Leaf Photosynthesis and Carbon Metabolism Adapt to Crop Load in ‘Gala’ Apple Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Photosynthetic Gas Exchange
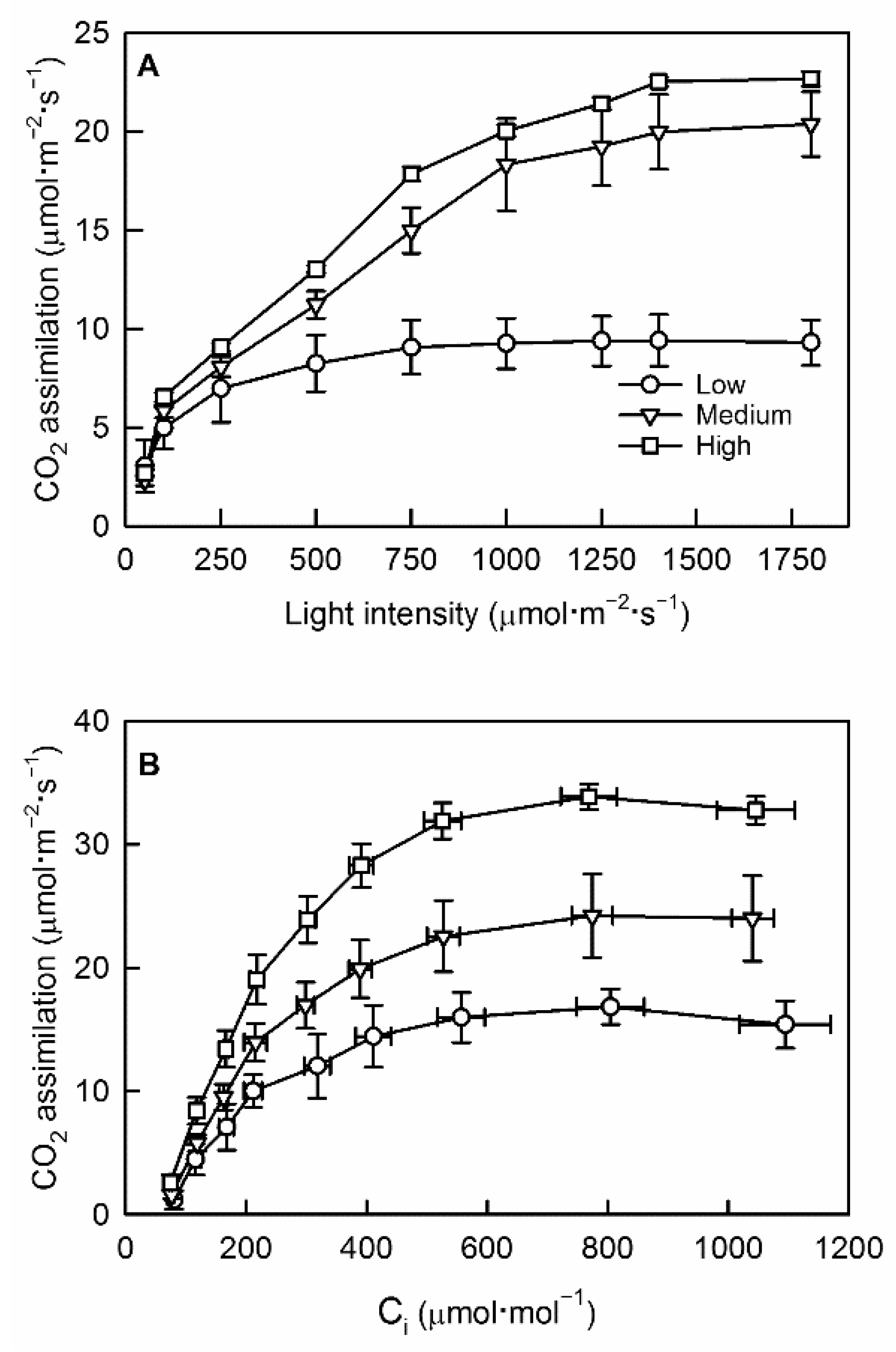
2.3. Light Response Curves
2.4. A/Ci Curves
2.5. Metabolites Analysis
2.6. Starch Analysis
2.7. Enzyme Activity Assays
2.8. Statistical Analysis
3. Results
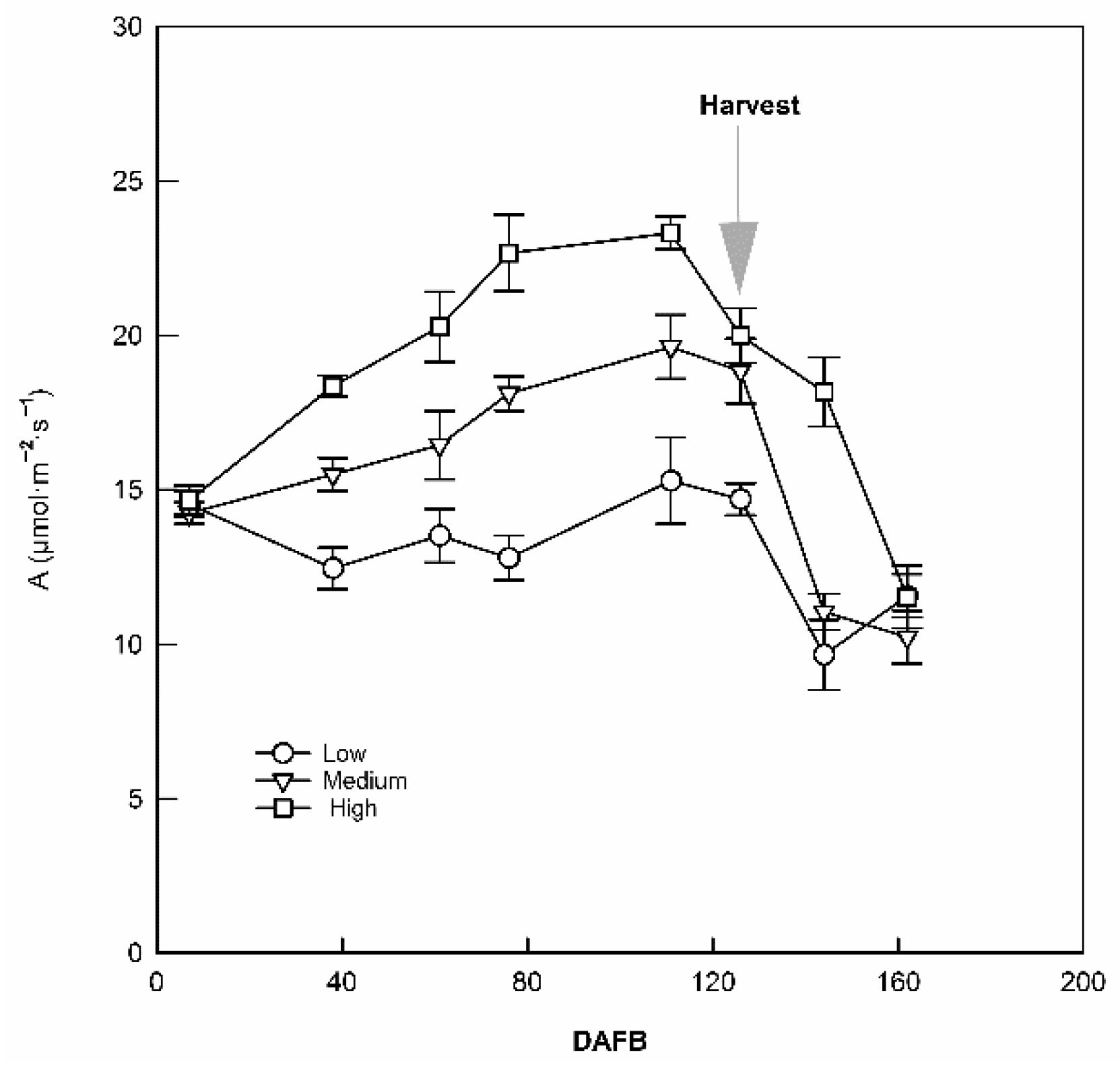
3.1. Crop Load Affected Photosynthetic Carbon Assimilation and Stomatal Conductance
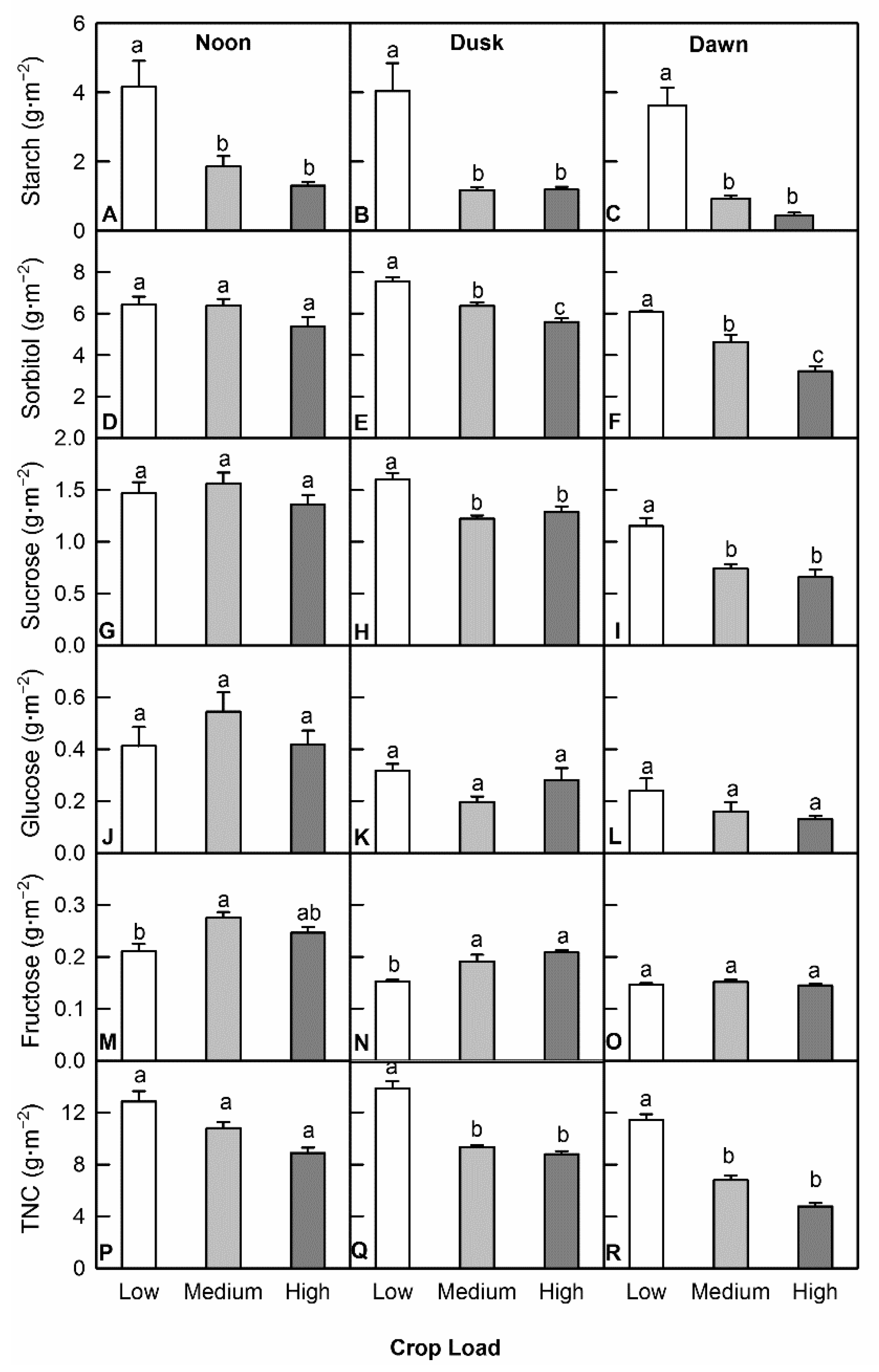
3.2. Crop Load Altered Accumulation of Non-Structural Carbohydrates (NSC)
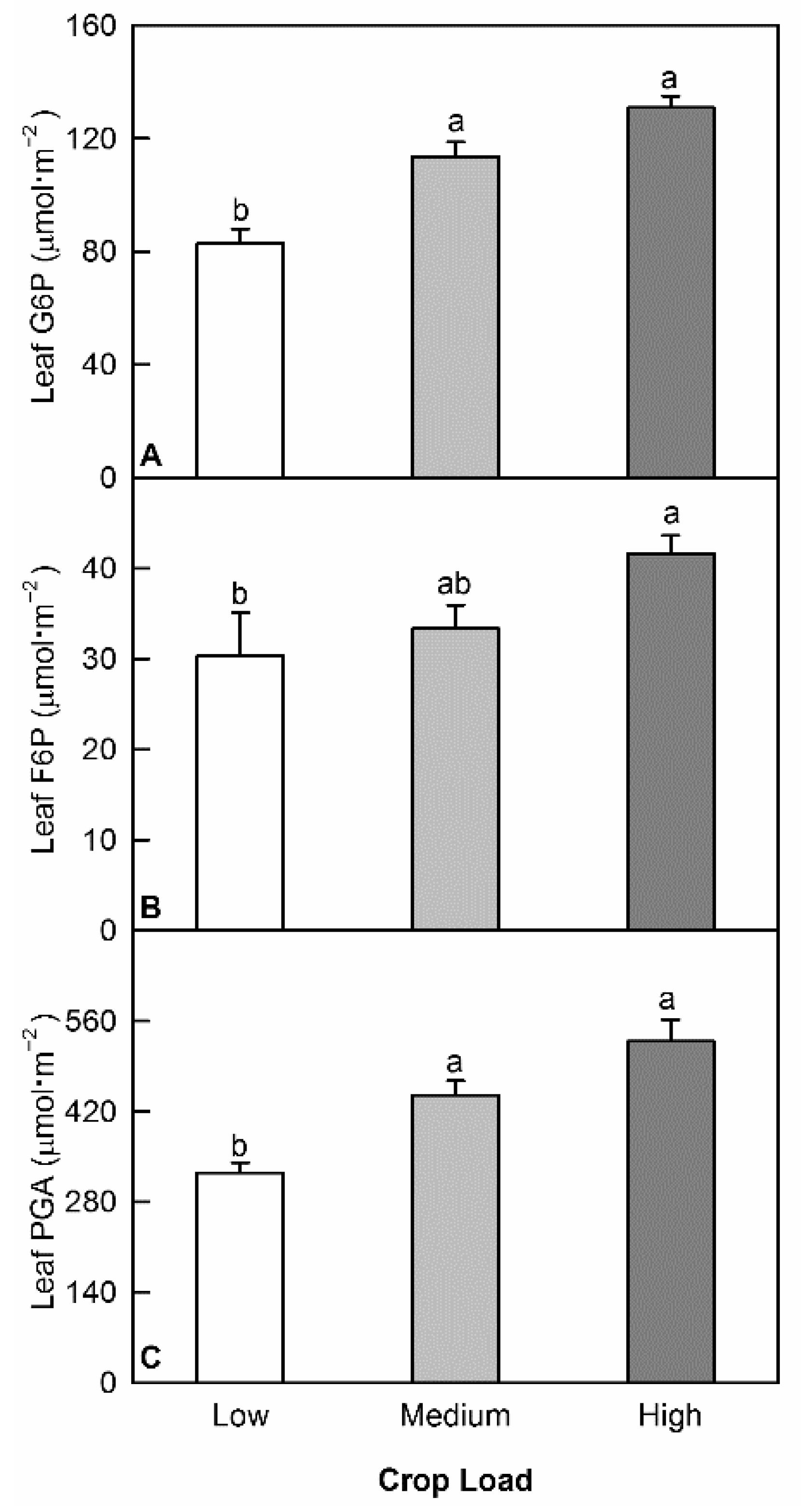
3.3. Crop Load Changed Activities of Key Enzymes in Carbon Assimilation and Metabolism
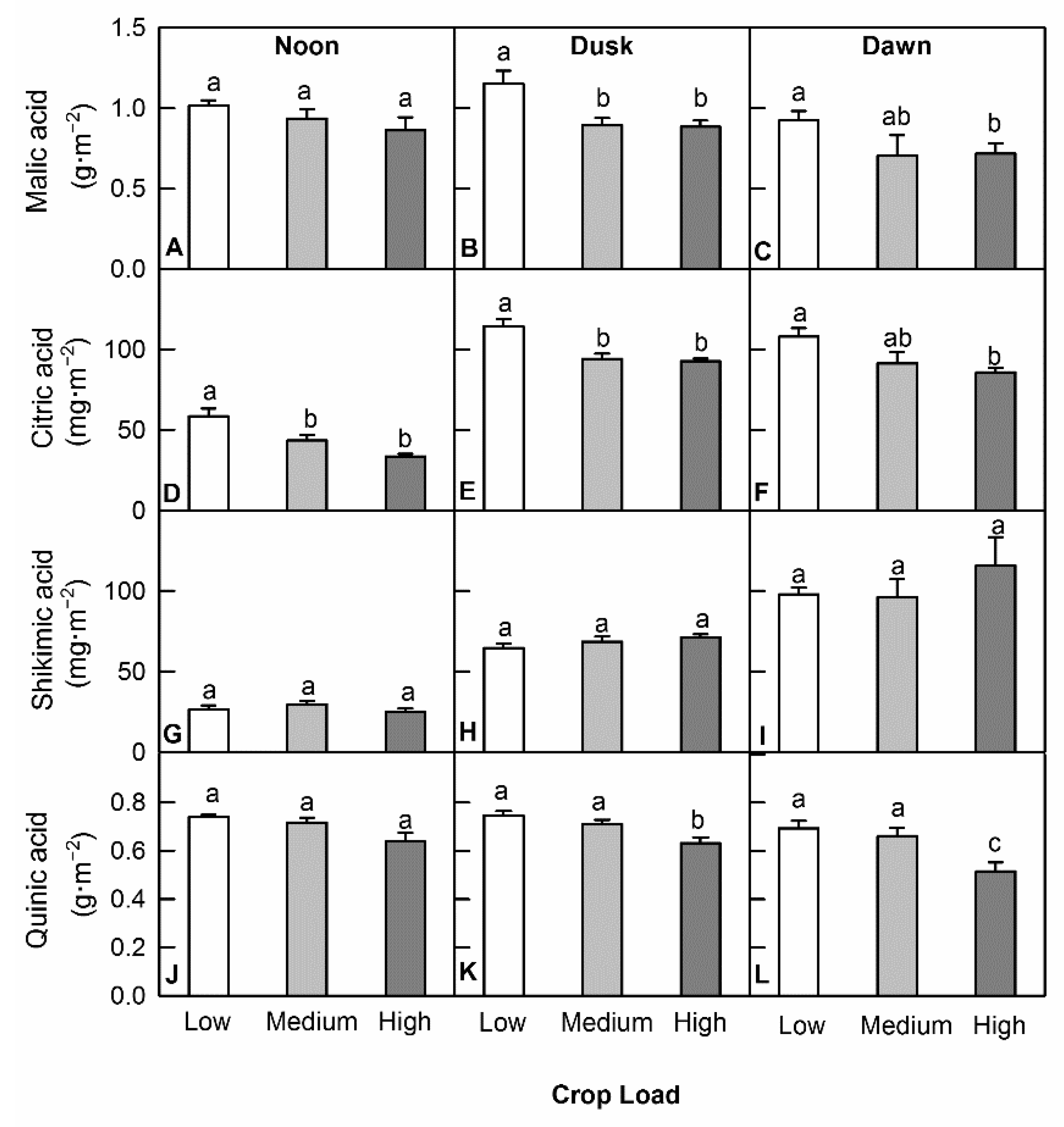
3.4. Crop Load Affected Organic Acid Levels to a Lesser Degree
4. Discussion
4.1. Crop Load Affected Carbon Assimilation in a Positive and Season-Dependent Manner
4.2. Reduced Leaf Assimilation Rate in Low Crop Load Trees Was Primarily a Result of Reduced Rubisco Activity
4.3. Low Crop Load Trees Accumulated a Higher Level of NSC and Organic Acids
4.4. Elevated Carbon Storage in Low Crop Load Trees and TPU Acclimation
4.5. Leaf Starch as an Indicator of Source–Sink Balance and Difference in Carbon Use between Apple and Herbaceous Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheen, J. Metabolic repression of transcription in higher plants. Plant Cell 1990, 2, 1027–1038. [Google Scholar]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Zhu, X.G.; de Sturler, E.; Long, S.P. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate, a numerical simulation using an evolutionary algorithm. Plant Physiol. 2007, 145, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Lo, S.F.; Ho, T.H.D. Source–sink communication, regulated by hormone; nutrient; and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Chang, T.G.; Zhu, X.G. Source–sink interaction, a century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana, the role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R. Next-generation strategies for understanding and influencing source–sink relations in crop plants. Curr. Opin. Plant Biol. 2018, 43, 63–70. [Google Scholar] [CrossRef]
