Decoupling of P from C, N, and K Elements in Cucumber Leaves Caused by Nutrient Imbalance under a Greenhouse Continuous Cropping System
Abstract
:1. Introduction
2. Materials and Methods
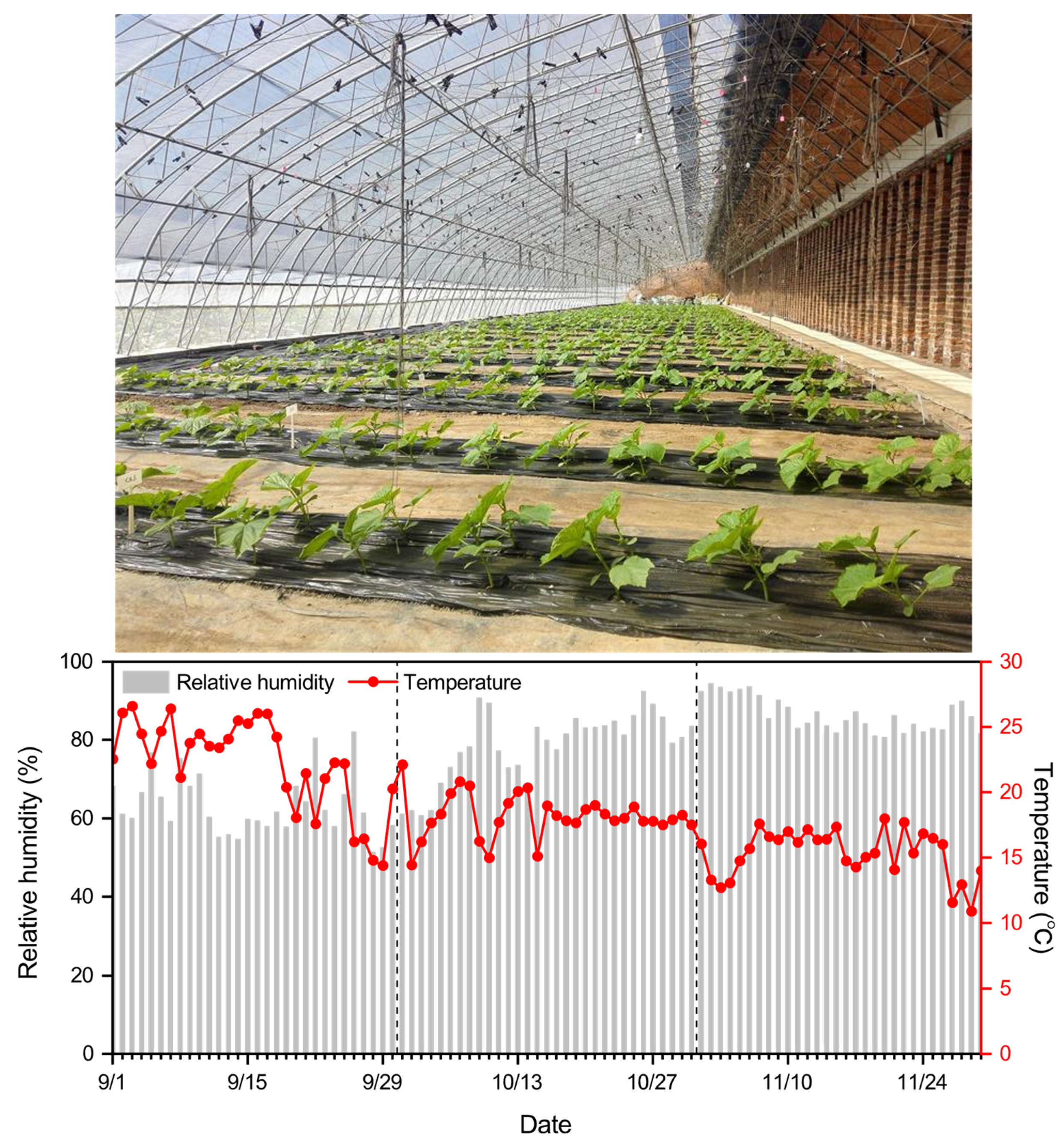
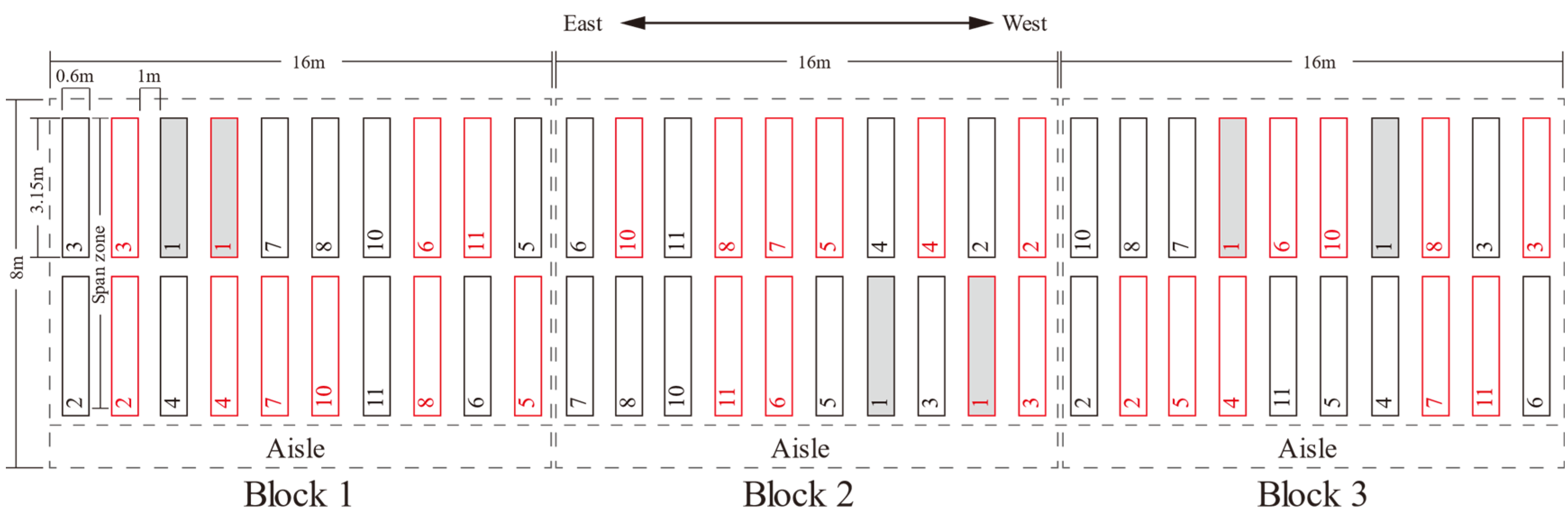
2.1. Study Site and Experimental Design
2.2. Sampling
2.3. Chemical Parameters Analysis in Soils and Leaves
2.4. Statistical Analysis
3. Results
3.1. Response of Elemental Stoichiometry to Planting Time
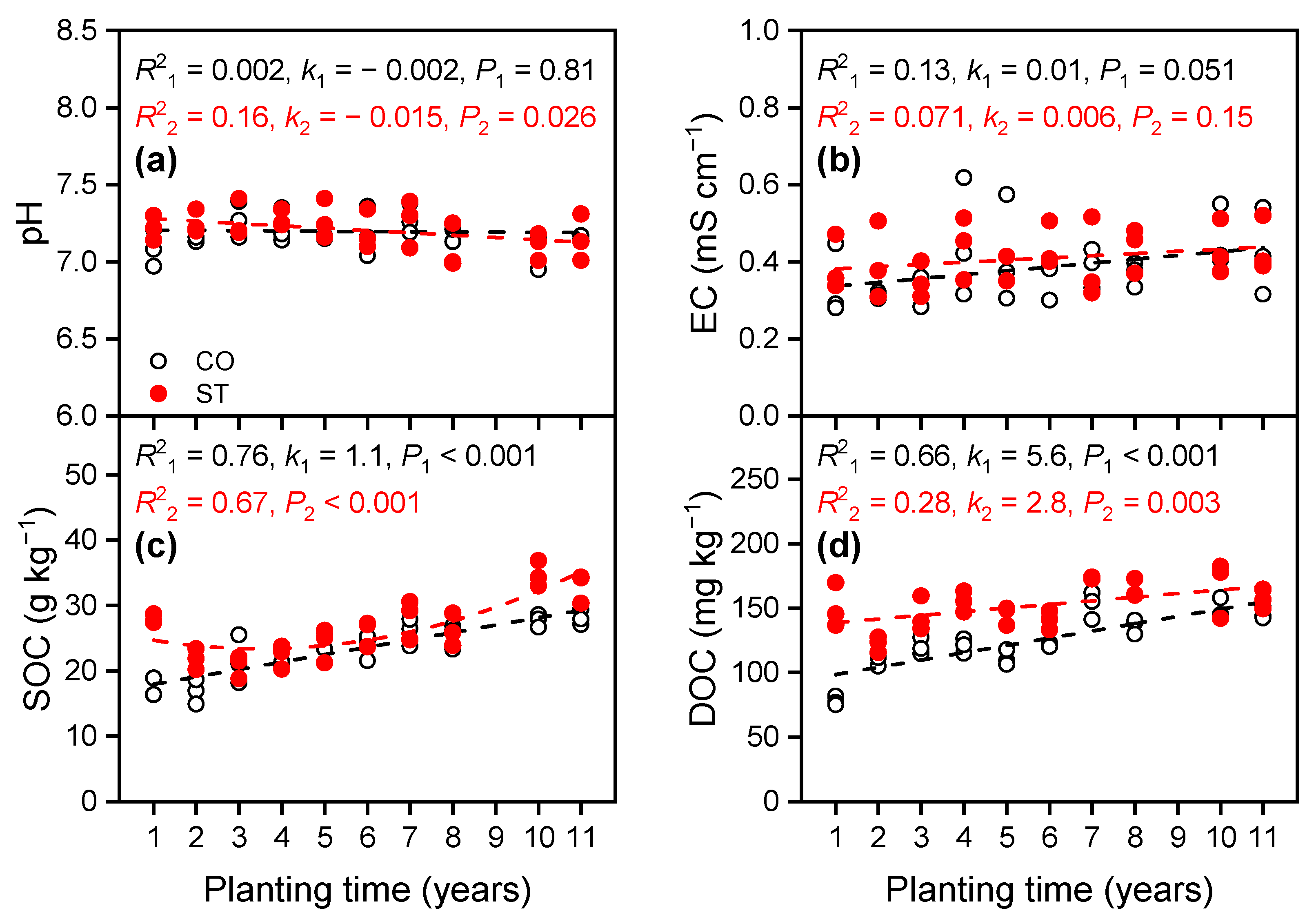
3.1.1. Variations in Chemical Properties in the Soil
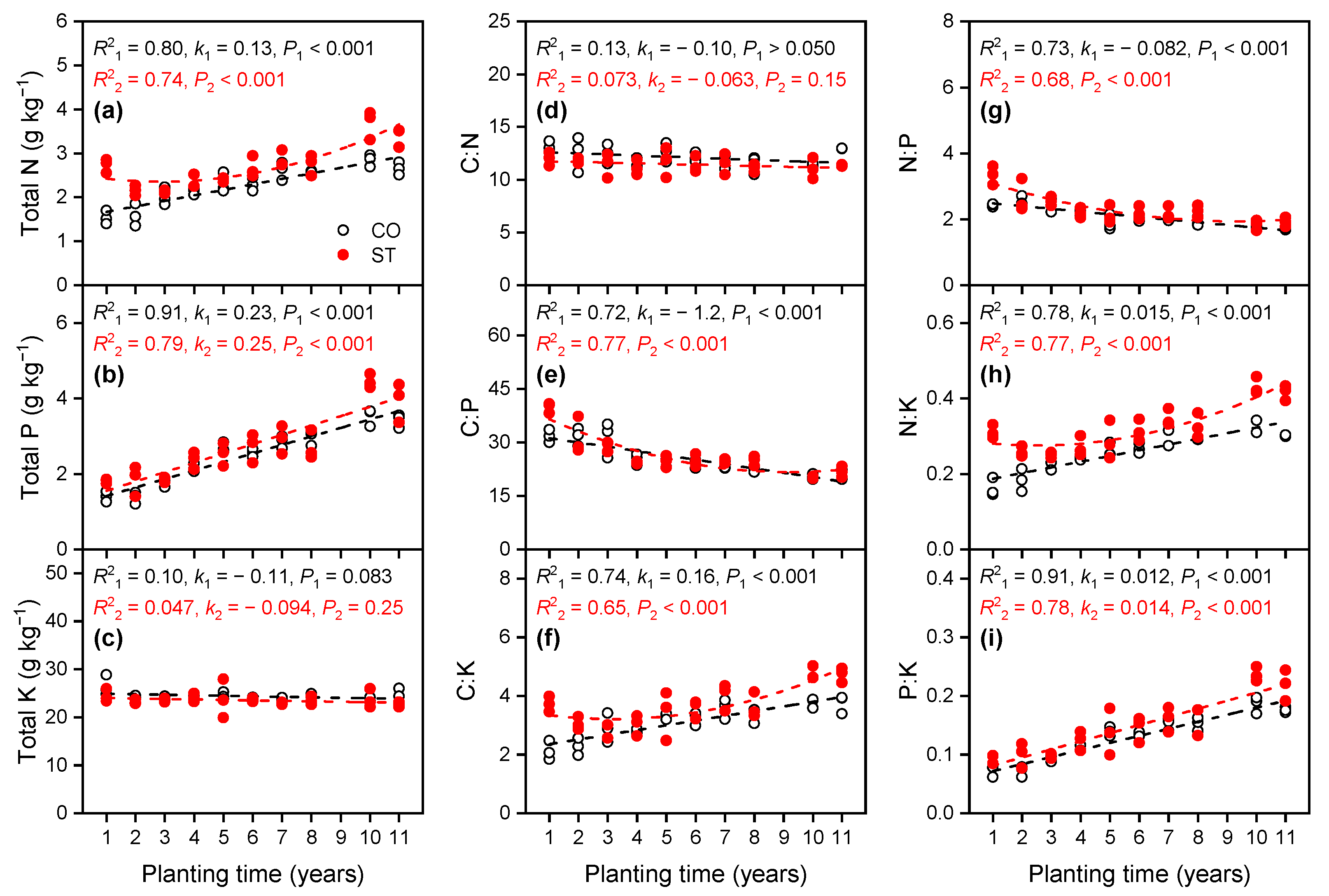
3.1.2. Variations in Total Nutrient Concentrations in the Soil
3.1.3. Variations in Available Nutrient Concentrations in the Soil
3.1.4. Variations in Nutrient Concentrations in Leaf
3.2. Response of Elemental Absorption in Leaf to Planting Time
3.3. The Relationships between Elements in the Leaf, Leaf Growth, and Soil Parameters
4. Discussion
4.1. Variation in the Stoichiometric Ratios of C, N, P, and K in the Soil and Cucumber Leaf with Increasing Planting Time
4.2. Decoupling of Element Utilization in Leaf with Increasing Planting Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements From Molecules to The Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning drives C:N:P stoichiometry and nutrient resorption in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Olde Venterink, H. Does phosphorus limitation promote species-rich plant communities? Plant Soil 2011, 345, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhan, S.; Wang, Y.; Zhu, Z.; Li, W.; Bai, Y. Nitrogen enrichment alters plant N:P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ. Exp. Bot. 2017, 134, 21–32. