Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Design
2.3. Soil Analyses
2.4. The Population of the Main Soil-Borne Pathogens: Fusarium oxysporum spp., Pythium spp. and Phytophthora spp.
2.5. Extraction of Soil DNA and Quantification of Fusarium oxysporum
2.6. Statistical Analyses
3. Results
3.1. Soil pH and EC
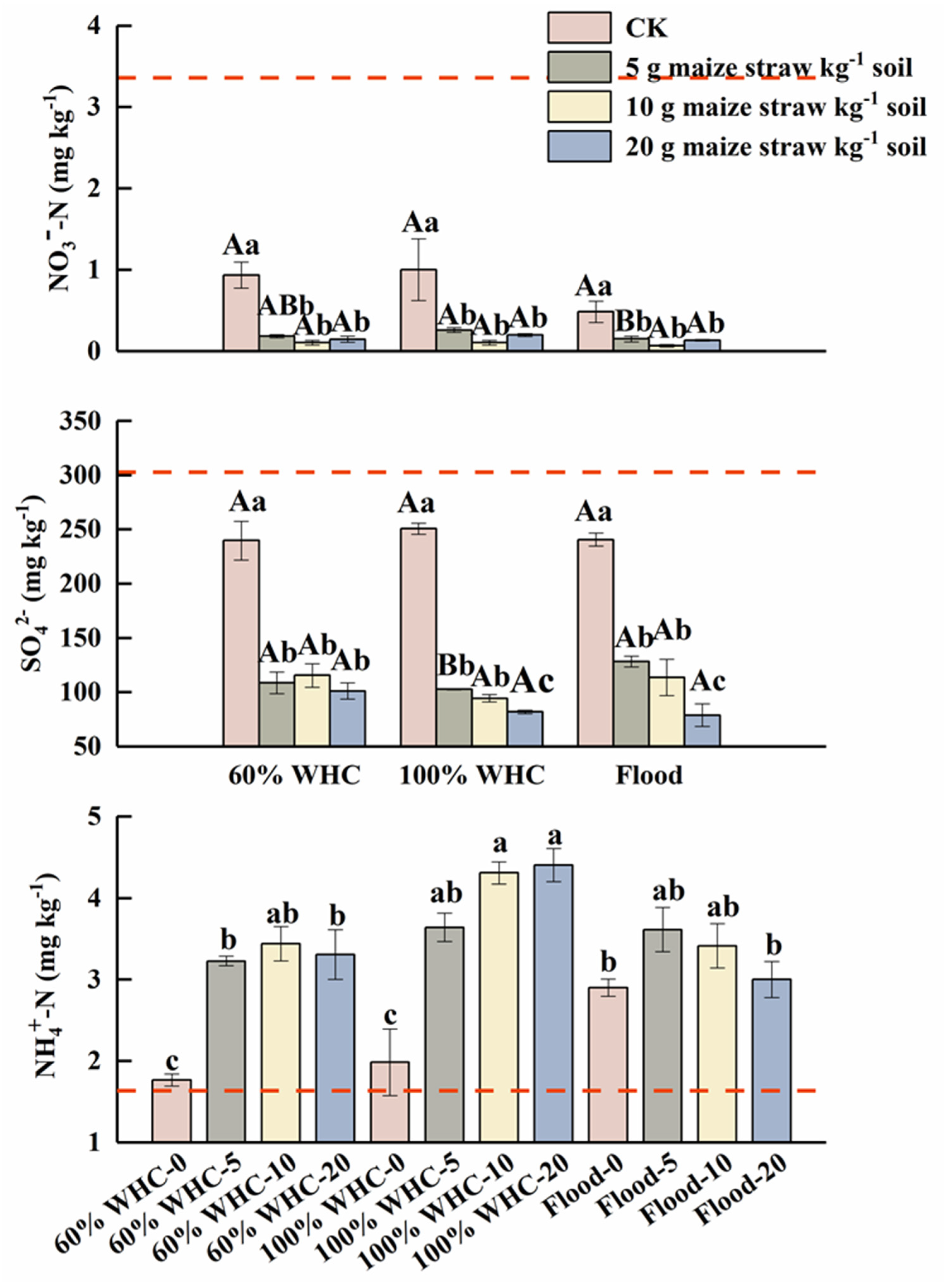
3.2. Soil SO42−, NH4+ and NO3− Contents
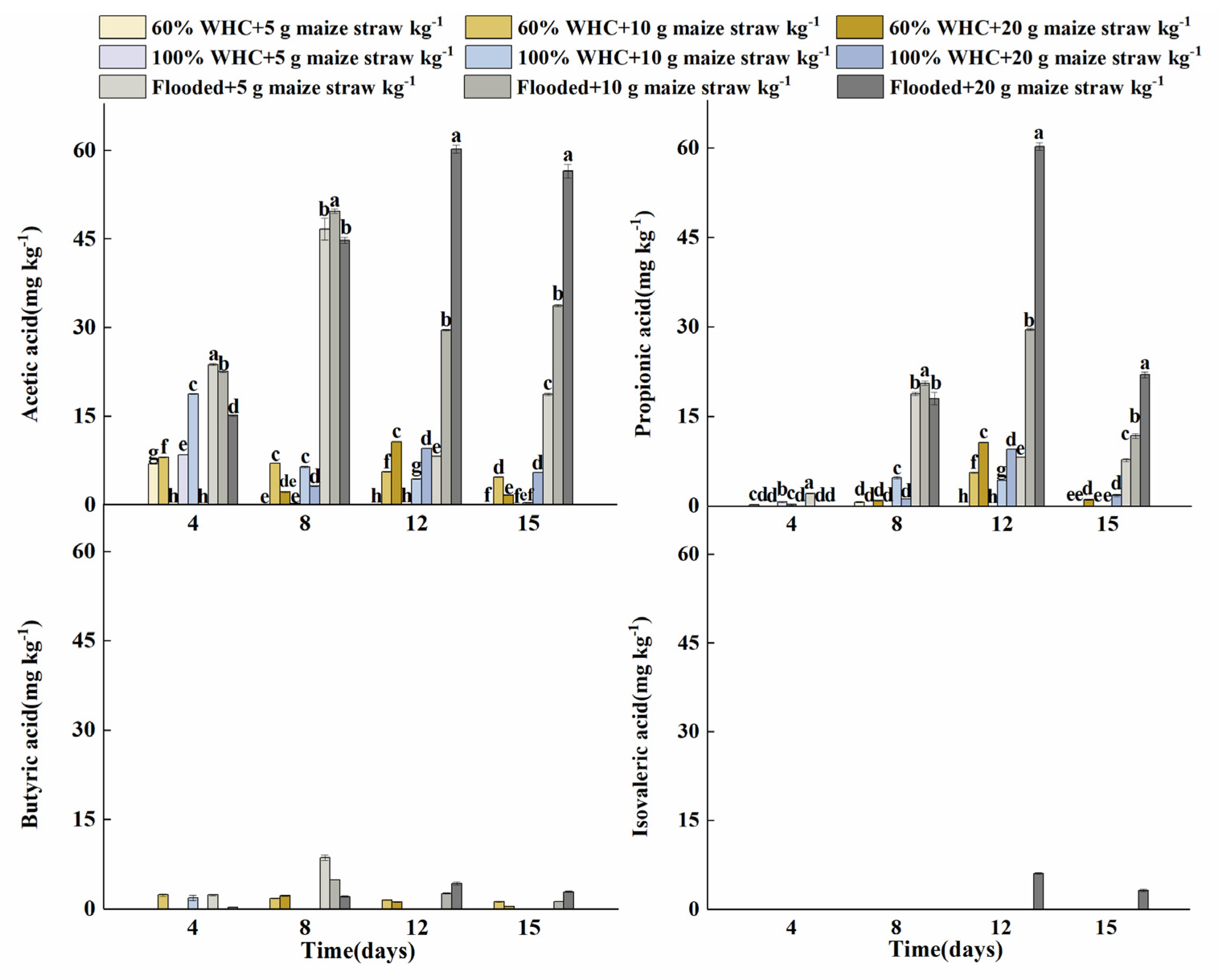
3.3. Dynamics of Organic Acids during the RSD Treatment
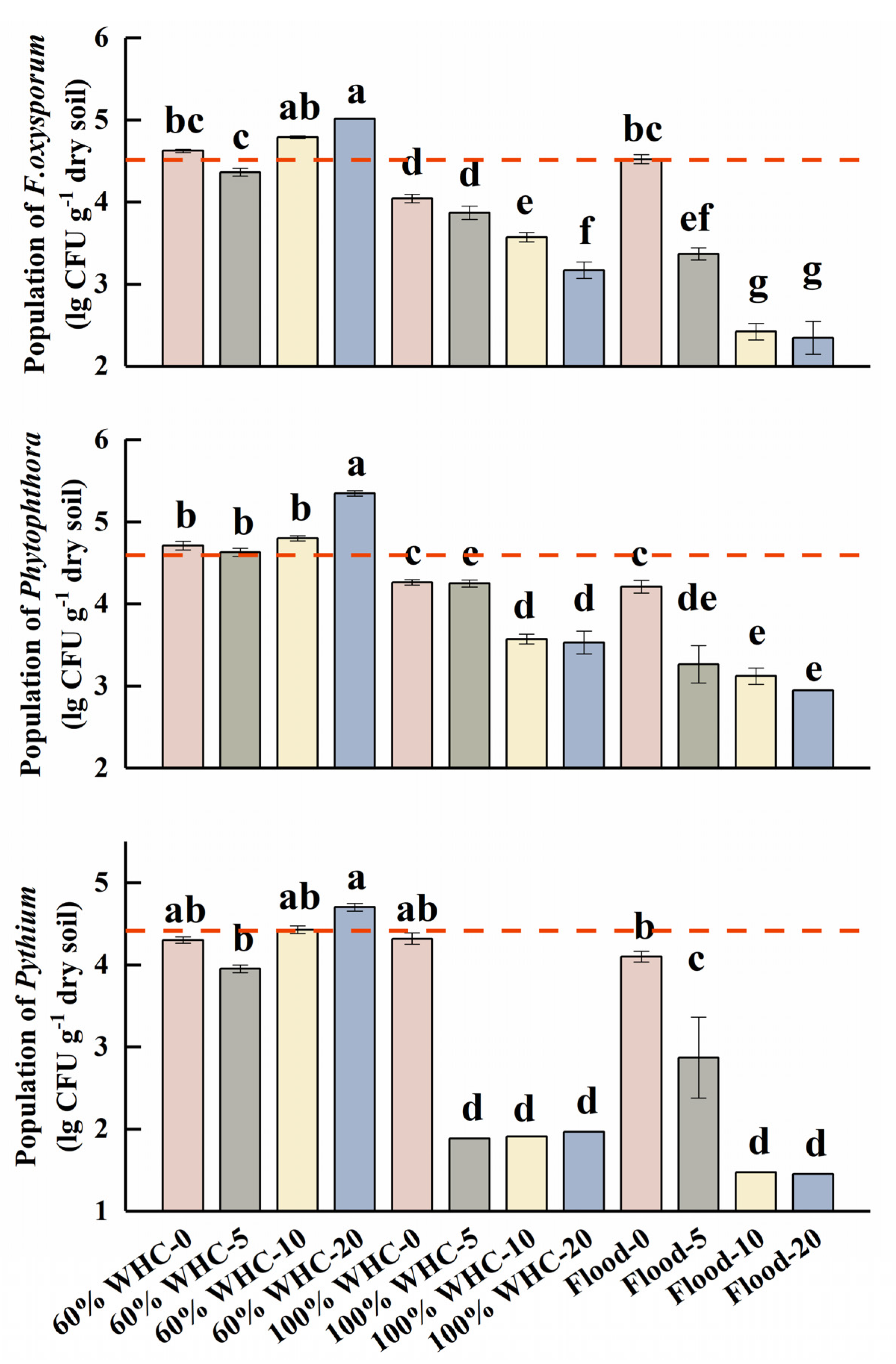
3.4. Community of Soil-Borne Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lal, R. Soil management in the developing countries. Soil Sci. 2000, 165, 57–72. [Google Scholar] [CrossRef]
- Ju, X.; Kou, C.; Zhang, F.; Christie, P. Nitrogen balance and groundwater nitrate contamination: Comparison among three intensive cropping systems on the North China Plain. Environ. Pollut. 2006, 143, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.M.; Yao, J.; Yan, F. Vegetable cultivation under greenhouse conditions leads to rapid accumulation of nutrients, acidification and salinity of soils and groundwater contamination in South-Eastern China. Nutr. Cycl. Agroecosyst. 2009, 83, 73–84. [Google Scholar] [CrossRef]
