Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. UV-B Radiation Experiments
2.3. Sampling for Biochemical Assays
2.4. Indicators and Methods
2.5. Data Analysis
3. Results
3.1. Yield and Quality
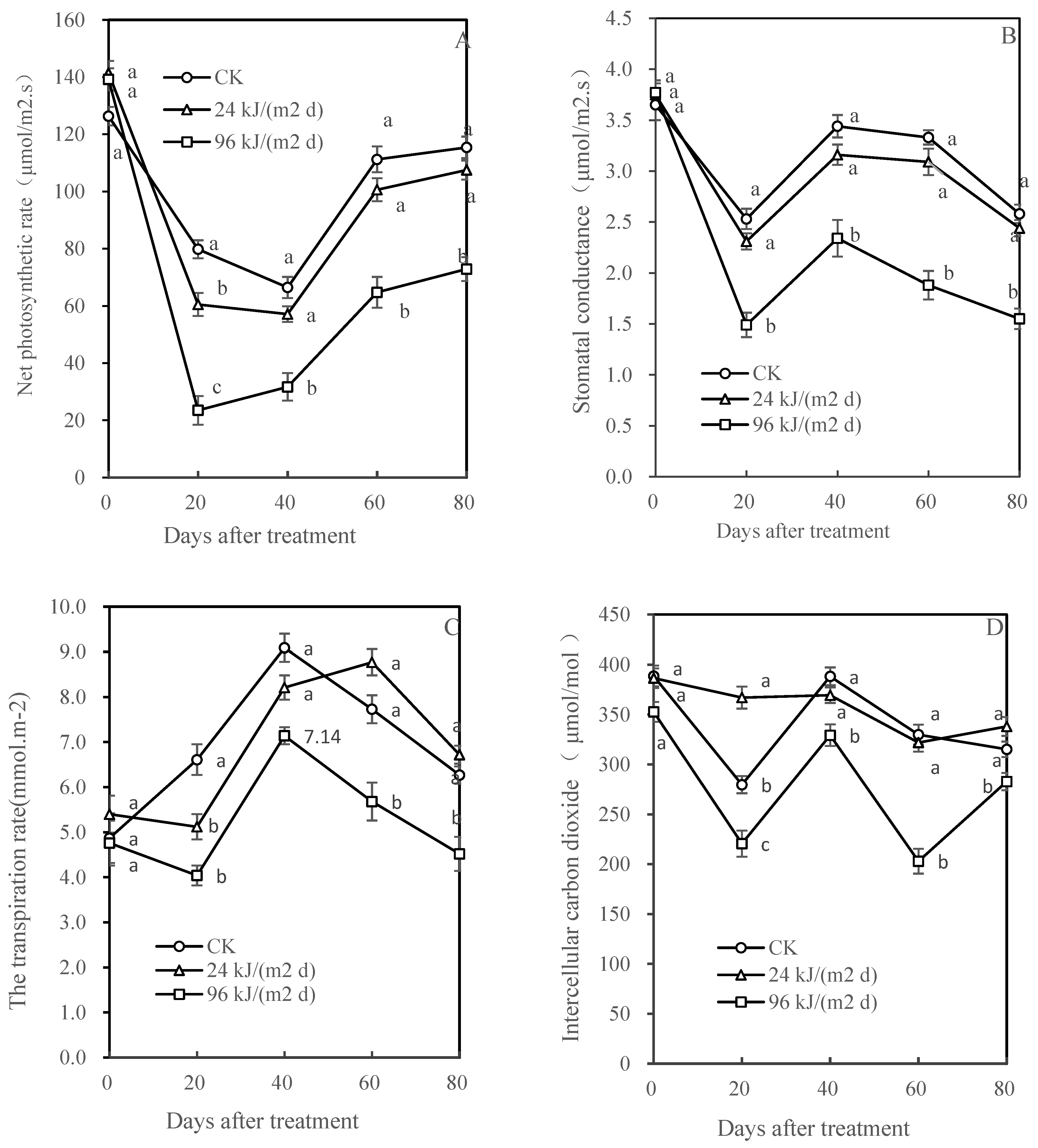
3.2. Pn, Sc, Tr, and Ci
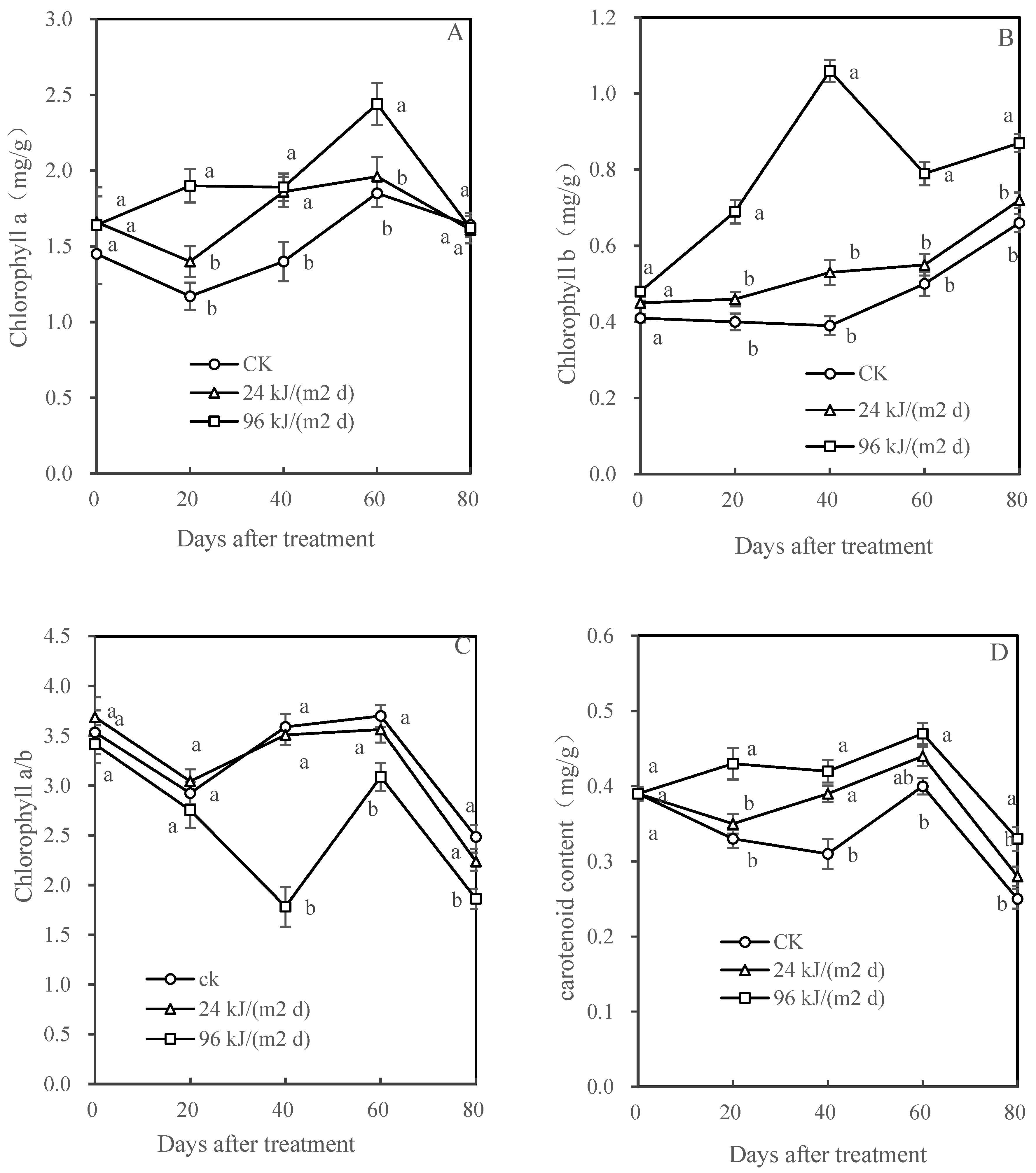
3.3. Chl a, Chl b, Chl a/b, and Carotenoids
3.4. Effects of UV-B Radiation on Light Reaction in Mango Leaves
Hill Activity and qP
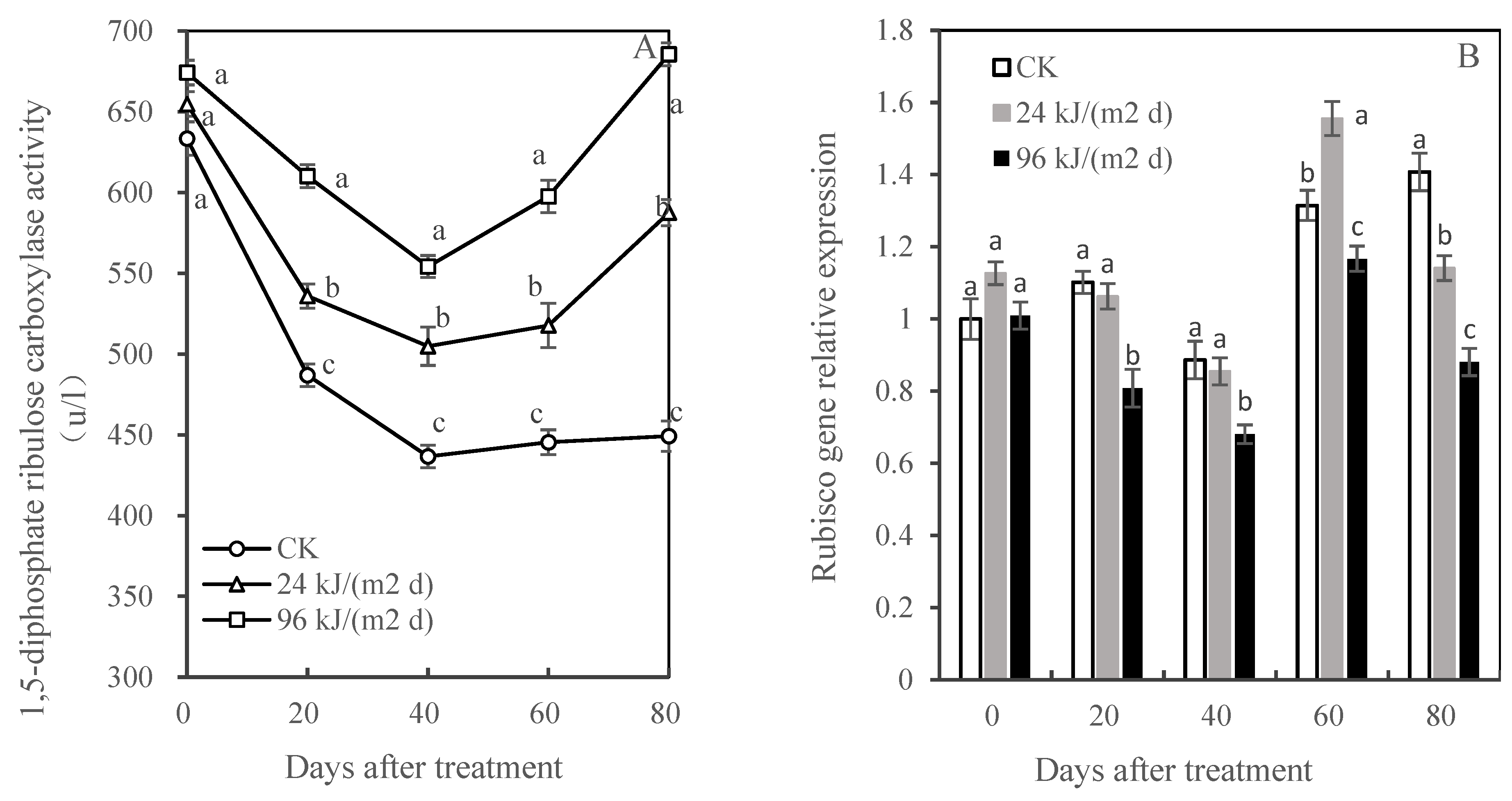
3.5. Effects of UV-B Radiation on the Carbon-Fixation Reaction in Mango Leaves
Rubisco Activity and Gene Expression Analysis
4. Discussion
4.1. Stomatal Limitation under Enhanced UV-B Radiation Inhibits Photosynthesis in Mango Trees
4.2. Non-Stomatal Limitation Caused by Enhanced UV-B Radiation Inhibits Photosynthesis and Causes Fruit Damage in Mango
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caldwell, M.M.; Teramura, A.H.; Tevini, M. The changing solar ultraviolet climate and the ecological consequences for higher plants. Trends Ecol. Evol. 1989, 4, 363–367. [Google Scholar] [CrossRef]
- McFarland, M.; Kaye, J. Chlorofluorocarbons and ozone. Photochem. Photobiol. 1992, 55, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Haapala, J.K.; Mörsky, S.K.; Saarnio, S.; Rinnan, R.; Suokanerva, H.; Kyrö, E.; Latola, K.; Martikanen, P.J.; Holopainen, T.; Silvola, J. Carbon dioxide balance of a fen ecosystem in northern Finland under elevated UV-B radiation. Glob. Chang. Biol. 2009, 15, 943–954. [Google Scholar] [CrossRef]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2001, 33, 179–197. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Swarna, K.; Bhanumathi, G.; Murthy, S.D.S. Studies on the UV-B radiation induced oxidative damage in thylakoid photofunctions and analysis of the role of antioxidant enzymes in maize primary leaves. Bioscan 2012, 7, 609–610. [Google Scholar]
- Rojas-Lillo, Y.; Alberdi, M.; Acevedo, P.; Inostroza-Blancheteau, C.; Rengel, Z.; Mora, M.L.; Reyes-Díaz, M. Manganese toxicity and UV-B radiation differentially influence the physiology and biochemistry of highbush blueberry (Vaccinium corymbosum L.) cultivars. Funct. Plant Biol. 2014, 41, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yanqun, Z.; Jianjun, C.; Haiyan, C. Intraspecific responses incrop growth and yield of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions. Field Crop. Res. 2000, 67, 25–33. [Google Scholar] [CrossRef]
- Lv, Z.W.; Feng, Q.; Lv, Y.W.; Liu, L.K.; Zhang, Y.J.; Li, B.Y.; Zhang, W.H. Response of 140 winter wheat varieties (lines) to UV-B radiation. J. Wheat Crop. 2017, 37, 841–845. (In Chinese) [Google Scholar]
- Teramura, A.H. Effects of ultraviolet-B radiation on thegrowth and yield of crop plants. Physiol. Plant 1983, 58, 415–427. [Google Scholar] [CrossRef]
- An, L.Z.; Feng, H.Y.; Wang, X.L. Enhance the effect of UV-B radiation on the growth of certain grains. J. Ecol. 2001, 21, 249–253. (In Chinese) [Google Scholar]
- Yuan, M.L.; Yue, K.; Wang, H.; Guo, Y.J.; Zhou, K.B. Effects of enhanced UV-B radiation treatment on photosynthesis, yield and conventional quality of adult mango tree. South. Agric. J. 2018, 49, 930–937. (In Chinese) [Google Scholar]