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Pan, Y.; Li, X.; Cong, R.; Lu, J. Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci. Rep. 2016, 6, 21725. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Dwivedi, B.S.; Yadvinder, S.; Singh, S.K.; Mishra, R.P.; Shukla, A.K.; Rathore, S.S.; Shekhawat, K.; Majumdar, K.; Jat, M.L. Effect of tillage and crop establishment, residue management and K fertilization on yield, K use efficiency and apparent K balance under rice-maize system in north-western India. Field Crops Res. 2018, 224, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Tillage Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Ma, Z.-W.; Li, L.-H. Soil nutrient contents and stoichiometry as affected by land-use in an agro-pastoral region of northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Wu, X.; Han, F.; Zhang, T. Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Tillage Res. 2010, 106, 268–274. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Malhi, S.S.; Nyborg, M.; Goddard, T.; Puurveen, D. Long-term tillage, straw management and N fertilization effects on quantity and quality of organic C and N in a Black Chernozem soil. Nutr. Cycl. Agroecosyst. 2011, 90, 227–241. [Google Scholar] [CrossRef]
- Zafar, M.; Abbasi, M.K.; Khaliq, A. Effect of different phosphorus sources on the growth, yield, energy content and phosphorus utilization efficiency in maize at Rawalakot Azad Jammu and Kashmir, Pakistan. J. Plant Nutr. 2013, 36, 1915–1934. [Google Scholar] [CrossRef]
- Das, D.; Dwivedi, B.S.; Datta, S.P.; Datta, S.C.; Meena, M.C.; Agarwal, B.K.; Shahi, D.K.; Singh, M.; Chakraborty, D.; Jaggi, S. Potassium supplying capacity of a red soil from eastern India after forty-two years of continuous cropping and fertilization. Geoderma 2019, 341, 76–92. [Google Scholar] [CrossRef]