- Zhou, D.P.; Chu, C.B.; Liu, F.F.; Fan, J.Q.; Jiang, Z.F.; Wu, S.H. Effect of asparagu’s cultivation years on physio-chemical properties, microbial community and enzyme activities in greenhouse soil. Plant Nutr. Fert. Sci. 2012, 2, 459–466. [Google Scholar]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Shinmura, A.; Sakamoto, N.; Abe, H. Control of Fusarium root rot of Welsh onion by soil reduction. Jpn. J. Phytopathol 1999, 65, 352–353. [Google Scholar]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Dang, Q.; Zhang, J.; Müller, C.; Cai, Z. Reductive soil disinfestation (RSD) alters gross N transformation rates and reduces NO and N2O emissions in degraded vegetable soils. Plant Soil 2014, 382, 269–280. [Google Scholar] [CrossRef]
- Wen, T.; Huang, X.; Zhang, J.; Zhu, T.; Meng, L.; Cai, Z. Effects of water regime, crop residues, and application rates on control of Fusarium oxysporum f. sp. cubense. J. Environ. Sci. 2015, 31, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Goud, J.K.C.; Termorshuizen, A.J.; Blok, W.J.; Van Bruggen, A.H. Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis. 2004, 88, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Albano, J.P.; Muramoto, J.; Shennan, C. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil 2012, 355, 149–165. [Google Scholar] [CrossRef]
- Kobara, Y.; Uematsu, S.; Tanaka-Miwa, C.; Sato, R.; Sato, M. Possibility of the new soil fumigation technique with ethanol solution. In Proceedings of the 2007 Annual Research Conference on Methyl Bromide Alternatives and Emissions Reduction, San Diego, CA, USA, 29 October–1 November 2007; p. 74. [Google Scholar]
