Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Field Measurements
2.2.1. Weather Data
2.2.2. Leaf Nutrient Composition, Vegetative Growth, Crop Yield, and Must Quality of Grapevines
2.2.3. Development and Reproductive Parameters of Planococcus ficus
2.3. Data Analysis
3. Results
3.1. Weather
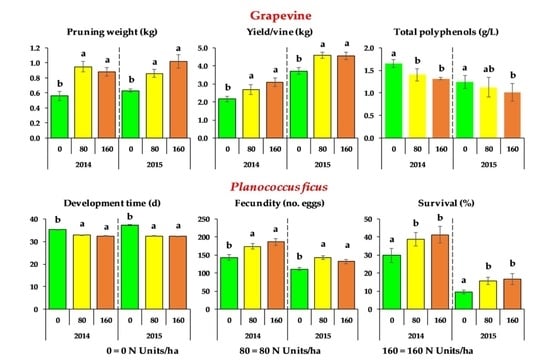
3.2. Plant Nitrogen Status, Vegetative Growth, Crop Yield, and Must Quality of Grapevines
3.3. Development and Reproductive Parameters of Planococcus ficus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, R.; Martin, P.; Del Alamo, M.; Gonzales, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Scagel, C.F. Nutrient uptake and distribution in a mature ‘Pinot Noir’ vineyard. HortScience 2006, 41, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, R.P. Nutrient uptake and distribution in young Pinot noir grapevines over two seasons. Am. J. Enol. Vitic. 2016, 67, 436–448. [Google Scholar] [CrossRef]
- Thiebeau, P.; Herre, C.; Doledec, A.-F.; Perraud, A.; Panigaï, L.; Mary, B.; Nicolardot, B. Incidence du mode de couverture du sol sur la fourniture en azote des sols de vigne en Champagne. J. Intern. Sci. Vigne Vin 2005, 39, 163–177. [Google Scholar]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Lacroux, F.; Trégoat, O.; van Leeuwen, C.; Pons, A.; Tominaga, T.; Lavigne-Cruege, V.; Dubourdieu, D. Effect of foliar nitrogen and sulphur application on aromatic expression of Vitis vinifera L. cv. Sauvignon blanc. Oeno One 2008, 42, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Arnink, K.J.; Hrazdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. I. Effects on grapevine growth, fruit development, and ripening. Am. J. Enol. Vitic. 1998, 49, 333–340. [Google Scholar]
- Spayd, S.; Wample, R.; Evans, R.; Stevens, R.; Seymour, B.; Nagel, C. Nitrogen fertilization of white Riesling grapes in Washington. Must and wine composition. Am. J. Enol. Vitic. 1994, 45, 34–41. [Google Scholar]
- Delas, J.; Molot, C.; Soyer, J.P. Effects of nitrogen fertilization and grafting on the yield and quality of the crop of Vitis vinifera cv. Merlot. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, USA, 18–19 June 1991; Rantz, J.M., Ed.; American Society for Enology and Viticulture: Davis, CA, USA, 1991; pp. 242–248. [Google Scholar]
- Sulas, L.; Mercenaro, L.; Campesi, G.; Nieddu, G. Different cover crops affect nitrogen fluxes in Mediterranean vineyard. Agron. J. 2017, 109, 2579–2585. [Google Scholar] [CrossRef]
- Shaahan, M.M.; El-Sayed, A.A.; Abou El-Nour, E.A.A. Predicting nitrogen, magnesium and iron nutritional status in some perennial crops using a portable chlorophyll meter. Sci. Hortic. 1999, 82, 339–348. [Google Scholar] [CrossRef]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, magnesium, nitrogen and potassium deficiency symptom discrimination by reflectance spectroscopy in grapevine leaves. Sci. Hortic. 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Vrignon-Brenas, S.; Aurélie, M.; Romain, L.; Shiva, G.; Alana, F.; Myriam, D.; Rolland, G.; Pellegrino, A. Gradual responses of grapevine yield components and carbon status to nitrogen supply. OENO One 2019, 53, 289–306. [Google Scholar] [CrossRef]
