Phenolics and Mineral Elements Composition in Underutilized Apple Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apple Fruits
2.3. Sampling Procedure, Polyphenols Extraction and LC-MS Analysis
2.4. Vitamin C Content and DPPH Assay
2.5. Cation Concentration
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
- (1)
- Promote quality products with higher nutritional properties (polyphenols, vitamin C and mineral nutrients);
- (2)
- Develop cultivars with recognized dietetic qualities and encourage fruit growers to re-introduce selected and underutilized germplasms;
- (3)
- Encourage plant breeders in investigating underutilized varieties as sources of traits for resistance to diseases or harsh environmental conditions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Felice, F.; Maragò, E.; Sebastiani, L.; Di Stefano, R. Apple juices from ancient Italian cultivars: A study on mature endothelial cells model. Fruits 2015, 70, 361–369. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus × domestica Borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Cantini, C.; Salusti, P.; Romi, M.; Francini, A.; Sebastiani, L. Sensory profiling and consumer acceptability of new dark cocoa bars containing Tuscan autochthonous food products. J. Food Sci. Nutr. 2018, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A. Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content. Soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Starowicz, M.; Achrem–Achremowicz, B.; Piskuła, M.K.; Zieliński, H. Phenolic Compounds from Apples: Reviewing their Occurrence, Absorption, Bioavailability, Processing, and Antioxidant Activity—A Review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Mureşan, E.A.; Muste, S.; Borşa, A.; Vlaic, R.A.; Mureşan, V. Evaluation of Physical-Chemical Indexes, Sugars, Pigments and Phenolic Compounds of Fruits from Three Apple Varieties at the End of Storage Period. Bull. UASVM Food Sci. Technol. 