- Momma, N.; Usami, T.; Amemiya, Y.; Shishido, M. Factors involved in the suppression of Fusarium oxysporum f. sp. lycopersici by soil reduction. Soil Microorg. (Jpn.) 2005, 59, 27–33. [Google Scholar]
- Shinmura, A. Principle and effect of soil sterilization method by reducing redox potential of soil. PSJ Soilborne Dis. Workshop Rep. 2004, 22, 2–12. [Google Scholar]
- Wen, T.; Huang, X.; Zhang, J.; Cai, Z. Effects of biological soil disinfestation and water regime on suppressing Artemisia selengensis root rot pathogens. J. Soils Sediments 2016, 16, 215–225. [Google Scholar] [CrossRef]
- Snyder, G.H. Agricultural flooding of organic soils. In Bulletin/University of Florida. Agricultural Experiment Station (USA); University of Florida, Agricultural Experiment Stations: Gainesville, FL, USA, 1987. [Google Scholar]
- Momma, N.; Momma, M.; Kobara, Y. Biological soil disinfestation using ethanol: Effect on Fusarium oxysporum f. sp. lycopersici and soil microorganisms. J. Gen. Plant Pathol. 2010, 76, 336–344. [Google Scholar] [CrossRef]
- Kubo, C. Analysis of factors involved in sterilization effect by soil reduction. Jpn. J. Phytopathol. 2005, 71, 281–282. [Google Scholar] [CrossRef]
- Matsuoka, K.; Moritsuka, N.; Kusaba, S.; Hiraoka, K. Concentrations of natural stable Cs in organs of blueberry bushes grown in three types of soils treated with acidification or fertilization. Hortic. J. 2019, 88, 31–40. [Google Scholar] [CrossRef]