- Gibon, Y.; Pyl, E.; Sulpice, R.; Lunn, J.E.; Hohne, M.; Gunther, M.; Stitt, M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ. 2009, 32, 859–874. [Google Scholar] [CrossRef]
- Graf, A.; Schlereth, A.; Stitt, M.; Smith, A.M. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. USA 2010, 107, 9458–9463. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and primary photosynthetic metabolism, more than the icing on the cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Prudent, M.; Sulpice, R.; Causse, M.; Fernie, A.R. The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population. Plant Physiol. 2010, 154, 1128–1142. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.L.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Marcelis, L.F. Quantifying the source–sink balance and carbohydrate content in three tomato cultivars. Front. Plant Sci. 2015, 6, 416. [Google Scholar]
- Paul, M.J.; Gonzalez-Uriarte, A.; Griffiths, C.A.; Hassani-Pak, K. The role of trehalose 6-phosphate in crop yield and resilience. Plant Physiol. 2018, 177, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Pyl, E.T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C.; et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Raba, R. Accumulation of macro- and micronutrients and nitrogen demand-supply relationship of ‘Gala’/M.26 trees grown in sand culture. J. Am. Soc. Hortic. Sci. 2009, 134, 3–13. [Google Scholar] [CrossRef]
- Xia, G.; Cheng, L.; Lakso, A.N.; Goffinet, M. Effects of nitrogen supply on source-sink balance and fruit size of ‘Gala’ apple trees. J. Am. Soc. Hortic. Sci. 2009, 134, 126–133. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, R.; Reidel, E.J.; Sharkey, T.D.; Dandekar, A.M. Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta 2005, 220, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Xiloyannis, C.; Flore, J.A. Gas exchange parameters; water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol. Plant. 1991, 83, 497–505. [Google Scholar] [CrossRef]
- Palmer, J.W. Effects of varying crop load on photosynthesis, dry-matter production and partitioning of ‘Crispin’/M.27 apple trees. Tree Physiol. 1992, 11, 19–33. [Google Scholar] [CrossRef]
