Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Drought Stress Conditions
2.2. Study Area
2.3. Experimental Treatments
2.4. Application of Biofertilizers
2.5. Protein Determination
2.6. Enzyme Extractions
2.7. Enzyme Assays
2.8. β-Sitosterol Assay
2.9. Grain Yield Assay
2.10. Statistical Analysis
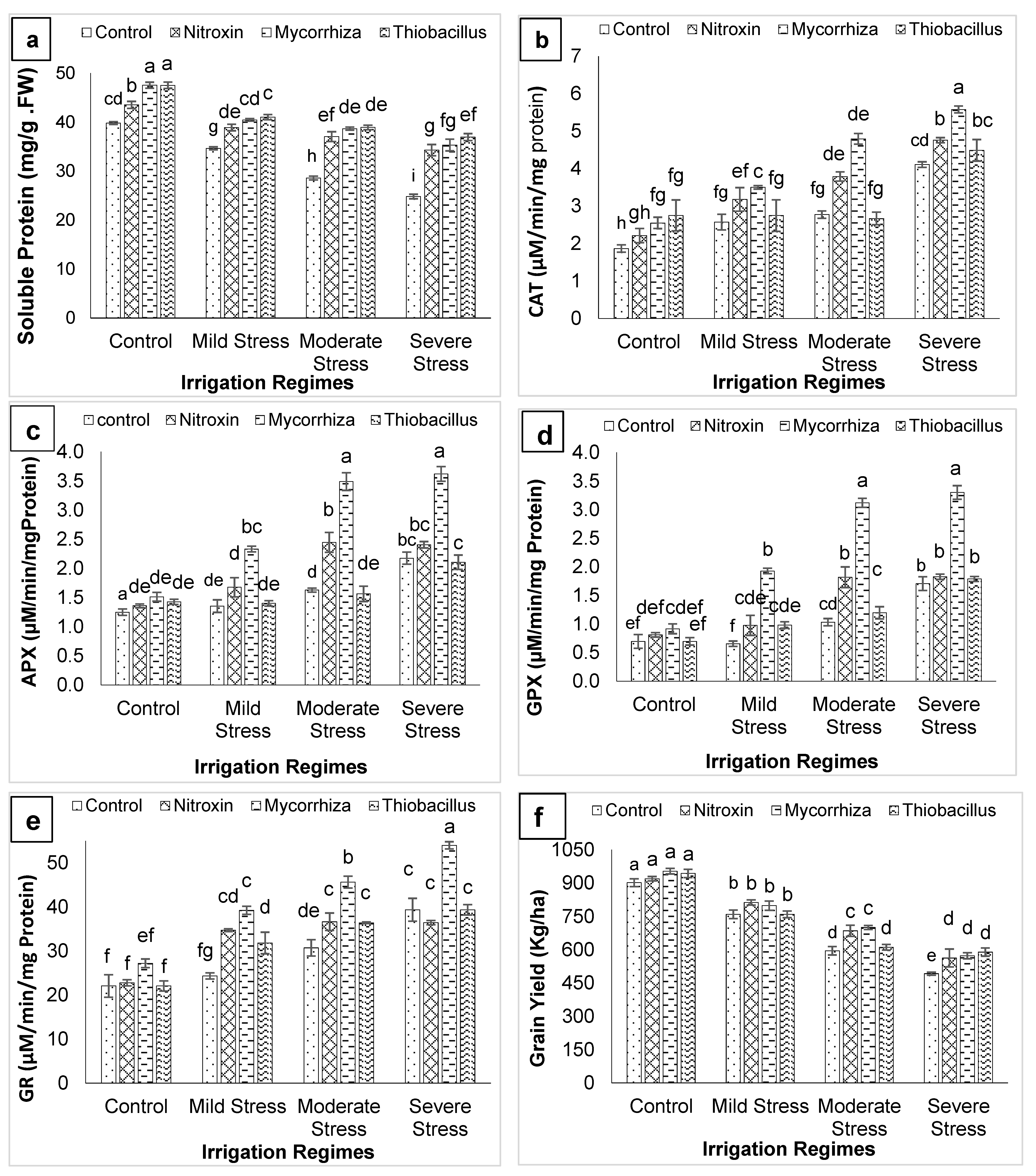
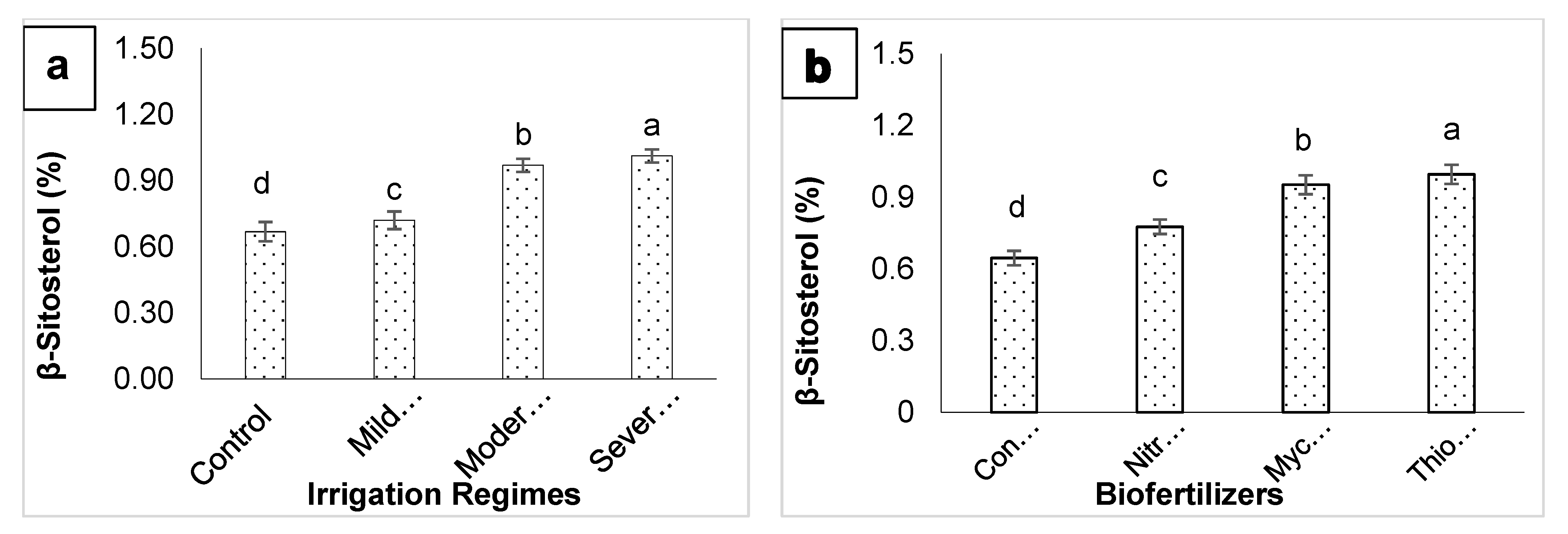
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plant. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Mittova, V.; Volokitam, G.Y.M.M.; Tal, M. Activities of SOD and the ascorbate-glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative (Lycopersicon pennellii). Physiol. Planta 2000, 110, 45–56. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kuhlmann, H.K.; Koetter, U.; Theurer, C. Sterol contents in medicinal pumpkin (Cucurbita pepo convar. citrullinina var. styriaca). Acta Hortic. 1999, 492, 175–178. [Google Scholar] [CrossRef]
- Younis, Y.M.H.; Al-Shihry, S.S. African (Cucurbita pepo L.) properties of seed and variability in fatty acid composition of seed oil. Phytochemistry 2000, 54, 71–75. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Ghaderifar, F.; Soltani, A.; Sadeghi Pour, H.R. Changes in Seed Quality during Seed Development and Maturation in Medicinal Pumpkin (Cucurbita pepo subsp. Pepo. Convar. Pepo var. Styriaca Greb). J. Herbs Spices Med. Plant 2011, 17, 249–257. [Google Scholar] [CrossRef]
- Chari, K.Y.; Polu, P.R.; Shenoy, R.R. An appraisal of pumpkin seed extract in 1,2-dimethylhydrazine induced colon cancer in Wistar rats. J. Toxicol. 2018, 2018, 6086490. [Google Scholar] [CrossRef] [Green Version]
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils, Process Safe. Environ. Protect. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Aroiee, H.; Omidbaigi, R. Effects of nitrogen fertilizer on productivity medicinal pumpkin. Acta Hortic. 2004, 629, 415–419. [Google Scholar] [CrossRef]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afri. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Zimmerman, J.R.D. Optimal nutrition for HIV/AIDS wellness. J. Am. Diet. Assoc. 1997, 97, A18. [Google Scholar] [CrossRef]
- Maleki Khezerlu, S.; Tahmasebi Sarvestani, Z.; Modarres Sanavi, S.A.M. Assessment of quantitative and qualitative traits in the pumpkin (Cucurbita pepo L.) under water deficit stress induction and nitrogen fertilizer. Iran. J. Med. Aromat. Plants Res. 2015, 31, 853–863. [Google Scholar]