- Zhou, K.B.; Li, S.J.; Yuan, M.L. The influences of enhanced UV-B radiation on yield and fruits quality and photosynthesis of mango trees. Chin. J. Trop. Crop. 2018, 39, 1102–1107. (In Chinese) [Google Scholar]
- Wang, J.; Jiang, L.; Wang, Y.; Li, S.S. UV-induced radiation sensitivity difference in plant leaves. Bull. Bot. 2007, 2, 189–193. (In Chinese) [Google Scholar]
- Luo, L.Q.; Chen, Z.Y.; Gu, J.; Zi, X.N. Effects of ultraviolet-B radiation on plant DNA and protein. J. Ecol. 2006, 5, 572–576. (In Chinese) [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wang, Y.L.; Yue, M. Effects of supplementary radia-tion of UV-B and red light on fruit quality of tomato inwinter plastic greenhouse. Acta Bot. Borealiocci-Dent. Sin. 2000, 20, 590–595. [Google Scholar]
- Zhang, S. Experimental Techniques of Plant Physiology; Science Press: Beijing, China, 2011; pp. 191–194. (In Chinese) [Google Scholar]
- Sullivan, J.H.; Teramura, A.H. The effects of ultraviolet-B radiation on loblolly pine. I. Growth, photosynthesis and pigment production in greenhouse-grown seedlings. Physiol. Plant. 1989, 77, 202–207. [Google Scholar] [CrossRef]
- Nogues, S.; Allen, D.J.; Morison, J.I.; Baker, N.R. Characterization of Stomatal Closure Caused by Ultraviolet-B Radiation. Plant Physiol. 1999, 121, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.X.; Li, Z.; Yang, K.J.; Zhao, C.J.; Yang, R.B.; Yu, G.B.; Huang, S.G.; Xu, J.Y.; He, L.; Zhao, Y.; et al. Effects of enhanced UV-B radiation on photosynthetic and antioxidant systems in sorghum seedlings. Spectrosc. Spectr. Anal. 2016, 36, 1389–1395. (In