- Daane, K.M.; Almeida, R.P.P.; Bell, V.A.; Walker, J.T.S.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; et al. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, Germany, 2012; pp. 271–307. [Google Scholar]
- Chiotta, M.L.; Ponsone, M.L.; Torres, A.M.; Combina, M.; Chulze, S.N. Influence of Planococcus ficus on Aspergillus section Nigri and ochratoxin A incidence in vineyards from Argentina. Lett. Appl. Microbiol. 2010, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Bordeu, E.; Troncoso, D.O.; Zaviezo, T. Influence of mealybug (Pseudococcus spp.)–infested bunches on wine quality in Carmenere and Chardonnay grapes. Int. J. Food Sci. Technol. 2012, 47, 232–239. [Google Scholar] [CrossRef]
- Cocco, A.; Lentini, A.; Serra, G. Mating disruption of Planococcus ficus (Hemiptera: Pseudococcidae) in vineyards using reservoir pheromone dispensers. J. Insect Sci. 2014, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.; Muscas, E.; Mura, A.; Iodice, A.; Savino, F.; Lentini, A. Influence of mating disruption on the reproductive biology of the vine mealybug, Planococcus ficus (Hemiptera: Pseudococcidae), under field conditions. Pest Manag. Sci. 2018, 74, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Marras, P.M.; Cocco, A.; Muscas, E.; Lentini, A. Laboratory evaluation of the suitability of vine mealybug, Planococcus ficus, as a host for Leptomastix dactylopii. Biol. Control 2016, 95, 57–65. [Google Scholar] [CrossRef]
- Lucchi, A.; Benelli, G. Towards pesticide-free farming? Sharing needs and knowledge promotes Integrated Pest Management. Environ. Sci. Pollut. Res. 2018, 14, 13439–13445. [Google Scholar] [CrossRef] [Green Version]
- European Union. Commission implementing regulation (EU) 2018/783. Off. J. Eur. Union L 2018, 132, 31–34. [Google Scholar]
- European Union. Commission implementing regulation (EU) 2018/785. Off. J. Eur. Union L 2018, 132, 40–44. [Google Scholar]
- European Union. Commission implementing regulation (EU) 2020/17. Off. J. Eur. Union L 2020, 7, 11–13. [Google Scholar]
- European Union. Commission implementing regulation (EU) 2020/18. Off. J. Eur. Union L 2020, 7, 14–16. [Google Scholar]
- Goodhue, R.; Mace, K.; Rudder, J.; Tolhurst, T.; Tregeagle, D.; Wei, H.; Grafton-Cardwell, B.; Grettenberger, I.; Wilson, H.; Van Steenwyk, R.; et al. Economic and Pest Management Evaluation of the Withdrawal of Chlorpyrifos: Six Major California Commodities. Available online: https://www.cdfa.ca.gov/files/pdf/ChlorpyrifosReport.pdf (accessed on 10 May 2020).