- Galloway, J.N.; Leach, A.M.; Bleeker, A.; Erisman, J.W. A chronology of human understanding of the nitrogen cycle. Philos. Trans. R. Soc. B 2013, 368, 20130120. [Google Scholar] [CrossRef] [Green Version]
- Shenker, M.; Bloom, P.R. Comments on “Amounts, Forms, and Solubility of Phosphorus in Soils Receiving Manure”. Soil Sci. Soc. Am. J. 2005, 69, 1353–1354. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, F.; Zhou, G.; Fang, K.; Zhang, D.; Li, C.; Yang, G.; Wang, G.; Wang, J.; Yang, Y. Linkages of plant stoichiometry to ecosystem production and carbon fluxes with increasing nitrogen inputs in an alpine steppe. Glob. Change Biol. 2017, 23, 5249–5259. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.-z.; Liu, Q.-z.; Zhang, Y.-t.; Li, X.-y.; Li, H.-q.; Li, W.-h. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Huang, S.W.; Tang, J.W.; Li, C.H.; Zhang, H.Z.; Yuan, S. Reducing potential of chemical fertilizers and scientific fertilization countermeasure in vegetable production in China. J. Plant Nutr. Fertil. 2017, 23, 1480–1493. (In Chinese) [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; FAO: Rome, Italy, 2006. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Al-Qurashi, A.D.; Awad, M.A.; Ismail, S.M. Growth, yield, fruit quality and nutrient uptake of tissue culture-regenerated ‘Barhee’ date palms grown in a newly established orchard as affected by NPK fertigation. Sci. Hortic. 2015, 184, 114–122. [Google Scholar] [CrossRef]