Chinese) [Google Scholar]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Zhu, Y.A. Research advances in Effect of enhanced UV-B radiation on growth and development of crops. Shaanxi Agric. Sci. 2007, 4, 113–116. (In Chinese) [Google Scholar]
- Mishra, V.; Srivastava, G.; Prasad, S.M.; Abraham, G. Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea (Vigna unguiculata) in response to UV-B and dimethoate. Pestic. Biochem. Physiol. 2008, 92, 30–37. [Google Scholar] [CrossRef]
- Surabhi, G.-K.; Reddy, K.R.; Singh, S.K. Photosynthesis, fluorescence, shoot biomass and seed weight responses of three cowpea (Vigna unguiculata (L.) Walp.) cultivars with contrasting sensitivity to UV-B radiation. Environ. Exp. Bot. 2009, 66, 160–171. [Google Scholar] [CrossRef]
- He, J.M.; She, X.P.; Liu, C.; Zhao, W.M. Stomatal and nonstomatal limitation of photosynthesis of mung b-ean under enhanced UV-B radiation and NaCl stress. J. Plant Physiol. Mol. Biol. 2004, 30, 53–58. (In Chinese) [Google Scholar]
- Gao, L.M.; Li, Y.F.; Han, R. Effects of He-Ne laser on photosynthesis of wheat seedlings expose-d to enhanced UV-B radiation. Guangxi Plant 2011, 31, 117–123. (In Chinese) [Google Scholar]
- Jordan, B.R.; Chow, W.S.; Strid, J.M.; Anderson, J.M. Reduction in cab and psb A RNA transcripts in response to supplementary ultraviolet-B radiation. FEBS Lett. 1991, 284, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Zhou, K.B.; Ding, S.; Cai, H.Z. Study on the damages and the protection response of adult leaves of mango (Mangifera indica L.) under enhanced UV-radiation. J. Mt. Agric. Biol. 2010, 29, 397–402. (In Chinese) [Google Scholar]