- Fernandes de Oliveira, A.; Serra, S.; Ligios, V.; Satta, D.; Nieddu, G. Assessing the effects of vineyard soil management on downy and powdery mildew development. Horticulturae 2021, 7, 209. [Google Scholar] [CrossRef]
- Cocco, A.; Marras, M.P.; Muscas, E.; Mura, A.; Lentini, A. Variation of life-history parameters of Planococcus ficus (Hemiptera: Pseudococcidae) in response to grapevine nitrogen fertilization. J. Appl. Entomol. 2015, 139, 519–528. [Google Scholar] [CrossRef]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruit quality, and the development of the vine mealybug under a Mediterranean climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Walton, V.M.; Pringle, K.L. Vine mealybug, Planococcus ficus(Sign.) (Hemiptera: Pseudococcidae), a key pest in South Africanvineyards. A review. S. Afr. J. Enol. Vitic. 2004, 25, 54–62. [Google Scholar]
- Pavlović, M.; Fantov, T.; Dević, S.; Marcelić, Š.; Franin, K.; Kos, T. The effectiveness of various measures for suppression of scale insect Planococcus ficus (Signoret) (Hemiptera: Coccoidea) on grapevine (Vitis vinifera L.) variety “Chardonnay” in the locality of Baštica, Zadar’s county hinterland. In Proceedings of the XV International Symposium on Scale Insect Studies, Zagreb, Croatia, 17–20 June 2019; p. 72. [Google Scholar]
- Pavan, F.; Cargnus, E.; Kiaeianmoosavi, S.; Bigot, G.; Tacoli, F.; Zandigiacomo, P. Bunch-zone leaf removal of grapevines to prevent damage by Lobesia botrana and grey mould. Bull. Insectol. 2016, 69, 107–115. [Google Scholar]
- Fornasiero, D.; Duso, C.; Pozzebon, A.; Tomasi, D.; Gaiotti, F.; Pavan, F. Effects of irrigation on the seasonal abundance of Empoasca vitis in North-Italian vineyards. J. Econ. Entomol. 2012, 105, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Zanettin, G.; Bullo, A.; Pozzebon, A.; Burgio, G.; Duso, C. Influence of vineyard inter-row groundcover vegetation management on arthropod assemblages in the vineyards of North-Eastern Italy. Insects 2021, 12, 349. [Google Scholar] [CrossRef]
- Cocco, A.; Pacheco da Silva, V.; Benelli, G.; Botton, M.; Lucchi, A.; Lentini, A. Sustainable management of the vine mealybug in organic vineyards. J. Pest Sci. 2021, 94, 153–185. [Google Scholar] [CrossRef]
- Mercenaro, L.; Nieddu, G.; Pulina, P.; Porqueddu, C. Sustainable management of an intercropped Mediterranean vineyard. Agric. Ecosyst. Environ. 2014, 192, 95–104. [Google Scholar] [CrossRef]
- Williams, D.W.; Andris, H.L.; Beede, R.H.; Luvisi, D.A.; Norton, M.V.K.; Williams, L.E. Validation of a model for the growth and development of the Thompson Seedless grapevine. II. Phenology. Am. J. Enol. Vitic. 1985, 36, 283–289. [Google Scholar]
- Walton, V.M.; Pringle, K.L. Developmental biology of vine mealybug, Planococcus ficus (Signoret) (Homoptera: Pseudococcidae), and its parasitoid Coccidoxenoides perminutus (Timberlake) (Hymenoptera: Encyrtidae). Afr. Entomol. 2005, 13, 143–147. [Google Scholar]