2014, 71, 45–50. [Google Scholar] [CrossRef][Green Version]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Francini, A.; Romeo, S.; Cifelli, M.; Gori, D.; Domenici, V.; Sebastiani, L. 1H NMR and PCA-based analysis revealed variety dependent changes in phenolic contents of apple fruit after drying. Food Chem. 2017, 221, 1206–1213. [Google Scholar] [CrossRef]
- Duralija, B.; Putnik, P.; Brdar, D.; Bebek Markovinovíc, A.; Zavadlav, S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Bursác Kovăcevíc, D. The Perspective of Croatian old apple cultivars in extensive farming for the production of functional foods. Foods 2021, 10, 708. [Google Scholar] [CrossRef]
- Iacopini, P.; Camangi, F.; Stefani, A.; Sebastiani, L. Antiradical potential of ancient Italian apple varieties of Malus x domestica Borkh. in a peroxynitrite-induced oxidative process. J. Food Compos. Anal. 2010, 23, 518–524. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2020, 246, 437–448. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Caprioli, G.; Maggi, F.; Dall’Acqua, S. Characterization of nutrients, polyphenols and volatile components of the ancient apple cultivar ‘Mela Rosa Dei Monti Sibillini’ from Marche region, central Italy. Int. J. Food Sci. Nutr. 2019, 70, 796–812. [Google Scholar] [CrossRef]
- Simonato, B.; Marangon, M.; Vincenzi, S.; Vegroban, M.; Pasini, G. Evaluation of the phenolic profile and immune reactivity of Mal d 3 allergen in ancient apple cultivars from Italy. J. Sci. Food Agric. 2020, 100, 4978–4986. [Google Scholar] [CrossRef] [PubMed]
