Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage
Abstract
:1. Introduction
2. Material and Methods
2.1. Vegetal Material
2.2. Extraction of Aloe Vera Gel and Edible Coating Formulation
- -
- EC1: 120 mL Aloe vera gel + 6 g HPMC + 3 g PSO were dissolved in 300 mL of water;
- -
- EC2: 120 mL Aloe vera gel + 3 g HPMC were dissolved in 300 mL of water.
2.3. Experimental Design
2.4. Physico-Chemical Analysis
2.5. Microbiological Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Analysis
3.1.1. Weight Loss
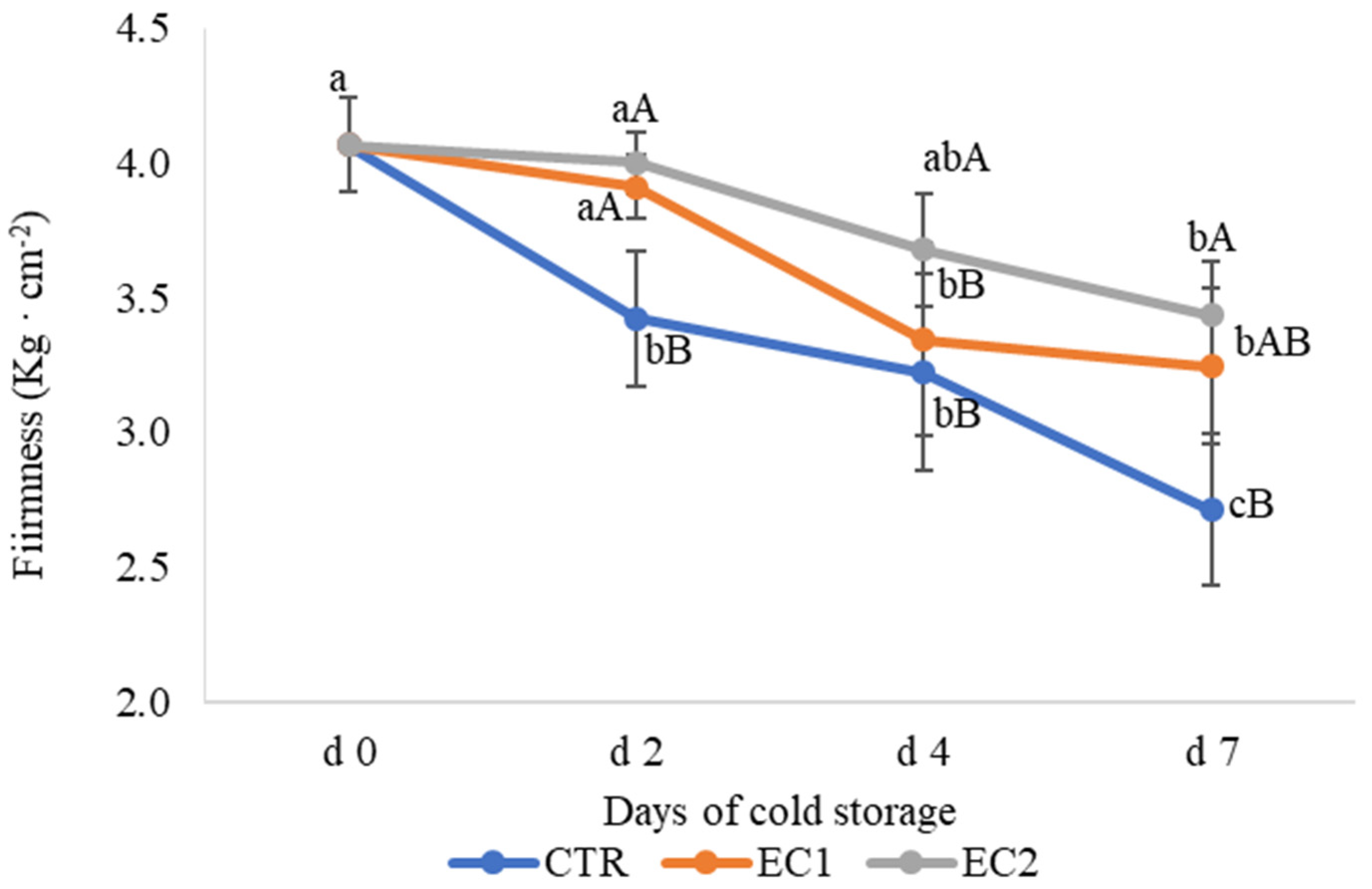
3.1.2. Firmness
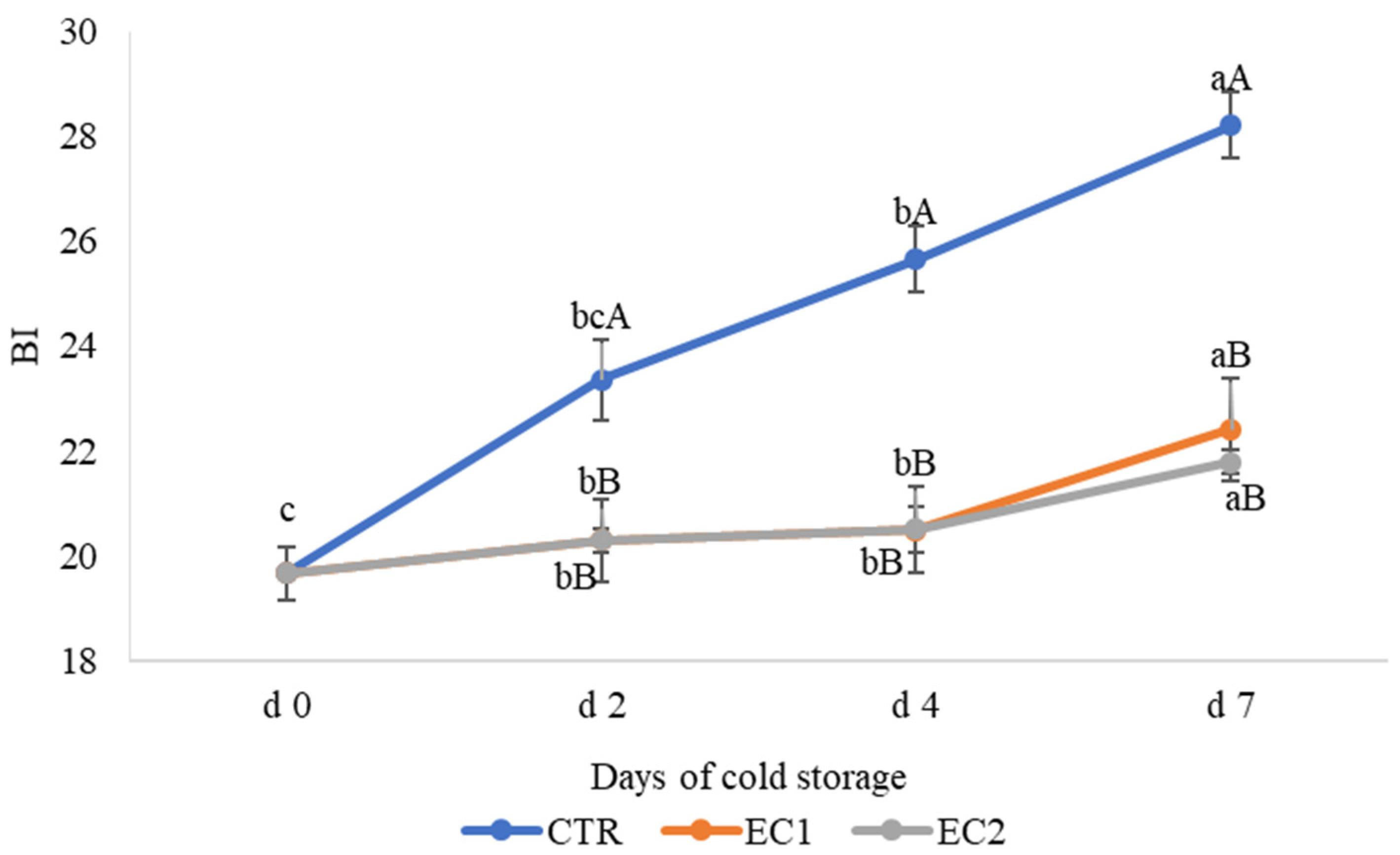
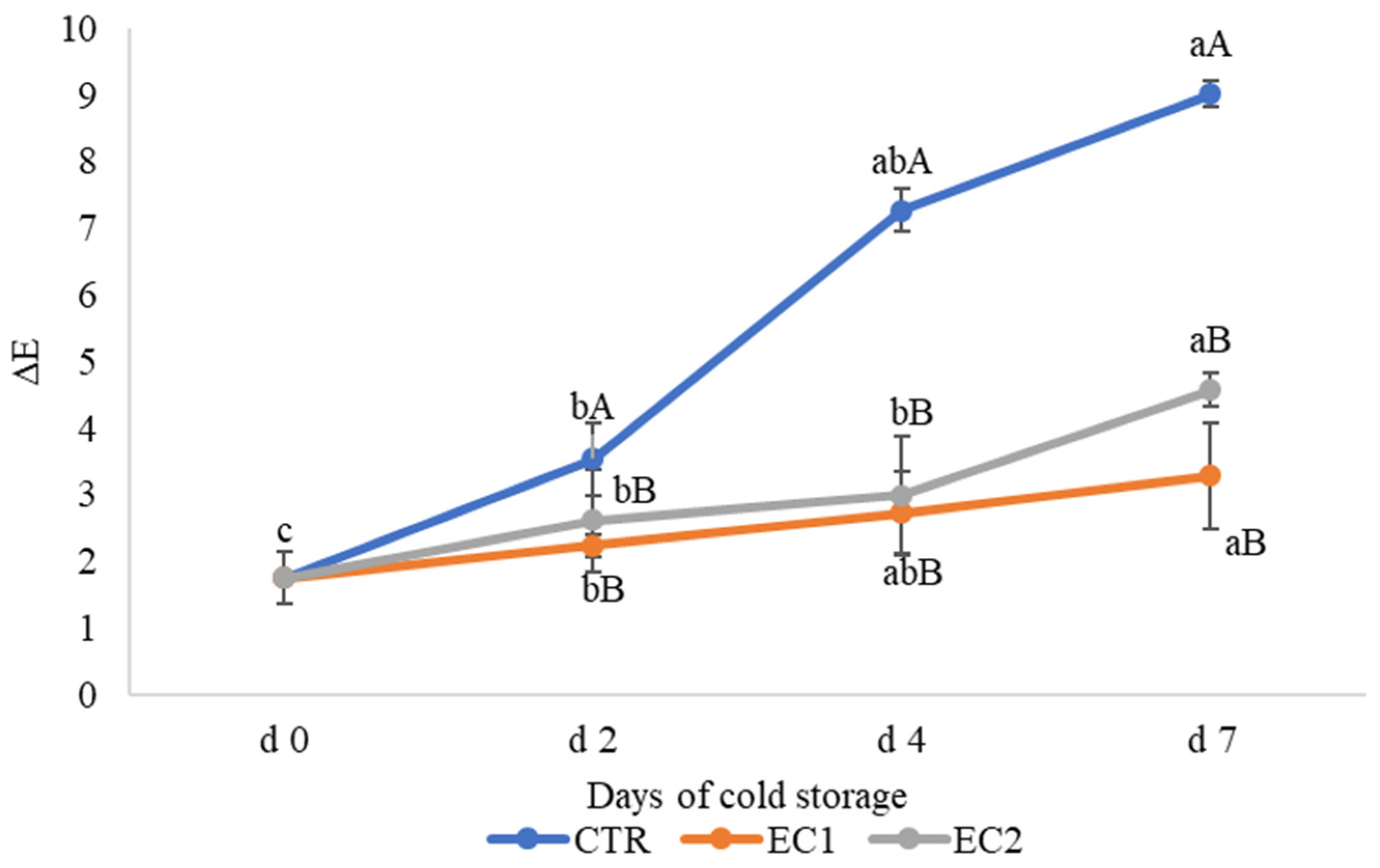
3.1.3. Flesh Colour
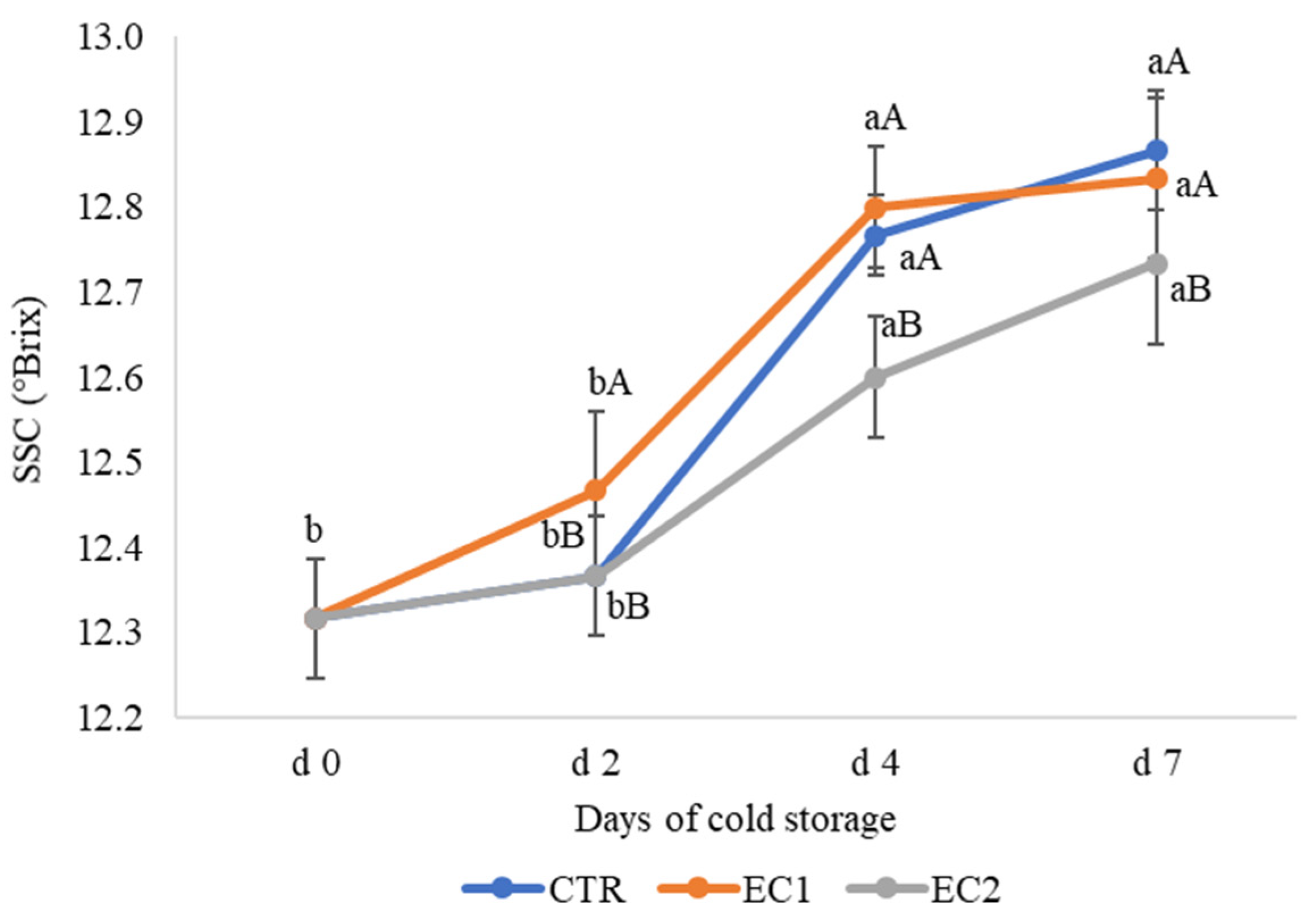
3.1.4. Soluble Solid Content
3.1.5. Microbiological Analyses
3.1.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nashima, K.; Shimizu, T.; Nishitani, C.; Yamamoto, T.; Takahashi, H.; Nakazono, M.; Shiratake, K. Microarray analysis of gene expression patterns during fruit development in European pear (Pyrus communis). Sci. Hortic. 2013, 164, 466–473. [Google Scholar] [CrossRef]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Loscos, J.; Dietz, K.J.; Aparicio-Tejo, P.M.; Becana, M. Function of antioxidant enzymes and metabolites during maturation of pea fruits. J. Exp. Bot. 2010, 61, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Anese, M.; Manzano, M.; Nicoli, M.C. Quality of minimally processed apple slices using different modified atmosphere conditions. J. Food Qual. 1997, 20, 359–370. [Google Scholar] [CrossRef]
- Williams, M.W.; Patterson, M.E. Storage effects on fruits, nonvolatile organic acids and core breakdown of Bartlett pears. J. Agric. Food Chem. 1964, 12, 80–83. [Google Scholar] [CrossRef]