- Liu, M.; Cao, B.; Zhou, S.; Liu, Y. Responses of the flavonoid pathway to UV-B radiation stress and the correlation with the lipid antioxidant characteristics in the desert plant Caryopteris mongolica. Mus. Soc. 2012, 32, 150–155. [Google Scholar] [CrossRef]
- Liu, M.; Li, R.G.; Du, G.C. Effects of Enhanced UV-B Radiation on photosynthetic pigments and some enzymes in Tobacco. Northwest Plant J. 2007, 27, 291–296. (In Chinese) [Google Scholar]
- Shi, S.B.; Ben, G.Y.; Zhao, X.Q.; Han, F. Effects of supplementary UV-B radiation on net photosyntheticrate in the Alpine plant Gentiana straminea. J. Plant Ecol. 2001, 5, 520–524. (In Chinese) [Google Scholar]
- Kolb, C.A.; Kaser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfundel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zheng, Y.; Slusser, J.R.; Heisler, G.M.; Grant, R.H.; Xu, J.; He, D. Effects of supplementary ultraviolet-B irradiation on maize yield and qualities: A field experiment. Photochem. Photobiol. 2004, 80, 127–131. [Google Scholar] [CrossRef]
- Kısa, D. Expressions of glutathione-related genes and activities of their corresponding enzymes in leaves of tomato exposed to heavy metal. Russ. J. Plant Physiol. 2017, 64, 876–882. [Google Scholar] [CrossRef]
- Chen, L.T.; Sun, A.Q.; Yang, M.; Chen, L.L.; Ma, X.L.; Li, M.L.; Yin, Y.P. Relation of wheat seed vigor and main related enzyme activities and gene expression under stress conditions. J. Appl. Ecol. 2016, 28, 609–619. (In Chinese) [Google Scholar]