- Francini, A.; Galleschi, L.; Saviozzi, F.; Pinzino, C.; Izzo, R.; Sgherri, C.; Navari-Izzo, F. Enzymatic and non-enzymatic protective mechanisms in recalcitrant seeds of Araucaria bidwillii subjected to desiccation. Plant Physiol. Biochem. 2006, 44, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialyzable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Ullah, C.; Unsicker, S.B.; Fellenberg, C.; Constabel, C.P.; Schmidt, A.; Gershenzon, J.; Hammerbacher, A. Flavan-3-ols Are an Effective Chemical Defense against Rust Infection. Plant Physiol. 2017, 175, 1560–1578. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Tian, J.; Yao, Y. Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front. Plant Sci. 2017, 8, 1286. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of apple phytochemicals, Phloretin and Phloridzin, in modulating processes related to intestinal inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Shiv, K.; Sinha, K.; Sharma, R.; Purohit, R.; Padwad, Y. Phloretin and phloridzin improve insulin sensitivity and enhance glucose uptake by subverting PPARγ/Cdk5 interaction in differentiated adipocytes. Exp. Cell Res. 2019, 383, 111480. [Google Scholar]
- Hrubá, M.; Baxant, J.; Čížková, H.; Smutná, V.; Kovařík, F.; Ševčík, R.; Hanušová, K.; Rajchl, A. Phloridzin as a marker for evaluation of fruit product’s authenticity. Czech. J. Food Sci. 2021, 39, 49–57. [Google Scholar] [CrossRef]
- Agnolet, S.; Ciesa, F.; Soini, E.; Cassar, A.; Matteazzi, A.; Guerra, W.; Robatscher, P.; Storti, A.; Baric, S.; Oberhuber, M.; et al. Dietary elements and quality parameters of 34 old and eight commercial apple cultivars grown at the same site in South Tyrol, Italy. Erwerbs-Obstbau 2017, 59, 171–183. [Google Scholar] [CrossRef]