- Porro, D.; Dorigatti, C.; Stefanini, M.; Ceschini, A. Use of SPAD meter in diagnosis of nutritional status in apple and grapevine. Acta Hort. 2001, 564, 243–252. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C. The grape phenolic determination. Riv. Vitic. Enol. 1991, 49, 37–45. [Google Scholar]
- Cocco, A.; Mura, A.; Muscas, E.; Lentini, A. Comparative development and reproduction of Planococcus ficus and Planococcus citri (Hemiptera: Pseudococcidae) on grapevine under field conditions. Agric. For. Entomol. 2018, 20, 104–112. [Google Scholar] [CrossRef]
- Brunetto, G.; Trentin, G.; Ceretta, C.A.; Girotto, E.; Lorensini, F.; Miotto, A.; Moser, G.R.Z.; de Melo, G.W. Use of the SPAD-502 in estimating nitrogen content in leaves and grape yield in grapevines in soils with different texture. Am. J. Plant Sci. 2012, 3, 1546–1561. [Google Scholar] [CrossRef] [Green Version]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M.L. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Petretto, G.L.; Mercenaro, L.; Urgeghe, P.P.; Fadda, C.; Valentoni, A.; Del Caro, A. Grape and wine composition in Vitis vinifera L. cv. Cannonau explored by GC-MS and sensory analysis. Foods 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaria, P.; Lopez, R.; Garde-Cerdan, T. Phenolic composition of Tempranillo grapes following foliar application of phenylalanine andurea: A two year study. Sci Hortic. 2017, 219, 191–199. [Google Scholar] [CrossRef]
- Hilbert, G.; Soyer, J.P.; Molot, C.; Giraudon, J.; Milin, S.; Gaudillere, J.P. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. Vitis 2003, 42, 69–76. [Google Scholar]
- Portu, J.; Lopez-Alfaro, I.; Gomez-Alonso, S.; Lopez, R.; Garde-Cerdan, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hogendorp, B.K.; Cloyd, R.A.; Swiader, J.M. Effect of nitrogen fertility on reproduction and development of citrus mealybug, Planococcus citri Risso (Homoptera: Pseudococcidae), feeding on two colors of coleus, Solenostemon scutellarioides L. Codd. Environ. Entomol. 2006, 35, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, S.A.; Turnbull, M.W.; Chong, J.H. Nitrogen fertilization of host plant influenced the nutritional status and life history of the Madeira mealybug (Hemiptera: Pseudococcidae). Environ. Entomol. 2019, 48, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Rae, D.J.; Jones, R.E. Influence of host nitrogen levels on development, survival, size and population dynamics of sugarcane mealybug, Saccharicoccus sacchari (Cockerell) (Hemiptera, Pseudococcidae). Aust. J. Zool. 1992, 40, 327–342. [Google Scholar] [CrossRef]
- Moon, C.E.; Lewis, B.E.; Murray, L.; Sanderson, S.M. Russian wheat aphid (Homoptera: Aphididae) development, reproduction, and longevity on hydroponically grown wheat with varying nitrogen levels. Environ. Entomol. 1995, 24, 367–371. [Google Scholar] [CrossRef]