- Hulme, A.C. Carbon dioxide injury and the presence of succinic acid in apples. Nature 1956, 178, 218–219. [Google Scholar] [CrossRef]
- Peppelenbos, H.W.; Oosterhaven, J. A theoretical approach on the role of fermentation in harvested plant products. In Proceedings of the ISHS Acta Horticulturae 464: International Postharvest Science Conference Postharvest 96, Taupo, New Zealand, 4–9 August 1996; pp. 381–386. [Google Scholar]
- Rawyler, A.; Pavelic, D.; Gianinazzi, C.; Oberson, J.; Braendle, R. Membrane lipid integrity relies on a threshold of ATP production rate in potato cell cultures submitted to anoxia. Plant. Physiol. 1999, 120, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biegańska-Marecik, R.; Czapski, J. The effect of selected compounds as inhibitors of enzymatic browning and softening of minimally processed apples. Acta Sci. Pol. Technol. Aliment. 2007, 6, 37–49. [Google Scholar]
- El-Shimi, N.M. Control of enzymatic browning in apple slices by using ascorbic acid under different conditions. Plant. Foods Hum. Nutr. 1993, 43, 71–76. [Google Scholar] [CrossRef]
- Arias, E.; López-Buesa, P.; Oria, R. Extension of fresh-cut “Blanquilla” pear (Pyrus communis L.) shelf-life by 1-MCP treatment after harvest. Postharvest Biol. Technol. 2009, 54, 53–58. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C.; Cacace, D.; Palmieri, L.; Carpi, G. Effetti di trattamenti in soluzioni zuccherine su cubetti di melone freschi destinati alla IV gamma. Industria Conserve 1993, 68, 425–430. [Google Scholar]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef] [Green Version]