- Macit, I.; Aydın, E.; Tas, A.; Gundogdu, M. Fruit quality properties of the local apple varieties of Anatolia. Sustainability 2021, 13, 6127. [Google Scholar] [CrossRef]
- Horsley, R.; Gökbel, H.; Özcan, M.M.; Harmankaya, M.; Şimşek, S. Monitoring of element contents of three different apple (Malus Spp.) varieties in an apple tree. J. Food Nutr. Res. 2014, 2, 127–129. [Google Scholar]
- Ozcan, M.M.; Harmankaya, M.; Gezgin, S. Mineral and heavy metal contents of the outer and inner tissues of commonly used fruits. Environ. Monit. Assess. 2012, 184, 313–320. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar]
- Todea, D.A.; Cadar, O.; Simedru, D.; Roman, C.; Tanaselia, C.; Suatean, I.; Naghiu, A. Determination of major-to-trace minerals and polyphenols in different. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 523–529. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, S.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic acid content in apple pulp, peel, and monovarietal cloudy juices of 64 different cultivars. Int. J. Food Prop. 2017, 20, 2626–2634. [Google Scholar] [CrossRef]
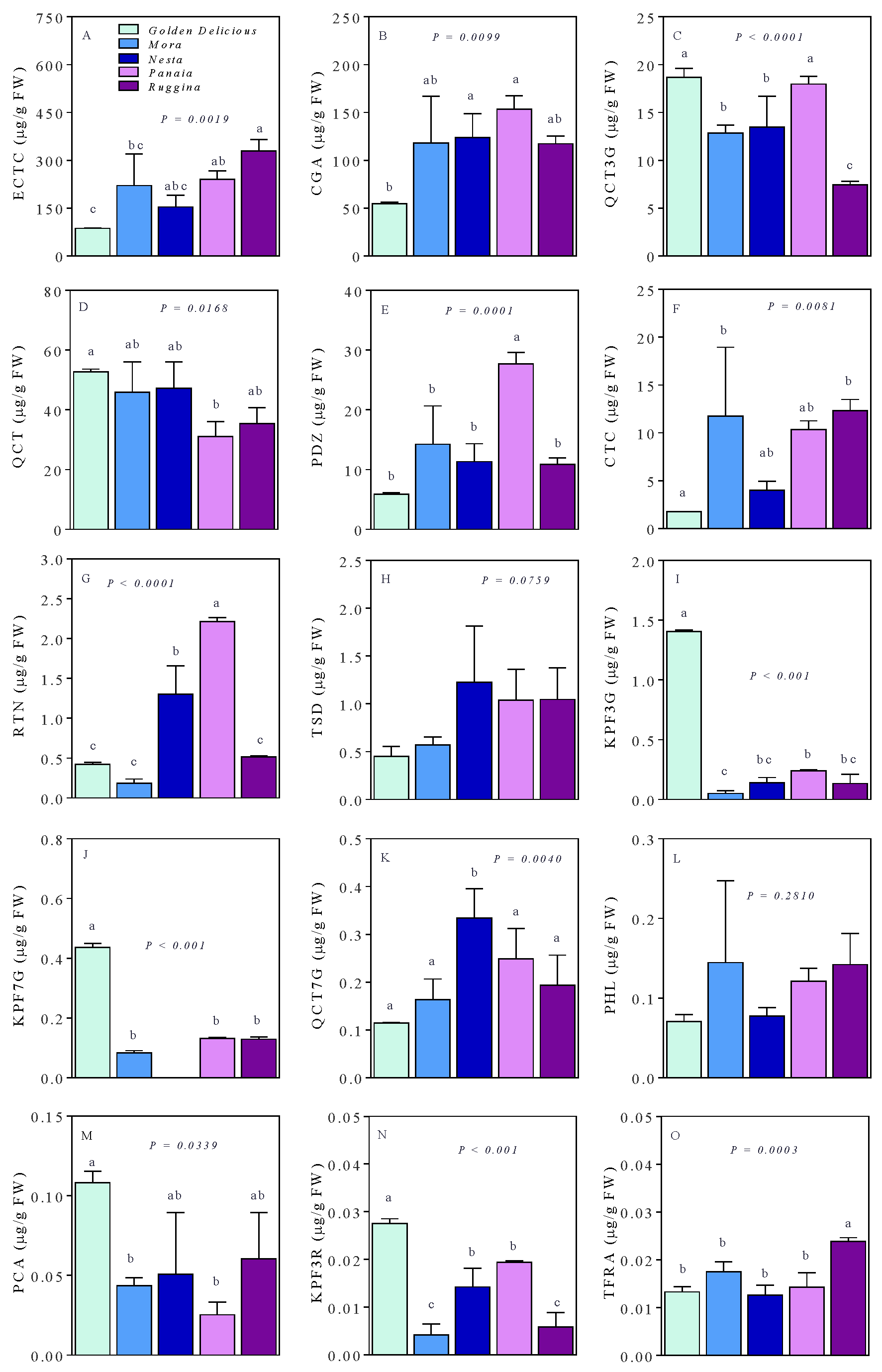
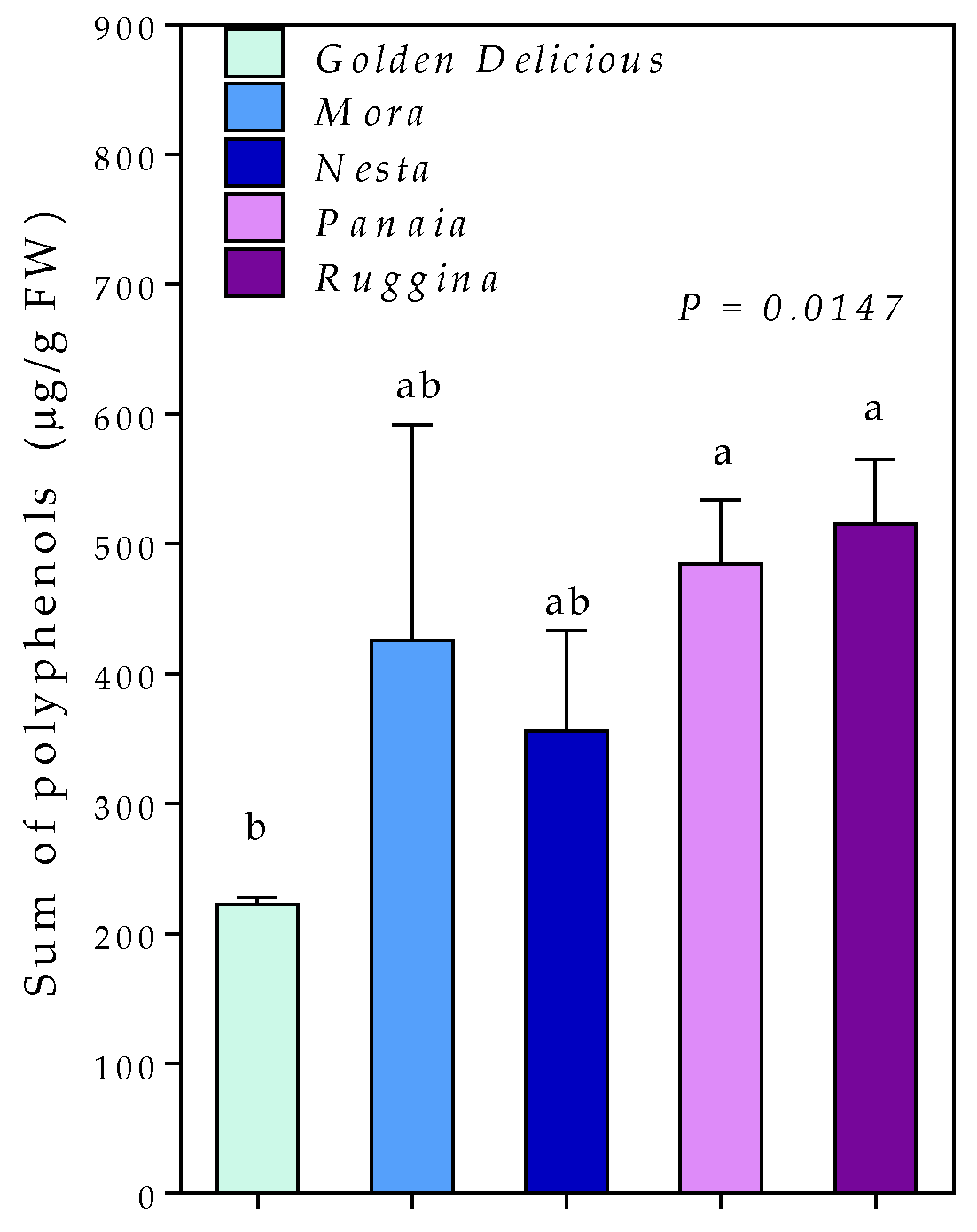

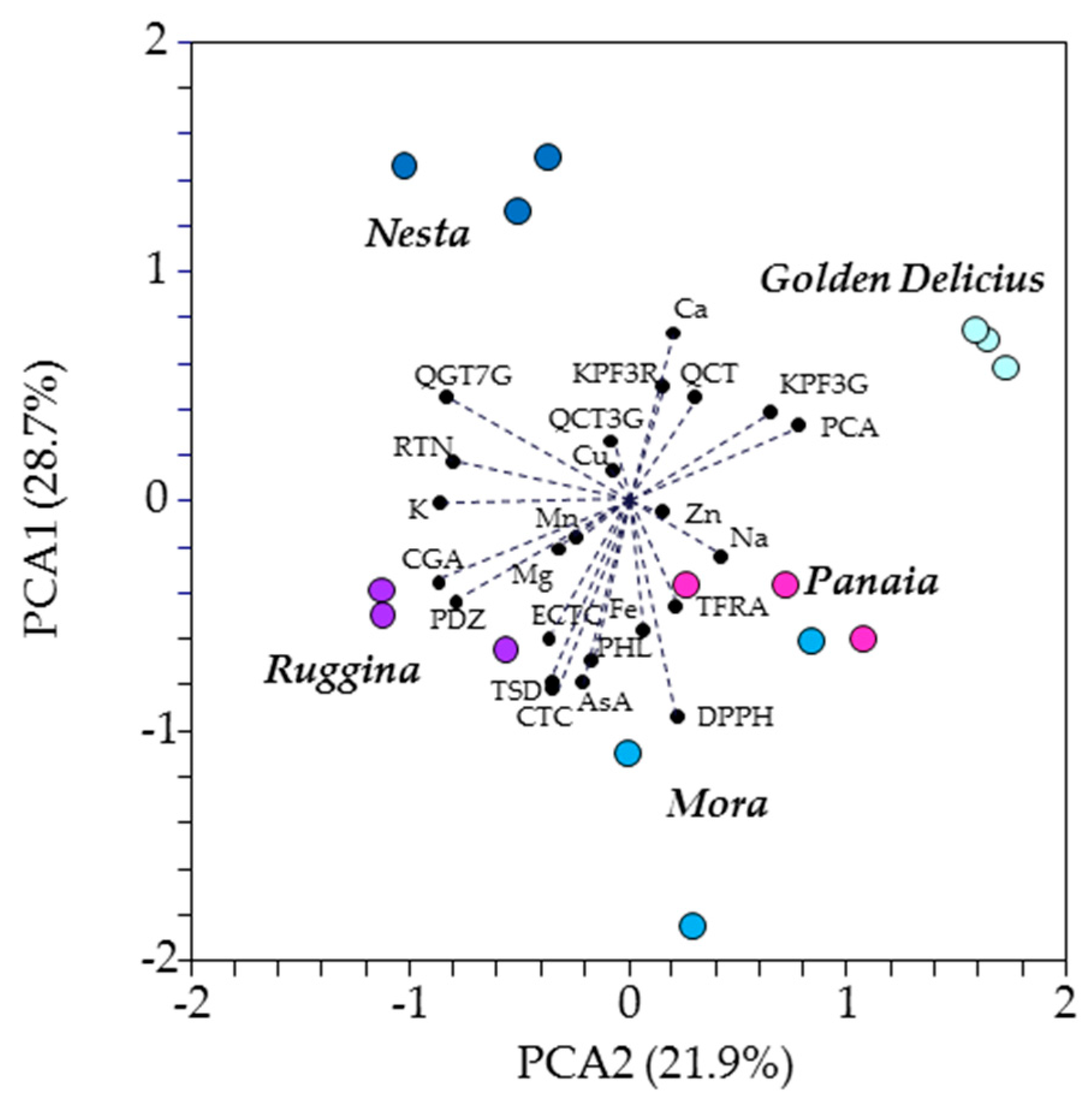
| Name | Acronym | RT (min) | Q1 | Q3 | DP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Catechin | CTC | 2.33 | 289.0 | 244.9 | −108 | −22 | −11 |
| Chlorogenic Acid | CGA | 2.44 | 353.0 | 191.0 | −61 | −24 | −9 |
| Epicatechin | ECTC | 2.63 | 289.0 | 244.9 | −108 | −22 | −11 |
| Quercetagetin 7-O-Glucoside | Q7G | 3.07 | 479.1 | 316.9 | −152 | −31 | −14 |
| p-Coumaric Acid | PCA | 3.09 | 163.0 | 119.0 | −65 | −18 | −11 |
| trans-Ferulic Acid | TFRA | 3.28 | 193.0 | 134.0 | −62 | −20 | −8 |
| Rutin | RTN | 3.30 | 609.2 | 299.9 | −154 | −48 | −11 |
| Quercetin 3-O-Glucoside | QCT3G | 3.40 | 463.1 | 300.0 | −154 | −37 | −5 |