- Ben Mimoun, M.; Longuenesse, J.J.; Génard, M. Pmax as related to leaf: Fruit ratio and fruit assimilate demand in peach. J. Hortic. Sci. 1996, 71, 767–775. [Google Scholar] [CrossRef]
- Naor, A.; Gal, Y.; Bravdo, B. Crop load affects assimilation rate; stomatal conductance; stem water potential; and water relations of field-grown Sauvignon blanc grapevines. J. Exp. Bot. 1997, 48, 1675–1680. [Google Scholar] [CrossRef]
- Palmer, J.W.; Giuliani, R.; Adams, H.M. Effect of crop load on fruiting and leaf photosynthesis of ‘Braeburn’/M.26 apple trees. Tree Physiol. 1997, 17, 741–746. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Palmer, J.W.; Greer, D.H. Effects of crop load on fruiting and gas-exchange characteristics of ‘Braeburn’/M.26 apple trees at full canopy. J. Am. Soc. Hortic. Sci. 2000, 125, 93–99. [Google Scholar]
- Di Vaio, C.; Petito, A.; Buccheri, M. Effect of girdling on gas exchanges and leaf mineral content in the ‘Independence’ nectarine. J. Plant Nutr. 2001, 24, 1047–1060. [Google Scholar] [CrossRef]
- Klages, K.; Donnison, H.; Wünsche, J.; Boldingh, H. Diurnal changes in non-structural carbohydrates in leaves; phloem exudate and fruit in ‘Braeburn’ apple. Funct. Plant Biol. 2001, 28, 131–139. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M. Regulation of photosynthesis through source, sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 2002, 116, 563–572. [Google Scholar] [CrossRef]
- Urban, L.; Léchaudel, M.; Lu, P. Effect of fruit load and girdling on leaf photosynthesis in Mangifera indica L. J. Exp. Bot. 2004, 55, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Vaast, P.; Angrand, J.; Franck, N.; Dauzat, J.; Génard, M. Fruit load and branch ring-barking affect carbon allocation and photosynthesis of leaf and fruit of Coffea arabica in the field. Tree Physiol. 2005, 25, 753–760. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Greer, D.H.; Laing, W.A.; Palmer, J.W. Physiological and biochemical leaf and tree responses to crop load in apple. Tree Physiol. 2005, 25, 1253–1263. [Google Scholar] [CrossRef]
- Franck, N.; Vaast, P.; Génard, M.; Dauzat, J. Soluble sugars mediate sink feedback down-regulation of leaf photosynthesis in field-grown Coffea arabica. Tree Physiol. 2006, 26, 517–525. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.; Araujo, W.L.; Fernie, A.R.; Moraes, G.A. In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef]