- Naeemi, M.; Akbari, G.A.; Shirani Rad, A.H.; Hassanloo, T.; Akbari, G.A. Effect of zeolite application and selenium spraying on water relations traits and antioxidant enzymes in medicinal pumpkin (Cucurbita pepo L.) under water deficit stress conditions. J. Crop. Improv. 2012, 14, 67–81. [Google Scholar]
- Aghai, A.H.; Ehsanzade, P. Effects of irrigation regime and nitrogen on yield and some physiological parameters in medicinal pumpkin. J. Iran. Hort. Sci. 2011, 42, 291–299. [Google Scholar]
- Zeynali, M.; Maleki Zanjani, B.; Moradi, P.; Shekari, F. Effects of field capacity based-irrigation levels on physiological and agronomic characteristics of medicinal pumpkin (Cucurbita pepo L.). Appl. Res. Field Crop. 2019, 31, 1–20. [Google Scholar]
- Zeynali, M.; Maleki Zanjani, B.; Moradi, P.; Shekari, F. Effects of drought stress on some physiological traits, yield and yield component in four varieties of pumpkin (Cucurbita pepo L.). J. Plant Res. 2019, 32, 439–452. [Google Scholar]
- Habibi, A.; Heidari, G.; Sohrabi, Y.; Badakhshan, H.; Mohammadi, K. Grain Quality of Medicinal Pumpkin (Cucurbita pepo subsp. Pepo var. styriaca) Affected by Type and amount of Fertilizers. J. Essen. Oil-Bear. Plants 2014, 17, 533–537. [Google Scholar] [CrossRef]
- Sharma, A.K. Biofertilizers for sustainable agriculture. Agrobios 2003, 12, 319–324. [Google Scholar]
- Marulanda, A.; Azcon, R.; Ruiz-Lozano, J.M. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol. Plant 2003, 119, 526–533. [Google Scholar] [CrossRef]
- Messick, D.L.; Fan, M.X. The role of sulfur fertilizer in oil crop production. In Proceedings of the Regional Conference for Asia and the Pacific, Kuala Lumpur, Malaysia, 26–28 April 1999. [Google Scholar]
- Galik, B.R.; Penrose, D.; Wenbo, M. Bacterial promotion of plant growth. Biotechnol. Adv. 2001, 19, 135–138. [Google Scholar]
- Biari, A.; Gholami, A.; Rahmani, H.A. Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth-promoting Rhizobacteria in arid region of Iran. J. Biol. Sci. 2008, 8, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Sajjadinik, N. Effect of nitroxin bio-fertilizer and bio-vermicompost on yield and yield components of sesame. In Proceedings of the Eleventh Congress of Plant Agriculture Reform in Iran, Tehran, Iran, 16–20 August 2010; pp. 1366–1369. (In Persian). [Google Scholar]
