.; Miyao, E.M.; Turini, T.; Cahn, M.D. Nitrogen in soil and subsurface drip-irrigated processing tomato plants (Solanum lycopersicum L.) as affected by fertilization level. Sci. Hortic. 2020, 261, 108999. [Google Scholar] [CrossRef]
- Tavallali, V.; Esmaili, S.; Karimi, S. Nitrogen and potassium requirements of tomato plants for the optimization of fruit quality and antioxidative capacity during storage. Food Meas. 2018, 12, 755–762. [Google Scholar] [CrossRef]
- Sainju, U.M.; Dris, R.; Singh, B. Mineral nutrition of tomato. Food Agric. Environ. 2003, 1, 176–183. [Google Scholar]
- Qiu, R.J.; Du, T.S.; Kang, S.Z.; Chen, R.Q.; Wu, L.S. Influence of Water and Nitrogen Stress on Stem Sap Flow of Tomato Grown in a Solar Greenhouse. J. Amer. Soc. Hort. Sci. 2015, 140, 111–119. [Google Scholar] [CrossRef]
- Mazzitelli, J.B.; Lopez-Lauri, F.; Laurent, S.; Vidal, V.; Urban, L.; Gautier, H.; Gomez, L.; Vercambre, G.; Sérino, S.; Mazollier, C. Substantial Reduction of Water and Nitrogen Supply Does Not Negatively Affect Quality or Yield of Organically Grown Tomatoes. Acta Hortic. 2014, 1041, 103–108. [Google Scholar] [CrossRef]
- Colla, G.; Battistelli, A.; Moscatello, S.; Proietti, S.; Casa, R.; Lo Cascio, B.; Leoni, C. Effects of reduced irrigation and nitrogen fertigation rate on yield, carbohydrate accumulation, and quality of processing tomatoes. Acta Hortic. 2001, 542, 187–196. [Google Scholar] [CrossRef]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef]
- Gómez, P.; Ferrer, M.Á.; Fernández-Trujillo, J.P.; Calderón, A.; Artés, F.; Egea-Cortines, M.; Weiss, J. Structural changes, chemical composition and antioxidant activity of cherry tomato fruits (cv. Micro-Tom) stored under optimal and chilling conditions. J. Sci. Food Agric. 2009, 89, 1543–1551. [Google Scholar] [CrossRef]
- Krumbein, A.; Peters, P.; Brückner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
| Cultivation Stage | Minimum Day Temperature (°C) | Minimum Night Temperature (°C) | Temperature Set Point for Ventilation (°C) |
|---|---|---|---|
| Sowing till germination | 24 | 20 | 26 |
| Germination till planting | 22 | 18 | 26 |
| First week after planting | 24 | 20 | 26 |
| From second week after planting | 20 | 16 | 22 |
| Variety | N Supply | Total Yield (kg m−2) | Proportion of Marketable Fruits (%) | Single Fruit Weight (g) |
|---|---|---|---|---|
| ‘Delioso’ | con | 4.27 ± 0.32 | 98.87 ± 0.51 | 39.67 ± 1.00 |
| Nred | 3.98 ± 0.38 | 98.14 ± 0.86 | 36.75 ± 1.42 * | |
| ‘Apresa’ | con | 3.13 ± 0.51 | 78.40 ± 0.71 | 33.88 ± 1.31 |
| Nred | 2.91 ± 0.04 | 82.72 ± 2.68 * | 32.77 ± 0.97 | |
| ‘Bombonera’ | con | 4.10 ± 0.07 | 36.59 ± 4.94 | 51.97 ± 1.18 |
| Nred | 3.40 ± 0.23 * | 50.31 ± 10.39 | 48.67 ± 1.71 * |