- Matsuoka, K.; Moritsuka, N.; Kusaba, S.; Hiraoka, K. Effects of soil type and soil treatment on solubilization of 13 elements in the root zone and their absorption by blueberry bushes. Hortic. J. 2018, 87, 155–165. [Google Scholar] [CrossRef]
- Meng, T.; Yang, Y.; Cai, Z.; Ma, Y. The control of Fusarium oxysporum in soil treated with organic material under anaerobic condition is affected by liming and sulfate content. Biol. Fertil. Soils 2018, 54, 295–307. [Google Scholar] [CrossRef]
- Lu, R.K. Soil and Agro-Chemistry Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Meng, T.; Zhu, T.; Zhang, J.; Cai, Z. Effect of liming on sulfate transformation and sulfur gas emissions in degraded vegetable soil treated by reductive soil disinfestation. J. Environ. Sci. 2015, 36, 112–120. [Google Scholar] [CrossRef]
- Schumacher, B.A. Methods for the determination of total organic carbon (TOC) in soils and sediments. US Environ Prot Agency. 2002, 32, 25. [Google Scholar]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lise, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agriculture systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Ling, N.; Huang, Q.; Guo, S.; Shen, Q. Paenibacillus polymyxa SQR-21 systemically affects root exudates of watermelon to decrease the conidial germination of Fusarium oxysporum f. sp. niveum. Plant Soil 2011, 341, 485–493. [Google Scholar] [CrossRef]