- Krizek, D.T. Methods of inducing water stress in plants. Hort. Sci. 1985, 20, 1027–1038. [Google Scholar]
- Noborio, K.; Horton, R.; Tan, C.S. Time domain reflectometry probe for simultaneous measurement of soil matric potential and water content. Soil. Sci. Soc. Am. J. 1999, 63, 1500–1505. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mainly, A.C.; Chance, B. The assay of catalase and peroxidase. In Methods of Biochemical Analysis; Click, D., Ed.; Interscience Inc.: New York, NY, USA, 1959; Volume 1, pp. 357–425. [Google Scholar]
- Asada, R.D. Ascorbate peroxidase-a hydrogen peroxide scavenging enzyme in plants. Physiol. Planta 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Chang, C.J.; Kao, C.H. H2O2 metabolism during senescence of rice Leaves: Change in enzyme activities in light and darkness. Plant Grow. Reg. 1998, 25, 11–15. [Google Scholar] [CrossRef]
- Di Baccio, D.; Navari-Izzo, F.; Izzo, R. Seawater irrigation: Antioxidant defense responses in leaves and roots of a sunflower (Helianthus annuus L.) ecotype. J. Plant Physiol. 2004, 161, 1359–1366. [Google Scholar] [CrossRef]
- Smith, R.S. Legume inoculants formulation and application. Can. J. Microbiol. 1992, 38, 485–492. [Google Scholar] [CrossRef]
- Bavec, F.L.; Gril, S.; Grobelnik, M.; Bavec, M. Production of Pumpkin For Oil; ASHS Rress: Alexandria, Egypt, 2002; pp. 187–190. [Google Scholar]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, B.; Joe, M.M.; Jaleel, C.A. Response of some medicinal plants to VAM inoculations. J. Sci. Res. 2009, 1, 381–386. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. (Eds.) Mycorrhizal Symbiosis; Academic Press Inc.: San Diego, CA, USA, 2008. [Google Scholar]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Levent-Tuna, A.; Cullu, M.A. The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pongrac, P.; Kump, P.; Nečemer, M.; Revgear, M. Colonization of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous (arbuscular mycorrhizal) fungal mixture induces changes in heavy metal and nutrient uptake. Env. Poll. 2006, 139, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; George, E. The effect of (arbuscular mycorrhizal) root colonization on growth and nutrient uptake of two different cowpeas (Vigna unguiculata L. Walp.) genotypes exposed to drought stress. Emir. J. Food Agric. 2009, 21, 1–17. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef]