| Parameter (unit) | Storage | ‘Delioso’ con | ‘Delioso’ Nred | ‘Apresa’ con | ‘Apresa’ Nred | ‘Bombonera’ con | ‘Bombonera’ Nred |
|---|---|---|---|---|---|---|---|
| Firmness (kg cm−2) | Before | 50.8 ± 4.3 a | 50.7 ± 6.2 a | 43.8 ± 4.8 a | 43.9 ± 8.2 a | 61.9 ± 6.0 a | 58.3 ± 7.8 a |
| After | 46.1 ± 5.8 b | 46.9 ± 4.2 b | 40.9 ± 4.7 b | 40.4 ± 5.4 b | 53.7 ± 7.6 b | 53.4 ± 8.0 b | |
| FLAV | Before | 1.48 ± 0.12 a | 1.51 ± 0.15 a | 1.62 ± 0.11 a | 1.60 ± 0.10 a | 2.19 ± 0.14 a | 2.19 ± 0.06 a |
| After | 1.27 ± 0.16 b | 1.26 ± 0.13 b | 1.48 ± 0.12 b | 1.44 ± 0.12 b * | 2.19 ± 0.06 a | 2.17 ± 0.07 a | |
| ANTH_RG | Before | 1.42 ± 0.07 a | 1.40 ± 0.10 a | 1.21 ± 0.12 a | 1.20 ± 0.10 a | 1.15 ± 0.16 a | 1.22 ± 0.14 a * |
| After | 1.34 ± 0.14 b | 1.33 ± 0.12 b | 1.23 ± 0.07 a | 1.20 ± 0.06 a | 1.32 ± 0.13 b | 1.34 ± 0.10 b | |
| SFR_G | Before | 1.26 ± 0.07 a | 1.24 ± 0.07 a | 1.35 ± 0.09 a | 1.36 ± 0.11 a | 1.28 ± 0.16 a | 1.23 ± 0.12 a |
| After | 1.21 ± 0.08 b | 1.20 ± 0.07 b | 1.33 ± 0.08 a | 1.31 ± 0.08 b | 1.27 ± 0.14 a | 1.26 ± 0.15 a |
| Mineral | ‘Delioso’ con | ‘Delioso’ Nred | ‘Apresa’ con | ‘Apresa’ Nred | ‘Bombonera’ con | ‘Bombonera’ Nred |
|---|---|---|---|---|---|---|
| N (g kg−1) | 2.90 ± 0.09 | 2.71 ± 0.23 | 3.15 ± 0.20 | 3.07 ± 0.35 | 2.87 ± 0.20 | 2.73 ± 0.07 |
| P (g kg−1) | 0.44 ± 0.02 | 0.44 ± 0.04 | 0.55 ± 0.02 | 0.54 ± 0.03 | 0.42 ± 0.04 | 0.40 ± 0.01 |
| K (g kg−1) | 5.05 ± 0.22 | 4.90 ± 0.41 | 6.36 ± 0.59 | 5.86 ± 0.46 | 5.29 ± 0.44 | 5.16 ± 0.28 |
| Ca (g kg−1) | 0.22 ± 0.03 | 0.21 ± 0.02 | 0.27 ± 0.06 | 0.21 ± 0.01 | 0.27 ± 0.09 | 0.20 ± 0.01 |
| Mg (g kg−1) | 0.19 ± 0.00 | 0.19 ± 0.02 | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.02 | 0.21 ± 0.01 |
| Fe (mg kg−1) | 5.88 ± 0.58 | 6.11 ± 0.67 | 7.78 ± 0.83 | 7.82 ± 1.75 | 5.91 ± 1.03 | 6.10 ± 0.73 |
| Zn (mg kg−1) | 3.60 ± 0.29 | 3.85 ± 0.53 | 4.40 ± 0.17 | 4.04 ± 0.40 | 5.34 ± 3.39 | 3.47 ± 0.09 |
| Mn (mg kg−1) | 1.12 ± 0.07 | 1.20 ± 0.09 | 1.41 ± 0.07 | 1.35 ± 0.10 | 1.29 ± 0.16 | 1.32 ± 0.09 |
| Cu (mg kg−1) | 1.79 ± 0.18 | 1.79 ± 0.26 | 2.16 ± 0.26 | 1.91 ± 0.26 | 1.46 ± 0.18 | 1.56 ± 0.08 |
| Parameter (unit) | Storage | ‘Delioso’ con | ‘Delioso’ Nred | ‘Apresa’ con | ‘Apresa’ Nred | ‘Bombonera’ con | ‘Bombonera’ Nred |
|---|---|---|---|---|---|---|---|
| Total phenolics (mg kg−1) | Before | 355.5 ± 32.5 a | 370.5 ± 35.3 a | 410.3 ± 14.2 a | 411.4 ± 17.7 a | 343.3 ± 85.2 a | 369.0 ± 54.1 a |
| After | 351.4 ± 24.8 a | 382.5 ± 33.6 a | 413.5 ± 20.6 a | 420.5 ± 36.5 a | 346.7 ± 5.4 a | 355.9 ± 13.5 a | |
| Total content of liposoluble pigments (mg kg−1) | Before | 4.60 ± 0.20 a | 4.45 ± 0.25 a | 3.31 ± 0.24 a | 3.00 ± 0.21 a | 7.28 ± 0.15 a | 7.04 ± 0.42 a |