- Scramin, J.A.; de Britto, D.; Forato, L.A.; Bernardes-Filho, R.; Colnago, L.A.; Assis, O.B. Characterisation of zein–oleic acid films and applications in fruit coating. Int. J. Food Sci. Technol. 2011, 46, 2145–2152. [Google Scholar] [CrossRef]
- Ju, Z.; Duan, Y.; Ju, Z. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol. Technol. 2000, 20, 243–250. [Google Scholar] [CrossRef]
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Carboxymethyl cellulose coating and low-dose gamma irradiation improves storage quality and shelf life of pear (Pyrus communis L., Cv. Bartlett/William). J. Food Sci. 2010, 75, M586–M596. [Google Scholar] [CrossRef] [PubMed]
- Nandane, A.S.; Dave, R.K.; Rao, T.V.R. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolau-Lapeña, I.; Aguiló-Aguayo, I.; Kramer, B.; Abadias, M.; Viñas, I.; Muranyi, P. Combination of ferulic acid with Aloe vera gel or alginate coatings for shelf-life prolongation of fresh-cut apples. Food Packag. Shelf Life 2021, 27, 100620. [Google Scholar] [CrossRef]
- Allegra, A.; Farina, V.; Inglese, P.; Gallotta, A.; Sortino, G. Qualitative Traits and Shelf Life of Fig Fruit (‘Melanzana’) Treated with Aloe Vera Gel Coating. Acta Hortic. 2021, 1310, 87–92. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Scuderi, D.; Saletta, F.; Gugliuzza, G.; Gallotta, A.; Sortino, G. Postharvest application of aloe vera gel-based edible coating to improve the quality and storage stability of fresh-cut papaya. J. Food Qual. 2020, 2020, 8303140. [Google Scholar] [CrossRef] [Green Version]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of Three Different Aloe vera Gel-Based Edible coatings on the Quality of Fresh-Cut “Hayward” Kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.A.; Yousef, A.; Ali, A. Application of Biodegradable Aloe vera gel and Linseed mucilage for extending the shelf life of Plums. Res. J. Pharm. Technol. 