- Sun, E.; Su, H.; Ko, W. Identification of Fusarium oxysporum f. sp. cubense race 4 from soil or host tissue by cultural characters [Bananas, wilt]. Phytopathology (USA) 1978, 68, 1672–1673. [Google Scholar]
- Dineen, S.M.; Aranda, R.; Anders, D.L.; Robertson, J.M. An evaluation of commercial DNA extraction kits for the isolation of bacterial spore DNA from soil. J. Appl. Microbiol. 2010, 109, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.; Lévesque, C.A.; Cammue, B.; Thomma, B.P. Quantitative assessment of phytopathogenic fungi in various substrates using a DNA macroarray. Environ. Microbiol. 2005, 7, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wen, T.; Zhang, J.; Meng, L.; Zhu, T.; Cai, Z. Toxic organic acids produced in biological soil disinfestation mainly caused the suppression of Fusarium oxysporum f. sp. cubense. BioControl 2015, 60, 113–124. [Google Scholar] [CrossRef]
- Muramoto, J.; Shennan, C.; Fitzgerald, A.; Koike, S.; Bolda, M.; Daugovish, O.; Rosskopf, E.; Kokalis-Burelle, N.; Butler, D. Effect of anaerobic soil disinfestation on weed seed germination. In Proceedings of the Annual International Research conference on Methyl Bromide Alternatives and Emissions Reductions, Orlando, FL, USA, 11–14 November 2008; Available online: https://mbao.org/prev_year (accessed on 25 October 2021).