| After | 5.75 ± 0.48 b | 6.18 ± 0.26 b | 4.19 ± 0.19 b | 4.05 ± 0.16 b | 7.05 ± 0.20 a | 7.11 ± 0.13 a | |
| Ascorbic acid (mg kg−1) | Before | 170.3 ± 19.9 a | 203.1 ± 12.0 a * | 203.1 ± 64.1 a | 191.2 ± 26.1 a | 173.1 ± 26.6 a | 167.8 ± 30.7 a |
| After | 185.8 ± 15.6 a | 216.4 ± 15.9 a* | 186.8 ± 17.8 a | 188.1 ± 23.1 a | 157.3 ± 2.8 a | 147.9 ± 7.2 a | |
| TEAC (mmol kg−1) | Before | 3.10 ± 0.08 a | 3.35 ± 0.10 a * | 3.40 ± 0.27 a | 3.55 ± 0.30 a | 3.63 ± 0.32 a | 3.67 ± 0.31 a |
| After | 3.28 ± 0.31 a | 3.57 ± 0.19 b | 3.79 ± 0.17 b | 3.91 ± 0.28 a | 3.36 ± 0.06 a | 3.55 ± 0.32 a |
| Parameter | Storage | ‘Delioso’ con | ‘Delioso’ Nred | ‘Apresa’ con | ‘Apresa’ Nred | ‘Bombonera’ con | ‘Bombonera’ Nred |
|---|---|---|---|---|---|---|---|
| Soluble solids (°Brix) | Before | 5.40 ± 0.50 a | 5.44 ± 0.40 a | 5.73 ± 0.18 a | 5.42 ± 0.46 a | 5.53 ± 1.23 a | 5.88 ± 0.76 a |
| After | 5.26 ± 0.37 a | 5.38 ± 0.49 a | 5.68 ± 0.23 a | 5.48 ± 0.37 a | 5.64 ± 0.07 a | 5.74 ± 0.18 a | |
| pH | Before | 4.15 ± 0.02 a | 4.16 ± 0.02 a | 4.18 ± 0.02 a | 4.16 ± 0.03 a | 4.22 ± 0.05 a | 4.18 ± 0.03 a |
| After | 4.24 ± 0.01 b | 4.25 ± 0.03 b | 4.25 ± 0.01 b | 4.23 ± 0.03 b | 4.22 ± 0.01 a | 4.19 ± 0.04 a | |
| Total acidity (g kg−1) | Before | 4.13 ± 0.38 a | 4.09 ± 0.21 a | 4.96 ± 0.47 a | 5.03 ± 0.22 a | 4.08 ± 0.88 a | 4.01 ± 0.54 a |
| After | 3.52 ± 0.27 a | 3.57 ± 0.12 b | 4.62 ± 0.27 a | 4.31 ± 0.39 a | 4.01 ± 0.03 a | 3.95 ± 0.13 a | |
| Chroma | Before | 22.14 ± 0.33 a | 21.85 ± 1.83 a | 24.34 ± 1.31 a | 25.37 ± 1.01 a | 14.16 ± 4.97 a | 10.28 ± 6.85 a |
| After | 19.10 ± 11.18 a | 19.82 ± 11.92 a | 4.83 ± 3.05 b | 4.47 ± 2.93 b | 10.33 ± 5.83 a | 19.02 ± 1.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, L.; Zinkernagel, J. Opportunities of Reduced Nitrogen Supply for Productivity, Taste, Valuable Compounds and Storage Life of Cocktail Tomato. Horticulturae 2021, 7, 48. https://doi.org/10.3390/horticulturae7030048
Schmidt L, Zinkernagel J. Opportunities of Reduced Nitrogen Supply for Productivity, Taste, Valuable Compounds and Storage Life of Cocktail Tomato. Horticulturae. 2021; 7(3):48. https://doi.org/10.3390/horticulturae7030048
Chicago/Turabian StyleSchmidt, Lilian, and Jana Zinkernagel. 2021. "Opportunities of Reduced Nitrogen Supply for Productivity, Taste, Valuable Compounds and Storage Life of Cocktail Tomato" Horticulturae 7, no. 3: 48. https://doi.org/10.3390/horticulturae7030048
APA StyleSchmidt, L., & Zinkernagel, J. (2021). Opportunities of Reduced Nitrogen Supply for Productivity, Taste, Valuable Compounds and Storage Life of Cocktail Tomato. Horticulturae, 7(3), 48. https://doi.org/10.3390/horticulturae7030048