- Duan, W.; Pei, G.F.; Li, J.W.; Wei, D.L.; Shu, T.Y.; Shao, H.L. Photosynthetic response to low sink demand after fruit removal in relation to photoinhibition and photoprotection in peach trees. Tree Physiol. 2008, 28, 123–132. [Google Scholar] [CrossRef]
- Bustan, A.; Avnu, A.; Lavee, S.; Zipori, I.; Yeselson, Y.; Schaffer, A.A.; Riov, J.; Dag, A. Role of carbohydrate reserves in yield production of intensively cultivated oil olive (Olea europaea L.) trees. Tree Physiol. 2011, 31, 519–530. [Google Scholar] [CrossRef]
- Pallas, B.; Bluy, S.; Ngao, J.; Martinez, S.; Clément-Vidal, A.; Kelner, J.J.; Costes, E. Growth and carbon balance are differently regulated by tree and shoot fruiting contexts, an integrative study on apple genotypes with contrasted bearing patterns. Tree Physiol. 2018, 38, 1395–1408. [Google Scholar] [CrossRef]
- Roper, T.R.; Keller, J.D.; Loescher, W.H.; Rom, C.R. Photosynthesis and carbohydrate partitioning in sweet cherry, fruiting effects. Physiol. Plant. 1998, 72, 42–47. [Google Scholar] [CrossRef]
- Nebauer, S.G.; Arenas, C.; Rodríguez-Gamir, J.; Bordón, Y.; Fortunato-Almeida, A.; Monerri, C.; Guardiola, G.L.; Molina, R.V. Crop load does not increase the photosynthetic rate in citrus leaves under regular cropping conditions. A study throughout the year. Sci. Hort. 2013, 160, 358–365. [Google Scholar] [CrossRef]
- Neilsen, D.; Neilsen, G.; Guak, S.; Forge, T. Consequences of deficit irrigation and crop load reduction on plant water relations, yield, and quality of ‘Ambrosia’ apple. HortScience 2016, 51, 98–106. [Google Scholar] [CrossRef]
- Saa, S.; Brown, P.H. Fruit presence negatively affects photosynthesis by reducing leaf nitrogen in almond. Funct. Plant Biol. 2014, 41, 884–891. [Google Scholar] [CrossRef]
- Ding, N.; Chen, Q.; Zhu, Z.; Peng, L.; Ge, S.; Jiang, Y. Effects of crop load on distribution and utilization of 13C and 15N and fruit quality for dwarf apple trees. Sci. Rep. 2017, 7, 14172. [Google Scholar] [CrossRef] [PubMed]