- Xue, X.; Lu, J.; Ren, T.; Li, L.; Yousaf, M.; Cong, R.; Li, X. Positional difference in potassium concentration as diagnostic index relating to plant K status and yield level in rice (Oryza sativa L.). Soil Sci. Plant Nutr. 2016, 62, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Lanzén, A.; Mijangos, I.; Garbisu, C. The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl. Soil Ecol. 2019, 135, 73–84. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Zheng, S.; Xia, Y.; Hu, Y.; Chen, X.; Rui, Y.; Gunina, A.; He, X.; Ge, T.; Wu, J.; Su, Y.; et al. Stoichiometry of carbon, nitrogen, and phosphorus in soil: Effects of agricultural land use and climate at a continental scale. Soil Tillage Res. 2021, 209, 104903. [Google Scholar] [CrossRef]
- Guo, W.L.; Dang, J.X.; Lv, J.L.; Guo, J.W.; Quan, D.G.; Liu, S.C.; Ma, Q.A. Soil properties and fertilization in vegetable greenhouse at different ages. Agric. Res. Arid. Areas 2005, 23, 85–89. (In Chinese) [Google Scholar]
- Ning, D.F.; Kong, L.Q.; Tang, N.; Zhou, P. Variations of Soil Nutrients in Vegetable Garden with Different Cultivation Years. J. Sichuan Agric. Univ. 2016, 34, 67–72. (In Chinese) [Google Scholar]
- Zhang, W.; Gao, D.; Chen, Z.; Li, H.; Deng, J.; Qiao, W.; Han, X.; Yang, G.; Feng, Y.; Huang, J. Substrate quality and soil environmental conditions predict litter decomposition and drive soil nutrient dynamics following afforestation on the Loess Plateau of China. Geoderma 2018, 325, 152–161. [Google Scholar] [CrossRef]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs—Theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Change Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Change 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Tian, D.; Reich, P.B.; Chen, H.Y.H.; Xiang, Y.; Luo, Y.; Shen, Y.; Meng, C.; Han, W.; Niu, S. Global changes alter plant multi-element stoichiometric coupling. New Phytol. 