2021, 14, 1579–1585. [Google Scholar]
- Ahmed, M.J.; Singh, Z.; Khan, A.S. Postharvest Aloe vera gel-coating modulates fruit ripening and quality of ‘Arctic Snow’ nectarine kept in ambient and cold storage. Int. J. Food Sci. Technol. 2009, 44, 1024–1033. [Google Scholar] [CrossRef]
- Dang, K.T.H.; Singh, Z.; Swinny, E.E. Edible coatings influence fruit ripening, quality, and aroma biosynthesis in mango fruit. J. Agric. Food Chem. 2008, 56, 1361–1370. [Google Scholar] [CrossRef]
- Ergun, M.; Satici, F. Use of Aloe vera gel as biopreservative for ‘Granny Smith’ and ‘Red Chief’ apples. J. Anim. Plant. Sci. 2012, 22, 363–368. [Google Scholar]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53. [Google Scholar] [CrossRef]
- Burdock, G.A. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the cross-linking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based composite edible films containing Origanum vulgare L. essential oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Perone, N.; Torrieri, E.; Cavella, S.; Masi, P. Effect of rosemary oil on functional properties of HPMC films at different concentration. Food Bioprocess. Technol. 2014, 7, 605–609. [Google Scholar] [CrossRef]
- Lucci, P.; Pacetti, D.; Loizzo, M.R.; Frega, N.G. Punica granatum cv. Dente di Cacallo seed ethanolic extract: Antioxidant and antiproliferative activities. Food Chem. 2015, 167, 475–483. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant acitivity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef]