- Bethke, J.A.; Redak, R.A.; Schuch, U.K. Melon aphid performance on chrysanthemum as mediated by cultivars, and different levels of fertilization and irrigation. Entomol. Exp. Appl. 1998, 88, 41–47. [Google Scholar] [CrossRef]
- Walton, V.M.; Daane, K.M.; Bentley, W.J.; Millar, J.G.; Larsen, T.E.; Malakar-Kuenen, R. Pheromone–based mating disruption of Planococcus ficus (Hemiptera: Pseudococcidae) in California vineyards. J. Econ. Entomol. 2006, 99, 1280–1290. [Google Scholar] [CrossRef]
- Waterworth, R.A.; Wright, I.M.; Millar, J.G. Reproductive biology of three cosmopolitan mealybug (Hemiptera: Pseudococcidae) species, Pseudococcus longispinus, Pseudococcus viburni, and Planococcus ficus. Ann. Entomol. Soc. Am. 2011, 104, 249–260. [Google Scholar] [CrossRef]
- Gonzalez Luna, M.F.; La Rossa, F.R. Parámetros biológicos y poblacionales de Planococcus ficus (Hemiptera: Pseudococcidae) sobre dos cultivares de Vitis vinifera. Rev. Soc. Entomol. Argent. 2016, 75, 45–54. [Google Scholar]
- Lentini, A.; Mura, A.; Muscas, E.; Nuvoli, M.T.; Cocco, A. Effects of delayed mating on the reproductive biology of the vine mealybug, Planococcus ficus (Hemiptera: Pseudococcidae). Bull. Entomol. Res. 2018, 108, 263–270. [Google Scholar] [CrossRef]
- White, T.C.R. The Inadequate Environment—Nitrogen and the Abundance of Animals; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–425. [Google Scholar]
- Bentz, J.A.; Reeves, J., III; Barbosa, P.; Francis, B. Nitrogen fertilizer effect on selection, acceptance, and suitability of Euphorbia pulcherrima (Euphorbiaceae) as a host plant to Bemisia tabaci (Homoptera: Aleyrodidae). Environ. Entomol. 1995, 24, 40–45. [Google Scholar] [CrossRef]
- Mgocheki, N.; Addison, P. Spatial distribution of ants (Hymenoptera: Formicidae), vine mealybugs and mealybug parasitoids in vineyards. J. Appl. Entomol. 2010, 134, 285–295. [Google Scholar] [CrossRef]
- Dalla Montà, L.; Duso, C.; Malagnini, V. Current status of scale insects (Hemiptera: Coccoidea) in the Italian vineyards. Boll. Zool. Agr. Bachic. 2001, 33, 343–350. [Google Scholar]
- Oliveira, M.D.; Barbosa, P.R.; Silva-Torres, C.S.; Silva, R.R.; Barros, E.M.; Torres, J.B. Reproductive performance of striped mealybug Ferrisia virgata Cockerell (Hemiptera: Pseudococcidae) on water-stressed cotton plants subjected to nitrogen fertilization. Arthropod-Plant Interact. 2014, 8, 461–468. [Google Scholar] [CrossRef]

| N Dose (Units) (kg ha−1) | SPAD Value (± SE) {9} | ||||||
|---|---|---|---|---|---|---|---|
| 2014 | 8 May (−17) | 20 May (−5) | 3 June (9) | 24 June (30) | 16 July (52) | 1 August (68) | |
| 0 | 34.49 ± 0.53 b | 36.49 ± 0.74 c | 37.58 ± 0.43 b | 38.49 ± 0.69 c | 32.79 ± 0.68 c | 33.79 ± 0.97 b | |
| 80 | 36.69 ± 0.66 a | 39.99 ± 0.54 b | 42.90 ± 0.94 a | 44.50 ± 0.87 b | 42.18 ± 0.95 b | 42.96 ± 0.67 a | |
| 160 | 38.17 ± 0.51 a | 41.61 ± 0.54 a | 44.47 ± 0.63 a | 46.60 ± 0.88 a | 45.20 ± 0.76 a | 44.73 ± 0.75 a | |