2019, 221, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, R.; Yu, Q.; Cao, Y.; Zhang, Y.; Yang, L.; Dijkstra, F.A.; Jiang, Y. Decoupling of plant and soil metal nutrients as affected by nitrogen addition in a meadow steppe. Plant Soil 2019, 443, 337–351. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Parzych, A.; Jonczak, J.; Sobisz, Z. Bioaccumulation of Macronutrients in the Herbaceous Plants of Mid-forest Spring Niches. Balt. For. 2017, 23, 384–393. [Google Scholar]
- Xing, W.; Wu, H.; Shi, Q.; Hao, B.; Liu, H.; Wang, Z.; Liu, G. Multielement stoichiometry of submerged macrophytes across Yunnan plateau lakes (China). Sci. Rep. 2015, 5, 10186. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Materials | pH | EC | Total C | Total N | Total P | Total K | Total C:Total N:Total P:Total K |
|---|---|---|---|---|---|---|---|
| mS cm−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | |||
| Control soil | 7.5 | 0.2 | 12 | 1.3 | 0.9 | 10.6 | 3.7:0.3:0.1:1 |
| Straw mixture soil | 6.9 | 0.9 | 22 | 2.8 | 1.7 | 11.6 | 6.1:0.7:0.2:1 |
| Straw | − | − | 413 | 11.0 | 0.5 | 17.4 | 77.0:1.8:0.04:1 |
| Chicken manure | − | − | 295 | 51.4 | 21.6 | 35.4 | 27.0:4.0:0.8:1 |
| C in Leaf | N in Leaf | P in Leaf | K in Leaf | ANL | APL | AKL | ||
|---|---|---|---|---|---|---|---|---|
| CO | pH | −0.258 | −0.109 | −0.101 | −0.208 | −0.021 | −0.003 | −0.087 |
| EC | 0.124 | 0.067 | 0.259 | 0.039 | 0.116 | 0.174 | 0.002 | |
| SOC | 0.160 | −0.212 | 0.411 * | −0.056 | 0.143 | 0.368 * | 0.078 | |
| Total N | 0.196 | −0.185 | 0.500 ** | −0.047 | 0.232 | 0.460 * | 0.149 | |
| Total P | 0.097 | −0.253 | 0.382 * | −0.087 | 0.151 | 0.372 * | 0.099 | |
| Total K | 0.111 | 0.310 | −0.259 | −0.029 | 0.101 | −0.143 | −0.045 | |
| NO3−−N | 0.041 | −0.145 | 0.205 | 0.064 | 0.053 | 0.246 | 0.138 | |