- Nebauer, S.G.; Renau-Morata, B.; Lluch, Y.; Baroja-Fernández, E.; Pozueta-Romero, J.; Molina, R.V. Influence of crop load on the expression patterns of starch metabolism genes in alternate-bearing citrus trees. Plant Physiol. Biochem. 2014, 80, 105–113. [Google Scholar] [CrossRef]
- Naschitz, S.; Naor, A.; Genish, S.; Wolf, S.; Goldschmidt, E.E. Internal management of non-structural carbohydrate resources in apple leaves and branch wood under a broad range of sink and source manipulations. Tree Physiol. 2010, 30, 715–727. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signaling systems underpinning source/sink interactions. BBA-Bioenergetics 2016, 1857, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Cheng, L. Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus x domestica Borkh.) with excessive accumulation of carbohydrates. Planta 2010, 232, 511–522. [Google Scholar] [CrossRef]
- Chen, L.S.; Lin, Q.; Nose, A. A comparative study on diurnal changes in metabolite levels in the leaves of three crassulacean acid metabolism (CAM) species, Ananas comosus, Kalanchoë daigremontiana and K. pinnata. J. Exp. Bot. 2002, 53, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Cheng, L. Carbon assimilation and carbohydrate metabolism of ‘Concord’ grape (Vitis labrusca L.) leaves in response to nitrogen supply. J. Am. Soc. Hortic. Sci. 2003, 128, 754–760. [Google Scholar] [CrossRef]
- Chen, L.S.; Qi, Y.P.; Nose, A. Diurnal changes in metabolite levels in the chlorenehyma and the water storage parenchyma of Ananas comosus leaves. Acta Phytophysiol. Sin. 2001, 27, 253–260. [Google Scholar]
- Jones, M.G.; Outlaw, W.H.; Lowry, O.H. Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977, 60, 379–383. [Google Scholar] [CrossRef]
- Cheng, L.; Fuchigami, L.H. Rubisco activation state decreases with increasing nitrogen content in apple leaves. J. Exp. Bot. 2000, 51, 1687–1694. [Google Scholar] [CrossRef]
- Leegood, R.C. Enzymes of the Calvin Cycle. In Methods in Plant Biochemistry; Lea, P.J., Ed.; Academic Press: New York, NY, USA, 1990; Volume 3, pp. 15–37. [Google Scholar]