- Rouhi, S.Z.T.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. BMC Complement. Altern. Med. 2017, 17, 156. [Google Scholar]
- Foss, S.R.; Nakamura, C.V.; Ueda-Nakamura, T.; Cortez, D.A.; Endo, E.H.; Dias Filho, B.P. Antifungal activity of pomegranate peel extract and isolated compound punicalagin against dermatophytes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 32. [Google Scholar] [CrossRef]
- Okan, O.T.; Kilic, A.; Onaran, A.; Öz, M.; Deniz, I. Determination of chemical composition, antioxidant and antifungal properties of pomegranate (Punica granatum L.) and Parsley (Petroselinum crispum) seed oil produced in industrial scale. Artvin Çoruh Üniversitesi Orman Fakültesi Dergisi 2020, 21, 143–153. [Google Scholar]
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. Sci. Asia 2012, 38, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Onias, E.A.; Araújo, R.H.C.R.; de Queiroga, T.B.; Teodosio, A.E.M.d.M.; Onias, E.A.; Ferreira, A.P.N. Coating guava postharvest with the use of starch of tamarind seed and pomegranate seed oil. J. Agric. Sci. 2018, 11, 313. [Google Scholar]
- Siraj, N.; Shabbir, M.A.; Khan, M.R.; Rehman, K.U. Preventing oxidation of canola and sunflower oils by addition of pomegranate seed oil. Acta Aliment. 2019, 48, 18–27. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Ruangchakpet, A.; Sajjaanantakul, T. Effect of browning on total phenolic, flavonoid content and antioxidant activity in Indian gooseberry (Phyllanthus emblica Linn.). Agric. Nat. Resour. 