| NH4+−N | 0.099 | −0.079 | 0.134 | 0.028 | 0.042 | 0.139 | 0.008 | |
| DOC | −0.003 | −0.048 | 0.554 ** | 0.071 | 0.203 | 0.413 * | 0.159 | |
| Inorganic N | 0.049 | −0.154 | 0.197 | 0.042 | 0.050 | 0.245 | 0.124 | |
| Available P | 0.083 | −0.339 | 0.200 | −0.112 | 0.039 | 0.244 | 0.018 | |
| Available K | −0.063 | −0.099 | 0.261 | −0.260 | 0.152 | 0.295 | −0.046 | |
| ST | pH | 0.017 | −0.272 | −0.607 ** | −0.198 | −0.126 | −0.340 | −0.201 |
| EC | 0.047 | 0.123 | 0.231 | −0.039 | 0.171 | 0.196 | 0.071 | |
| SOC | 0.027 | 0.080 | 0.487 ** | 0.068 | 0.296 | 0.498 ** | 0.390 * | |
| Total N | 0.032 | 0.062 | 0.379 * | −0.039 | 0.348 | 0.469 ** | 0.359 | |
| Total P | 0.015 | −0.236 | 0.348 | −0.165 | 0.385 * | 0.562 ** | 0.375 * | |
| Total K | 0.207 | 0.289 | −0.048 | 0.172 | 0.039 | −0.034 | −0.038 | |
| NO3−−N | 0.128 | −0.035 | 0.176 | 0.035 | 0.113 | 0.108 | 0.141 | |
| NH4+−N | 0.112 | 0.206 | 0.363 * | 0.378 * | −0.360 | −0.137 | −0.161 | |
| DOC | −0.075 | −0.185 | 0.430 * | −0.123 | 0.091 | 0.322 | 0.173 | |
| Inorganic N | 0.112 | −0.043 | 0.192 | 0.036 | 0.094 | 0.100 | 0.123 | |
| Available P | 0.127 | −0.091 | −0.046 | 0.036 | 0.347 | 0.215 | 0.264 | |
| Available K | −0.049 | −0.016 | 0.195 | −0.141 | 0.204 | 0.297 | 0.137 |
| CO | ST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LB | PLB | TPB | TY | HI | LB | PLB | TPB | TY | HI | |
| pH | 0.029 | −0.323 | 0.283 | 0.359 | 0.308 | −0.196 | −0.304 | 0.248 | 0.372 * | 0.249 |
| EC | 0.226 | 0.453 * | −0.148 | −0.424 * | −0.504 ** | 0.152 | 0.151 | −0.050 | −0.163 | −0.180 |
| Total N | 0.450 * | 0.457 * | 0.090 | −0.269 | −0.470 ** | 0.446 * | 0.363 * | 0.082 | −0.160 | −0.364 * |
| Total P | 0.451 * | 0.341 | 0.176 | −0.174 | −0.383 * | 0.565 ** | 0.553 ** | 0.011 | 259 | −0.574 ** |