- Bianchi, E.; Xie, X.; Zhang, H.; Pare, P.W. Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J. Agric. Food Chem. 2009, 57, 653–657. [Google Scholar] [CrossRef]
- Gupta, M.L.; Prasad, A.; Ram, M.; Kumar, S. Effect of the vesicular-arbuscular mycorrhizal (VAM) fungus Glomus fasiculatum on the essential oil yield-related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour. Technol. 2002, 81, 77–79. [Google Scholar] [CrossRef]
- Khaosaad, T.; Vierheilig, H.; Nell, M.; Zittel- Eglseer, K.; Novak, J. Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp. Lamiaceae) Mycorrhiza 2006, 16, 443–446. [Google Scholar] [CrossRef]
- Coretta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth-promoting rhizobacteria in crop plant against pest and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Bastami, A.; Majidian, M. Effects of Mycorrhiza, Phosphatic Biofertilizer on Photosynthetic Pigments and Yield in Coriander (Coriandrum Sativum L.). J. Plant Prod. (Agron. Breed. Hortic.) 2016, 38, 49–60. [Google Scholar]
- Rukmani, R. Physical, Chemical and Biological Regulation of Fruit characters and Yield in Okra (Abelmesehus esculents L.); Department of Floriculture, College of Horticulture, Kerala Agriculture Universit: Thrissur, India, 1990. [Google Scholar]
- Yasar, F.; Kusvuran, S.; Ellialtioglu, S. Determination of antioxidant activities in some melon (Cucumis melo L.) varieties and cultivars under salt stress. J. Hortic. Sci. Biotechnol. 2006, 81, 627–630. [Google Scholar] [CrossRef]
- Youssef, A.A.; Edris, A.E.; Gomaa, A.M. A comparative Study between some plant growth regulators and certain growth hormones producing microorganisms on growth and essential oil composition of Salvia of officinalis L. Plant Ann. Agric. Sci. 2004, 49, 299–311. [Google Scholar]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free-living plant growth promoting rhizobacteria. Antonie Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dabaghian, Z.; Pirdashti, H.; Abasian, A.; Bahari Saravi, S.H. The effect of biofertilizers, Thiobacillus, Azotobacter, Azospirillum, and organic sulfur on nodulation process and yield of soybean (Glycine max L. Merr). Agron. J. 2014, 107, 17–25. [Google Scholar]