2007, 41, 331–337. [Google Scholar]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-methylcyclopropene and aloe arborescens edible coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Seyed, R.H.; Rastegar, S.; Faramarzi, S. Impact of edible coating derived from a combination of Aloe vera gel, chitosan and calcium chloride on maintain the quality of mango fruit at ambient temperature. J. Food Meas. Charact. 2021, 15, 2932–2942. [Google Scholar] [CrossRef]
- Misir, J.; Brishti, F.H.; Hoque, M.M. Aloe vera gel as a novel edible coating for fresh fruits: A review. Am. J. Food Sci. Technol. 2014, 2, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Deka, B.C.; Singh, A.; Patel, R.K.; Paul, D.; Misra, L.K.; Ojha, H. Extension of shelf life of pear fruits using different packaging materials. J. Food Sci. Technol. 2012, 49, 556–563. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Massa, A.; Perez-Gago, M.B. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno). LWT Food Sci. Technol. 2011, 44, 2328–2334. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant. Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.; Dubey, N. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Brishti, F.H.; Misir, J.; Sarker, A. Effect of Biopreservatives on storage life of Papaya fruit (Carica Papaya, L.). Int. J. Food Stud. 2013, 2, 126–136. [Google Scholar] [CrossRef]
- Olivas, G.I.; Rodriguez, J.J.; Barbosa-Cánovas, G.V. Edible coatings composed of methylcellulose, stearic acid, and additives to preserve quality of pear wedges. J. Food Process. Preserv. 2003, 27, 299–320. [Google Scholar] [CrossRef]