- Chen, L.S.; Qi, Y.P. Dithiothreitol decreases in vitro activity of ADP-glucose pyrophosphorylase from leaves of apple (Malus x domestica Borkh.) and many other plant species. Phytochem. Anal. 2007, 18, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Cheng, L. CO2 assimilation: Carbohydrate metabolism, xanthophyll cycle and the antioxidant system of ‘Honeycrisp’ apple (Malus x domestica Borkh.) leaves with zonal chlorosis. J. Am. Soc. Hortic. Sci. 2004, 129, 729–737. [Google Scholar] [CrossRef]
- Hansen, P. 14C studies on apple trees. VI. The influence of the fruit on the photosynthesis of leaves; and the relative photosynthetic yields of fruits and leaves. Physiol. Plant 1970, 23, 805–810. [Google Scholar] [CrossRef]
- Layne, D.R.; Flore, J.A. End-product inhibition of photosynthesis in Prunus cerasus L. in response to whole-plant source-sink manipulation. J. Am. Soc. Hortic. Sci. 1995, 120, 583–599. [Google Scholar] [CrossRef]
- Kaps, M.L.; Cahoon, G.A. Berry thinning and cluster thinning influence vegetative growth, yield, fruit composition, and net photosynthesis of ‘Seyval Blanc’ grapes. J. Am. Soc. Hortic. Sci. 1989, 114, 20–24. [Google Scholar]
- Edson, C.E.; Howell, G.S.; Flore, J.A. Influence of fruit load on photosynthesis and dry matter partitioning of Seyval grapevines. II. Seasonal changes in single leaf and whole vine photosynthesis. Am. J. Enol. Vitic. 1995, 46, 469–477. [Google Scholar]
- Naor, A.; Naschitz, S.; Peres, M.; Gal, Y. Responses of apple fruit size to tree water status and crop load. Tree Physiol. 2008, 28, 1255–1261. [Google Scholar] [CrossRef]
- Samuolienė, G.; Čeidaitė, A.; Sirtautas, R.; Duchovskis, P.; Kvikly, D. Effect of crop load on phytohormones, sugars, and biennial bearing in apple trees. Biol. Plant 2016, 60, 394–400. [Google Scholar] [CrossRef]
- Pan, Y.H.; Lu, Z.F.; Lu, J.W.; Li, X.K.; Cong, R.H.; Ren, T. Effects of low sink demand on leaf photosynthesis under potassium deficiency. Plant Physiol. Biochem. 2017, 113, 110–121. [Google Scholar] [CrossRef]
- Zhang, W.W.; Fu, X.Z.; Peng, L.Z.; Ling, L.L.; Cao, L.; Ma, X.H.; Xie, F.; Li, C. Effects of sink demand and nutrient status on leaf photosynthesis of spring-cycle shoot in ‘Newhall’ navel orange under natural field conditions. Sci. Hort. 2013, 150, 80–85. [Google Scholar] [CrossRef]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signaling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef]