| Total K | −0.080 | 0.160 | −0.182 | −0.221 | −0.211 | −0.143 | −0.220 | 0.118 | 0.121 | 0.248 |
| C:N | −0.360 | −0.521 ** | 0.101 | 0.345 | 0.527 ** | −0.075 | 0.193 | −0.253 | −0.236 | −0.139 |
| C:P | −0.458 * | −0.347 | −0.156 | 0.170 | 0.390 * | −0.425 * | −0.485 ** | 0.076 | 0.296 | 0.543 ** |
| N:P | −0.380 * | −0.193 | −0.223 | 0.055 | 0.249 | −0.495 ** | −0.597 ** | 0.071 | 0.309 | 0.609 ** |
| C:K | 0.391 * | 0.210 | 0.238 | −0.035 | −0.215 | 0.402 * | 0.469 ** | −0.070 | −0.279 | −0.444 * |
| N:K | 0.446 * | 0.370 * | 0.137 | −0.181 | −0.374 * | 0.369 * | 0.409 * | −0.059 | −0.257 | −0.402 * |
| P:K | 0.453 * | 0.336 | 0.178 | −0.161 | −0.374 * | 0.484 ** | 0.517 ** | −0.051 | −0.275 | −0.538 ** |
| NO3−−N | 0.407 * | 0.356 | 0.158 | −0.151 | −0.380 * | 0.191 | 0.148 | −0.098 | −0.255 | −0.277 |
| NH4+−N | 0.197 | 0.364 * | −0.141 | −0.350 | −0.363 * | −0.320 | −0.088 | −0.362 * | −0.207 | 0.175 |
| Inorganic N | 0.405 * | 0.354 | 0.159 | −0.148 | −0.375 * | 0.173 | 0.133 | −0.111 | −0.257 | −0.257 |
| Available P | 0.328 | 0.230 | 0.051 | −0.159 | −0.222 | 0.385 * | 0.152 | 0.211 | 0.004 | −0.281 |
| Available K | 0.324 | 0.352 | 0.032 | −0.224 | −0.425 * | 0.257 | 0.143 | 0.059 | −0.055 | −0.139 |
| Available C:N | −0.170 | −0.046 | −0.168 | −0.055 | 0.057 | −0.008 | 0.051 | 0.055 | 0.138 | 0.094 |
| Available C:P | 0.029 | 0.161 | 0.034 | −0.018 | −0.176 | −0.190 | 0.031 | −0.266 | −0.162 | 0.082 |
| Available N:P | 0.150 | 0.086 | 0.203 | 0.074 | −0.119 | −0.143 | −0.028 | −0.264 | −0.251 | −0.007 |
| Available C:K | 0.114 | 0.154 | 0.061 | −0.038 | −0.095 | −0.071 | 0.022 | −0.095 | −0.060 | −0.035 |
| Available N:K | 0.234 | 0.104 | 0.221 | 0.037 | −0.100 | −0.030 | 0.026 | −0.142 | −0.187 | −0.119 |