- Sapers, G.M.; Miller, R.L. Browning inhibition in fresh-cut pears. J. Food Sci. 1998, 63, 342–346. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, L.; Zhao, Z.; Zhi, H.; Guan, J. Effects of 1-MCP on reactive oxygen species, polyphenol oxidase activity, and cellular ultra-structure of core tissue in ‘Yali’pear (Pyrus bretschneideri Rehd.) during storage. Hortic. Environ. Biotechnol. 2015, 56, 207–215. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality changes in fresh-cut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Eccher Zerbini, P.; Rizzolo, A.; Brambilla, A.; Cambiaghi, P.; Grassi, M. Loss of ascorbic acid during storage of Conference pears in relation to the appearance of brown heart. J. Sci. Food Agric. 2002, 82, 1007–1013. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate-and gellan-based edible films for probiotic coatings on fresh-cut fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT Food Sci. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Goncalves, E.D.; Antunes, P.L.; Brackmann, A. Controlled atmosphere storage of Asian pears cv. Nijisseiki. Rev. Bras. Frutic. 2000, 22, 226–231. [Google Scholar]
- Park, Y.M. Relationship between instrumental and sensory analysis of quality factors in apple and pear fruits. Korean J. Hortic. Sci. Technol. 2002, 20, 394–398. [Google Scholar]
- Alfonzo, A.; Gaglio, R.; Miceli, A.; Francesca, N.; Di Gerlando, R.; Moschetti, G.; Settanni, L. Shelf-life evaluation of fresh-cut red chicory subjected to different minimal processes. Food Microbiol. 2018, 73, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Fierer, N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 2013, 8, e59310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miceli, A.; Settanni, L. Influence of agronomic practices and pre-harvest conditions on the attachment and development of Listeria monocytogenes in vegetables. Ann. Microbiol. 