- Poirier-Pocovi, M.; Lothier, J.; Buck-Sorlin, G. Modelling temporal variation of parameters used in two photosynthesis models, influence of fruit load and girdling on leaf photosynthesis in fruit-bearing branches of apple. Ann. Bot. 2018, 121, 821–832. [Google Scholar] [CrossRef]
- Sharkey, T.D. Photosynthesis in intact leaves of C3 plants, physics, physiology and rate limitations. Bot. Rev. 1985, 51, 53–105. [Google Scholar] [CrossRef]
- Gago, J.; de Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism, a multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef]
- Lima, V.F.; Medeiros, D.B.; Dos Anjos, L.; Gago, J.; Fernie, A.R.; Daloso, D.M. Toward multifaceted roles of sucrose in the regulation of stomatal movement. Plant Signal. Behav. 2018, 13, e1494468. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Matthews, J. Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 2020, 71, 273–302. [Google Scholar] [CrossRef] [PubMed]
- Wünsche, J.N.; Ferguson, I.B. Crop load interactions in apple. Hortic. Rev. 2005, 31, 231–290. [Google Scholar]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.P.; Bligny, R.; Gout, E.; Mahé, A.; Nogués, S.; Hodges, M.; Tcherkez, G.G. In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus. New Phytol. 2010, 185, 988–999. [Google Scholar] [CrossRef]
- Tcherkez, G.; Gauthier, P.; Buckley, T.N.; Busch, F.A.; Barbour, M.M.; Bruhn, D.; Heskel, M.A.; Gong, X.Y.; Crous, K.Y.; Griffin, K.; et al. Leaf day respiration, low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 2017, 216, 986–1001. [Google Scholar] [CrossRef]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Muller-Rober, B.T.; Kossman, J.; Hannah, L.C.; Willmitzer, L.; Sonnewald, U. ADPG-pyrophosphorylase genes from potato, mode of RNA expression and its relation to starch synthesis. In Phloem Transport and Assimilate Compartmentation; Bonnemain, J.L., Ed.; Quest Editions: Nantes, France, 1991; pp. 204–208. [Google Scholar]
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013, 36, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Kolbe, A.; Tiessen, A. Redox regulation of carbon storage and partitioning in response to light and sugars. J. Exp. Bot. 2005, 56, 1469–1479. [Google Scholar] [CrossRef]