- Huang, X.; Liu, L.; Wen, T.; Zhu, R.; Zhang, J.; Cai, Z. Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f. sp. cubense infected soil during and after reductive soil disinfestation. Microbiol. Res. 2015, 181, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Momma, N. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. JARQ 2008, 42, 7–12. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. In The Chemistry of Submerged Soils; Academic Press: Cambridge, MA, USA, 1972; Volume 24, pp. 29–96. [Google Scholar]
- Chandrasekaran, S.; Yoshida, T. Effect of organic acid transformations in submerged soils on growth of the rice plant. Soil Sci. Plant Nutr. 1973, 19, 39–45. [Google Scholar] [CrossRef][Green Version]
- Na, P.; Kairong, W.; Buresh, R. Effect of rice straw incorporation on concentration of organic acids and available phosphorus in soil under different water regimes. Acta Pedol. Sin. 2006, 43, 347. [Google Scholar]
- Simmons, C.W.; Higgins, B.; Staley, S.; Joh, L.D.; Simmons, B.A.; Singer, S.W.; Stapleton, J.J.; VanderGheynst, J.S. The role of organic matter amendment level on soil heating, organic acid accumulation, and development of bacterial communities in solarized soil. Appl. Soil Ecol. 2016, 106, 37–46. [Google Scholar] [CrossRef]
- Henry, P.M.; Haugland, M.; Lopez, L.; Munji, M.; Watson, D.C.; Gordon, T.R. The potential for Fusarium oxysporum f. sp. fragariae, cause of fusarium wilt of strawberry, to colonize organic matter in soil and persist through anaerobic soil disinfestation. Plant Pathol. 2020, 69, 1218–1226. [Google Scholar] [CrossRef]
- Momma, N.; Yamamoto, K.; Simandi, P.; Shishido, M. Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J. Gen. Plant Pathol 2006, 72, 247–252. [Google Scholar] [CrossRef]
- Mowlick, S.; Hirota, K.; Takehara, T.; Kaku, N.; Ueki, K.; Ueki, A. Development of anaerobic bacterial community consisted of diverse clostridial species during biological soil disinfestation amended with plant biomass. Soil Sci. Plant Nutr. 2012, 58, 273–287. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Klarer, E.; Reed, A.J.; Leisso, R.; Poirier, B.; Honaas, L.; Rudell, D.R.; Mazzola, M. Temporal dynamics of the soil metabolome and microbiome during simulated anaerobic soil disinfestation. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef]


| Sample | Total C (g kg−1) | Total N (g kg−1) | EOC (mg kg−1) | C/N |
|---|---|---|---|---|
| Maize straw | 505.1 | 10.64 | 1.75 | 47.47 |
| Soil | 23.65 | 2.68 | 0.05 | 8.82 |
| Number | Treatment |
|---|---|
| 1 | CK–60% WHC |
| 2 | 60% WHC + 5 g maize straw kg−1 soil |
| 3 | 60% WHC + 10 g maize straw kg−1 soil |
| 4 | 60% WHC + 20 g maize straw kg−1 soil |
| 5 | CK–100% WHC |
| 6 | 100% WHC + 5 g maize straw kg−1 soil |
| 7 | 100% WHC + 10 g maize straw kg−1 soil |
| 8 | 100% WHC + 20 g maize straw kg−1 soil |
| 9 | CK–continuous flooding |
| 10 | Continuous flooding + 5 g maize straw kg−1 soil |
| 11 | Continuous flooding + 10 g maize straw kg−1 soil |
| 12 | Continuous flooding + 20 g maize straw kg−1 soil |
| Variable | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | EC | SO42− | NH4+ | NO3− | F. oxysporum | Phytophthora | Pythium | |
| Straw rate (S) | <0.001 | 0.239 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Water content (W) | <0.001 | 0.266 | 0.365 | 0.002 | 0.132 | <0.001 | <0.001 | <0.001 |
| S × W | 0.018 | 0.300 | 0.217 | 0.001 | 0.447 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Huang, X.; Zhang, J.; Cai, Z.; Li, X.; Wen, T. Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate. Horticulturae 2021, 7, 559. https://doi.org/10.3390/horticulturae7120559
Zhu R, Huang X, Zhang J, Cai Z, Li X, Wen T. Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate. Horticulturae. 2021; 7(12):559. https://doi.org/10.3390/horticulturae7120559
Chicago/Turabian StyleZhu, Rui, Xinqi Huang, Jinbo Zhang, Zucong Cai, Xun Li, and Teng Wen. 2021. "Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate" Horticulturae 7, no. 12: 559. https://doi.org/10.3390/horticulturae7120559
APA StyleZhu, R., Huang, X., Zhang, J., Cai, Z., Li, X., & Wen, T. (2021). Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate. Horticulturae, 7(12), 559. https://doi.org/10.3390/horticulturae7120559