2019, 69, 185–199. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.C.; Martín-Belloso, O. Microbiological and biochemical changes in minimally processed fresh-cut Conference pears. Eur. Food Res. Technol. 2003, 217, 4–9. [Google Scholar] [CrossRef]

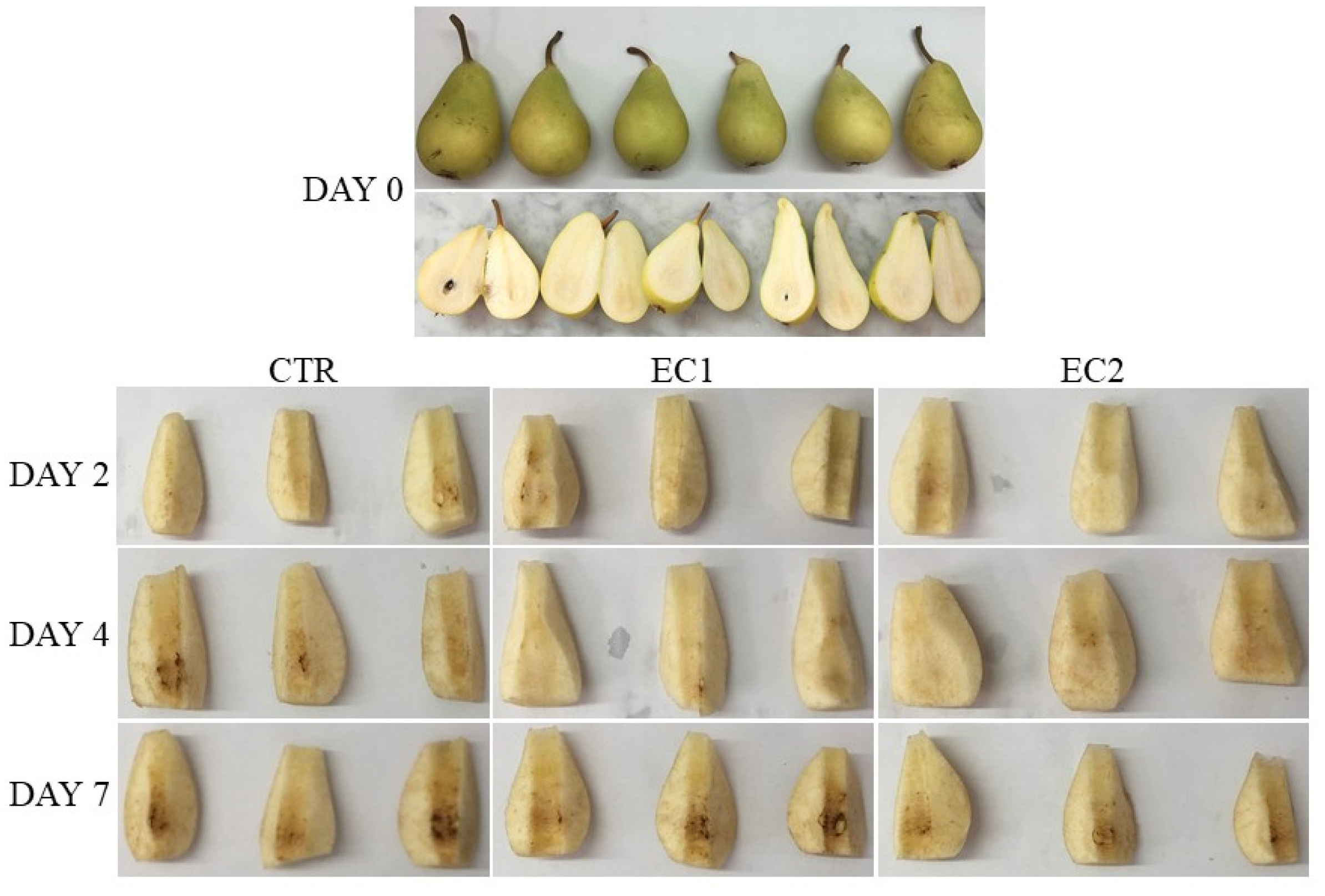
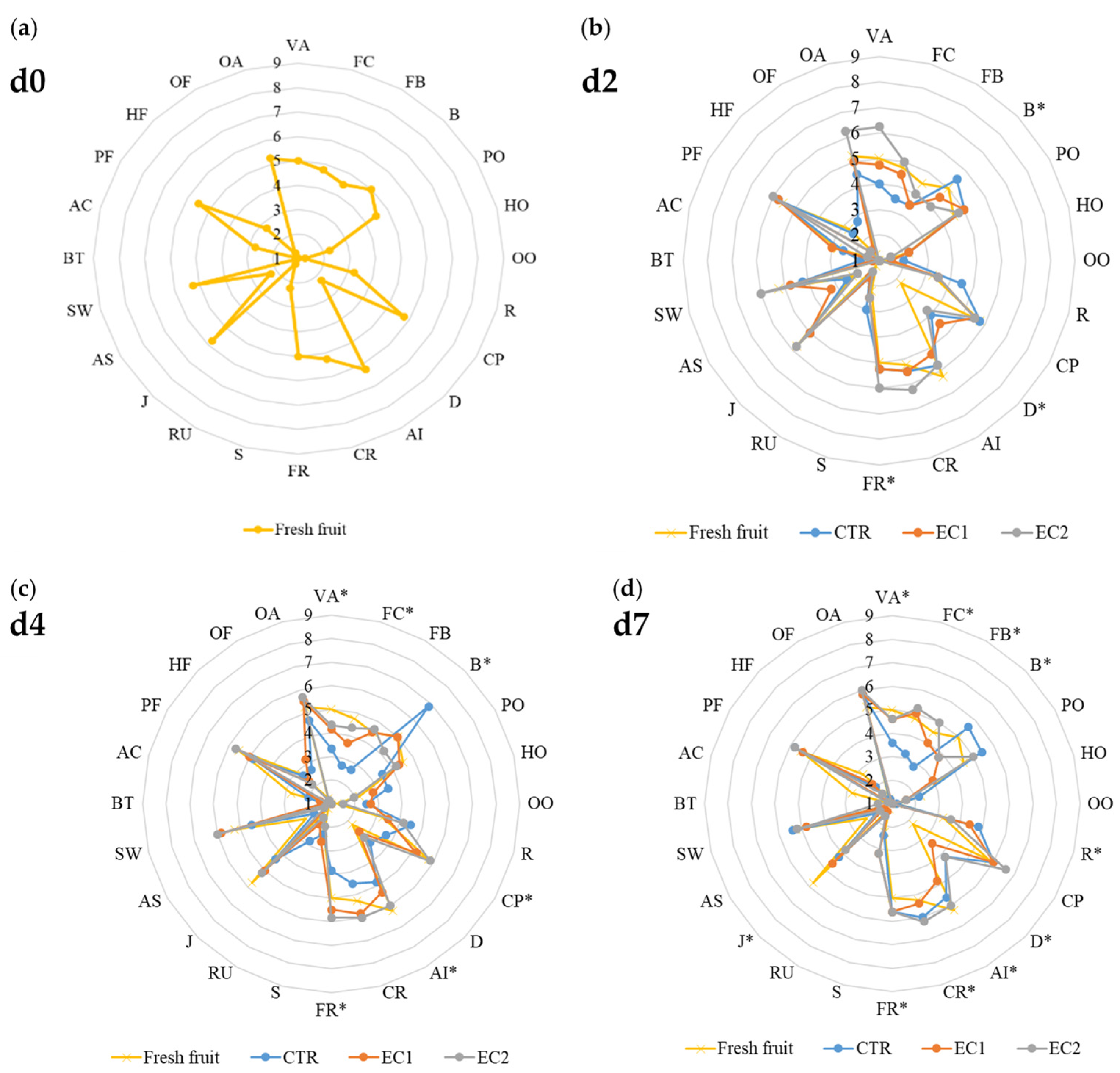
| Treatments | d2 | d4 | d7 |
|---|---|---|---|
| CTR | 1.14 ± 0.03 A | 1.7 ± 0.06 A | 2.1 ± 0.12 A |
| EC1 | 0.8 ± 0.03 B | 1.3 ± 0.05 AB | 1.6 ± 0.1 AB |
| EC2 | 0.7 ± 0.03 B | 1.0 ± 0.05 B | 1.2 ± 0.1 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passafiume, R.; Gugliuzza, G.; Gaglio, R.; Busetta, G.; Tinebra, I.; Sortino, G.; Farina, V. Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae 2021, 7, 581. https://doi.org/10.3390/horticulturae7120581
Passafiume R, Gugliuzza G, Gaglio R, Busetta G, Tinebra I, Sortino G, Farina V. Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae. 2021; 7(12):581. https://doi.org/10.3390/horticulturae7120581
Chicago/Turabian StylePassafiume, Roberta, Giovanni Gugliuzza, Raimondo Gaglio, Gabriele Busetta, Ilenia Tinebra, Giuseppe Sortino, and Vittorio Farina. 2021. "Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage" Horticulturae 7, no. 12: 581. https://doi.org/10.3390/horticulturae7120581
APA StylePassafiume, R., Gugliuzza, G., Gaglio, R., Busetta, G., Tinebra, I., Sortino, G., & Farina, V. (2021). Aloe-Based Edible Coating to Maintain Quality of Fresh-Cut Italian Pears (Pyrus communis L.) during Cold Storage. Horticulturae, 7(12), 581. https://doi.org/10.3390/horticulturae7120581