- Santin, M.; Giordani, T.; Cavallini, A.; Bernardi, R.; Castagna, A.; Hauser, M.-T.; Ranieri, A. UV-B exposure reduces the activity of several cell wall-dismantling enzymes and affects the expression of their biosynthetic genes in peach fruit (Prunus persica L., cv. Fairtime, melting phenotype). Photochem. Photobiol. Sci. 2019, 18, 1280–1289. [Google Scholar] [CrossRef]

| Treatment | Yield/Kg | Soluble Sugar/% | Titratable Acid/% | Sugar–Acid Ratio | Vitamin C/(mg/100 g) |
|---|---|---|---|---|---|
| CK | (26.20 ± 1.66) a | (13.85 ± 0.57) a | (0.49 ± 0.03) b | (28.27 ± 0.82) a | (131.7 ± 0.23) b |
| 24 kJ/(m2·d) | (31.42 ± 2.73) a | (14.39 ± 0.56) a | (0.58 ± 0.08) b | (24.81 ± 1.05) a | (182.3 ± 0.60) a |
| 96 kJ/(m2·d) | (17.32 ± 2.02) b | (11.16 ± 0.79) b | (0.83 ± 0.05) a | (13.45 ± 1.22) b | (168.8 ± 1.12) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, Y.; Zhu, J.; Yue, K.; Zhou, K. Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation. Horticulturae 2021, 7, 557. https://doi.org/10.3390/horticulturae7120557
Wang H, Guo Y, Zhu J, Yue K, Zhou K. Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation. Horticulturae. 2021; 7(12):557. https://doi.org/10.3390/horticulturae7120557
Chicago/Turabian StyleWang, Hong, Yujian Guo, Jianjun Zhu, Kun Yue, and Kaibing Zhou. 2021. "Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation" Horticulturae 7, no. 12: 557. https://doi.org/10.3390/horticulturae7120557
APA StyleWang, H., Guo, Y., Zhu, J., Yue, K., & Zhou, K. (2021). Characteristics of Mango Leaf Photosynthetic Inhibition by Enhanced UV-B Radiation. Horticulturae, 7(12), 557. https://doi.org/10.3390/horticulturae7120557