| Kaempferol 3-O-Rutinoside | KPF3R | 3.53 | 593.2 | 284.9 | −138 | −40 | −5 |
| Kaempferol 3-O-Glucoside | KPF3G | 3.62 | 447.1 | 284.1 | −202 | −39 | −11 |
| Kaempferol 7-O-Glucoside | KPF7G | 3.66 | 447.1 | 284.9 | −158 | −38 | −5 |
| Phloridzin | PDZ | 3.71 | 435.1 | 272.9 | −135 | −23 | −5 |
| Quercetin | QCT | 4.30 | 301.0 | 150.9 | −113 | −38 | −8 |
| Tiliroside | TSD | 4.41 | 593.2 | 284.9 | −138 | −40 | −5 |
| Phloretin | PHL | 4.64 | 273.0 | 167.0 | −103 | −38 | −11 |
| Name | DPPH (%) | Vitamin C (mg 100 g−1 FW) |
|---|---|---|
| Golden Delicious | 23.7 ± 3.43 c | 0.39 ± 0.140 c |
| Mora | 55.1 ± 5.23 a | 3.43 ± 0.054 a |
| Nesta | 3.30 ± 2.63 d | 1.27 ± 0.056 b |
| Panaia | 33.3 ± 5.48 bc | 3.06 ± 0.125 a |
| Ruggina | 41.1 ± 3.93 b | 3.30 ± 0.343 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francini, A.; Fidalgo-Illesca, C.; Raffaelli, A.; Sebastiani, L. Phenolics and Mineral Elements Composition in Underutilized Apple Varieties. Horticulturae 2022, 8, 40. https://doi.org/10.3390/horticulturae8010040
Francini A, Fidalgo-Illesca C, Raffaelli A, Sebastiani L. Phenolics and Mineral Elements Composition in Underutilized Apple Varieties. Horticulturae. 2022; 8(1):40. https://doi.org/10.3390/horticulturae8010040
Chicago/Turabian StyleFrancini, Alessandra, Carmen Fidalgo-Illesca, Andrea Raffaelli, and Luca Sebastiani. 2022. "Phenolics and Mineral Elements Composition in Underutilized Apple Varieties" Horticulturae 8, no. 1: 40. https://doi.org/10.3390/horticulturae8010040
APA StyleFrancini, A., Fidalgo-Illesca, C., Raffaelli, A., & Sebastiani, L. (2022). Phenolics and Mineral Elements Composition in Underutilized Apple Varieties. Horticulturae, 8(1), 40. https://doi.org/10.3390/horticulturae8010040