- Turgeon, R. The puzzle of phloem pressure. Plant Physiol. 2010, 154, 578–581. [Google Scholar] [CrossRef]
- Ruan, Y. Sucrose metabolism, gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Herold, A. Regulation of photosynthesis by sink activity–the missing link. New Phytol. 1980, 86, 131–144. [Google Scholar] [CrossRef]
- McClain, A.M.; Sharkey, T.D. Triose phosphate utilization and beyond, from photosynthesis to end product synthesis. J. Exp. Bot. 2019, 70, 1755–1766. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars, trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef]
- Zhang, W.; Lunn, J.E.; Feil, R.; Wang, Y.; Zhao, J.; Tao, H.; Guo, Y.; Zhao, Z. Trehalose 6-phosphate signal is closely related to sorbitol in apple (Malus x domestica Borkh. cv. Gala). Biol. Open 2017, 6, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Reidel, E.J.; Rennie, E.A.; Amiard, V.; Cheng, L.; Turgeon, R. Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 2009, 149, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef]
- Usadel, B.; Bläsing, O.E.; Gibon, Y.; Retzlaff, K.; Höhne, M.; Günther, M.; Stitt, M. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008, 146, 1834–1861. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cheng, L.; Guo, Y.; Turgeon, R. Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiol. 2011, 157, 1518–1527. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Chen, L.-S.; Cheng, L. Leaf Photosynthesis and Carbon Metabolism Adapt to Crop Load in ‘Gala’ Apple Trees. Horticulturae 2021, 7, 47. https://doi.org/10.3390/horticulturae7030047
Yang X, Chen L-S, Cheng L. Leaf Photosynthesis and Carbon Metabolism Adapt to Crop Load in ‘Gala’ Apple Trees. Horticulturae. 2021; 7(3):47. https://doi.org/10.3390/horticulturae7030047
Chicago/Turabian StyleYang, Xiaohua, Li-Song Chen, and Lailiang Cheng. 2021. "Leaf Photosynthesis and Carbon Metabolism Adapt to Crop Load in ‘Gala’ Apple Trees" Horticulturae 7, no. 3: 47. https://doi.org/10.3390/horticulturae7030047
APA StyleYang, X., Chen, L.-S., & Cheng, L. (2021). Leaf Photosynthesis and Carbon Metabolism Adapt to Crop Load in ‘Gala’ Apple Trees. Horticulturae, 7(3), 47. https://doi.org/10.3390/horticulturae7030047