| 2015 | 20 May (−2) | 5 June (14) | 19 June (28) | 24 July (63) | 7 August (77) | 2 September (103) | |
| 0 | 39.44 ± 0.77 c | 44.97 ± 0.65 b | 46.24 ± 0.54 c | 44.07 ± 0.93 b | 44.70 ± 1.06 b | 43.80 ± 0.92 b | |
| 80 | 43.53 ± 0.37 b | 49.06 ± 0.39 a | 50.31 ± 0.43 b | 49.71 ± 0.59 a | 49.72 ± 0.74 a | 49.14 ± 0.71 a | |
| 160 | 46.23 ± 0.44 a | 50.71 ± 0.88 a | 51.51 ± 0.37 a | 51.59 ± 0.38 a | 51.19 ± 0.64 a | 49.10 ± 0.69 a | |
| Statistics 1 | |||||||
| N dose | ** | ** | ** | ** | ** | ** | ** |
| Year | ** | ** | ** | ** | ** | ||
| N dose × year | n.s. | n.s. | n.s. | ** | * | ||
| N Dose (Units) (kg ha−1) | Primary Leaf Area (PLA) (m2) {12} | Lateral Leaf Area (LLA) (m2) {12} | Total Leaf Area (TLA) (m2) {12} | Pruning Weight (kg) {12} | Berry Weight (g) {18} | Cluster Weight (g) {18} | Cluster Vine−1 (no.) {18} | Yield Vine−1 (kg) {18} |
|---|---|---|---|---|---|---|---|---|
| 2014 | ||||||||
| 0 | 2.06 ± 0.12 b | 1.50 ± 0.09 b | 3.55 ± 0.17 b | 0.56 ± 0.06 b | 2.63 ± 0.11 a | 245.15 ± 14.37 b | 8.44 ± 0.65 a | 2.16 ± 0.15 b |
| 80 | 2.71 ± 0.16 a | 2.57 ± 0.13 a | 5.28 ± 0.16 a | 0.95 ± 0.07 a | 2.59 ± 0.10 a | 280.91 ± 18.07 b | 9.33 ± 0.78 a | 2.68 ± 0.26 a |
| 160 | 2.71 ± 0.12 a | 2.49 ± 0.15 a | 5.20 ± 0.19 a | 0.88 ± 0.06 a | 2.64 ± 0.13 a | 389.19 ± 24.14 a | 7.78 ± 0.36 a | 3.09 ± 0.24 a |
| 2015 | ||||||||
| 0 | 3.10 ± 0.07 b | 1.27 ± 0.05 b | 4.36 ± 0.09 b | 0.63 ± 0.02 b | 2.75 ± 0.10 a | 269.11 ± 17.55 b | 12.78 ± 0.89 a | 3.72 ± 0.18 b |
| 80 | 3.65 ± 0.18 a | 3.00 ± 0.13 a | 6.65 ± 0.28 a | 0.86 ± 0.05 a | 2.93 ± 0.09 a | 366.03 ± 13.82 a | 11.56 ± 0.78 a | 4.59 ± 0.17 a |
| 160 | 3.34 ± 0.25 a | 2.86 ± 0.23 a | 6.21 ± 0.44 a | 1.02 ± 0.09 a | 3.04 ± 0.10 a | 374.83 ± 14.57 a | 11.11 ± 0.61 a | 4.55 ± 0.21 a |
| Statistics 1 | ||||||||
| N dose | ** | ** | ** | ** | n.s. | ** | n.s. | ** |
| Year | ** | n.s. | ** | n.s. | ** | * | ** | ** |
| N dose × year | n.s. | * | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| N Dose (Units) (kg ha−1) | Primary Shoot {9} | Lateral Shoot {9} | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaves (no.) | Length N1 (cm) | Length N2 (cm) | Length N3 (cm) | Leaves (no.) | Length N1 (cm) | Length N2 (cm) | Length N3 (cm) | |
| 2014 | ||||||||
| 0 | 10.9 ± 0.5 a | 15.5 ± 0.9 b | 12.3 ± 0.7 a | 12.9 ± 0.7 a | 21.6 ± 3.1 b | 7.0 ± 0.6 b | 6.1 ± 0.4 a | 6.6 ± 0.6 b |
| 80 | 11.4 ± 0.4 a | 15.5 ± 0.3 ab | 12.8 ± 0.4 a | 12.8 ± 0.3 a | 29.6 ± 2.0 a | 9.9 ± 0.5 a | 8.5 ± 0.2 b | 8.4 ± 0.3 a |
| 160 | 13.2 ± 0.7 a | 16.3 ± 0.2 a | 12.8 ± 0.2 a | 13.1 ± 0.4 a | 33.2 ± 1.7 a | 8.2 ± 0.2 a | 6.7 ± 0.4 a | 7.1 ± 0.2 a |
| 2015 | ||||||||
| 0 | 17.3 ± 1.0 a | 14.0 ± 0.4 b | 12.2 ± 0.7 a | 13.3 ± 0.4 a | 19.3 ± 2.3 b | 7.2 ± 0.7 b | 6.1 ± 0.4 c | 6.2 ± 0.4 b |
| 80 | 16.3 ± 1.4 a | 15.1 ± 0.8 ab | 13.7 ± 1.1 a | 12.4 ± 1.2 a | 35.7 ± 2.2 a | 7.7 ± 0.2 a | 7.3 ± 0.3 b | 7.0 ± 0.2 a |