- Yadegari, M.; Barzegar, R. Effect of sulfur and thiobacillus on the absorption of nutrients, growth, and production of oil in lemon balm (Melissa officinalis L.). J. Herb. Drugs 2010, 1, 35–40. [Google Scholar]
- Carlot, M.; Giacomini, A.; Casella, S. Aspects of plant-microbe interactions in heavy metal polluted soil. Acta Biotechnol. 2002, 22, 13–20. [Google Scholar] [CrossRef]
| Source of Variation | df | MS | ||||||
|---|---|---|---|---|---|---|---|---|
| CAT | APX | GPX | GR | SP | GY | β-S | ||
| Year | 1 | 4.05 n | 0.34 ns | 0.96 ns | 31.51 ns | 72.60 ns | 1443.05 ns | 0.47 ns |
| Block (year) | 4 | 0.0037 ns | 0.046 ns | 0.006 ns | 27.72 ns | 2.89 ns | 1852.51 ns | 0.0 ns |
| Irrigation regimes (a) | 3 | 24.5 ns | 7.07 ** | 9.30 ** | 1531.42 ** | 603.77 ** | 639,122.2 ** | 0.72 ** |
| year * a | 3 | 3.45 ** | 0.24 ns | 0.11 ns | 18.30 ns | 10.05** | 856.96 ns | 0.017 ns |
| a * block (year) | 12 | 0.008 * | 0.064 ns | 0.03 ns | 18.50 ns | 1.23 ns | 3058.99 ns | 0.001 ns |
| Biofertilizers (b) | 3 | 7.09 * | 6.77 ** | 8.18 ** | 674.76 ** | 419.97 ** | 22,164.35 ns | 0.629 * |
| a * b | 9 | 1.05 * | 0.79 ** | 0.87 ** | 66.06 ** | 11.58 ** | 4390.07 * | 0.07 ns |
| year*b | 3 | 0.45 ns | 0.15 ns | 0.15 ns | 12.14 ns 5.37 ns | 0.47 ns | 4753.2 * | 0.015 ns |
| year * a * b | 9 | 0.31 ** | 0.09 ** | 0.144 ** | 5.37 ns | 0.42 ns | 1116.99 ns | 0.72 ** |
| Error | 48 | 0.006 | 0.03 | 0.023 | 11.33 | 1.7 | 1917.16 | 0.0007 |
| CV (%) | 2.28 | 3.87 | 10.34 | 9.92 | 3.43 | 6 | 3.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najafi, S.; Nazari Nasi, H.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588. https://doi.org/10.3390/horticulturae7120588
Najafi S, Nazari Nasi H, Tuncturk R, Tuncturk M, Sayyed RZ, Amirnia R. Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae. 2021; 7(12):588. https://doi.org/10.3390/horticulturae7120588
Chicago/Turabian StyleNajafi, Solmaz, Hossein Nazari Nasi, Ruveyde Tuncturk, Murat Tuncturk, Riyaz Z. Sayyed, and Reza Amirnia. 2021. "Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca)" Horticulturae 7, no. 12: 588. https://doi.org/10.3390/horticulturae7120588
APA StyleNajafi, S., Nazari Nasi, H., Tuncturk, R., Tuncturk, M., Sayyed, R. Z., & Amirnia, R. (2021). Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae, 7(12), 588. https://doi.org/10.3390/horticulturae7120588