| 160 | 14.0 ± 1.3 a | 16.0 ± 0.2 a | 14.1 ± 0.4 a | 12.8 ± 0.6 a | 32.7 ± 4.0 a | 8.6 ± 0.6 a | 8.3 ± 0.4 a | 7.6 ± 0.5 a |
| Statistics 1 | ||||||||
| N dose | n.s. | * | n.s. | n.s. | ** | ** | ** | * |
| Year | ** | n.s. | n.s. | n.s. | n.s. | * | n.s. | * |
| N dose × year | ** | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. |
| N Dose (Units) (kg ha−1) | Total Soluble Solids (°Brix) {9} | pH {9} | Tritatable Acidity (g L−1) {9} | Total Anthocyanins (mg L−1) {9} | Total Polyphenols (mg L−1) {9} |
|---|---|---|---|---|---|
| 2014 | |||||
| 0 | 22.1 ± 1.2 a | 3.63 ± 0.03 a | 5.28 ± 0.20 a | 436.4 ± 54.1 a | 1651.5 ± 86.1 a |
| 80 | 20.8 ± 0.8 a | 3.53 ± 0.01 a | 5.60 ± 0.26 a | 406.0 ± 63.2 a | 1400.6 ± 136.2 b |
| 160 | 20.9 ± 0.9 a | 3.47 ± 0.10 a | 5.40 ± 0.17 a | 470.9 ± 37.5 a | 1317.3 ± 25.3 b |
| 2015 | |||||
| 0 | 20.1 ± 0.2 a | 3.68 ± 0.06 a | 4.80 ± 0.09 a | 596.6 ± 28.7 a | 1248.9 ± 144.2 a |
| 80 | 19.4 ± 0.4 a | 3.70 ± 0.05 a | 5.00 ± 0.26 a | 522.3 ± 34.8 a | 1127.7 ± 213.0 ab |
| 160 | 19.0 ± 0.2 a | 3.68 ± 0.02 a | 5.10 ± 0.09 a | 618.3 ± 80.2 a | 1016.4 ± 197.6 b |
| Statistics 1 | |||||
| N dose | n.s. | n.s. | n.s. | n.s. | * |
| Year | * | * | ** | * | ** |
| N dose × year | n.s. | n.s. | n.s. | n.s. | * |
| N Dose (Units) (kg ha−1) | Development Time (Days) {90} 1 2 | Fecundity (no. eggs) {90} 1 | Fertility (%) {90} 2 | Survival (%) {9} 3 |
|---|---|---|---|---|
| 2014 | ||||
| 0 | 35.31 ± 0.20 b | 143.8 ± 7.0 b | 95.67 ± 0.62 a | 29.8 ± 3.9 b |
| 80 | 32.82 ± 0.11 a | 174.4 ± 7.5 a | 95.59 ± 0.91 a | 38.8 ± 3.6 a |
| 160 | 32.44 ± 0.12 a | 186.8 ± 9.4 a | 95.96 ± 0.60 a | 41.3 ± 4.5 a |
| 2015 | ||||
| 0 | 37.27 ± 0.32 b | 110.9 ± 4.4 b | 95.05 ± 1.04 a | 9.5 ± 1.1 b |
| 80 | 32.43 ± 0.15 a | 143.0 ± 5.6 a | 95.37 ± 0.66 a | 15.8 ± 2.0 a |
| 160 | 32.28 ± 0.18 a | 132.1 ± 6.3 a | 95.16 ± 0.68 a | 16.7 ± 3.2 a |
| Statistics 4 | ||||
| N dose | ** | ** | n.s | ** |
| Year | ** | ** | * | ** |
| N dose × year | ** | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, A.; Mercenaro, L.; Muscas, E.; Mura, A.; Nieddu, G.; Lentini, A. Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards. Horticulturae 2021, 7, 530. https://doi.org/10.3390/horticulturae7120530
Cocco A, Mercenaro L, Muscas E, Mura A, Nieddu G, Lentini A. Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards. Horticulturae. 2021; 7(12):530. https://doi.org/10.3390/horticulturae7120530
Chicago/Turabian StyleCocco, Arturo, Luca Mercenaro, Enrico Muscas, Alessandra Mura, Giovanni Nieddu, and Andrea Lentini. 2021. "Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards" Horticulturae 7, no. 12: 530. https://doi.org/10.3390/horticulturae7120530
APA StyleCocco, A., Mercenaro, L., Muscas, E., Mura, A., Nieddu, G., & Lentini, A. (2021). Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards. Horticulturae, 7(12), 530. https://doi.org/10.3390/horticulturae7120530