| Available P:K | 0.059 | −0.036 | −0.014 | −0.016 | 0.102 | 0.112 | −0.013 | 0.145 | 0.068 | −0.117 |
| N in leaf | −0.066 | 0.357 | −0.386 * | −0.481 ** | −0.337 | −0.011 | 0.137 | −0.119 | −0.275 | −0.122 |
| P in leaf | 0.358 | 0.471 ** | 0.073 | −0.212 | −0.474 ** | 0.234 | 0.489 ** | −0.321 | −0.522 ** | −0.395 * |
| K in leaf | −0.257 | 0.047 | −0.248 | −0.240 | −0.105 | 0.049 | 0.114 | −0.146 | −0.191 | −0.057 |
| C:N in leaf | 0.033 | −0.224 | 0.284 | 0.332 | 0.213 | 0.133 | −0.143 | 0.216 | 0.317 | 0.053 |
| C:P in leaf | −0.358 | −0.395 * | −0.101 | 0.160 | 0.406 * | −0.243 | −0.529 ** | 0.321 | 0.522 ** | 0.401 * |
| N:P in leaf | −0.349 | −0.270 | −0.229 | −0.026 | 0.266 | −0.300 | −0.543 ** | 0.270 | 0.470 ** | 0.421 * |
| C:K in leaf | 0.261 | 0.030 | 0.203 | 0.174 | 0.021 | −0.031 | −0.128 | 0.171 | 0.207 | 0.055 |
| N:K in leaf | 0.188 | 0.068 | 0.047 | 0.028 | −0.010 | −0.126 | −0.082 | 0.091 | 0.070 | 0.030 |
| P:K in leaf | 0.562 ** | 0.346 | 0.321 | 0.052 | −0.299 | 0.219 | 0.449 * | −0.180 | −0.381 * | −0.414 * |
| ANL | 0.710 ** | 0.608 ** | 0.226 | −0.117 | −0.481 ** | 0.911 ** | 0.512 ** | 0.629 ** | 0.179 | −0.523 ** |
| APL | 0.794 ** | 0.589 ** | 0.392 * | 0.001 | −0.484 ** | 0.816 ** | 0.733 ** | 0.327 | −0.119 | −0.657 ** |
| AKL | 0.563 ** | 0.441 * | 0.305 | 0.007 | −0.366 * | 0.881 ** | 0.576 ** | 0.516 ** | 0.107 | −0.523 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Bian, T.; Wang, S.; Zhang, X.; Li, X.; Zhang, Y.; Fu, H.; Sun, Z. Decoupling of P from C, N, and K Elements in Cucumber Leaves Caused by Nutrient Imbalance under a Greenhouse Continuous Cropping System. Horticulturae 2021, 7, 528. https://doi.org/10.3390/horticulturae7120528
Zheng S, Bian T, Wang S, Zhang X, Li X, Zhang Y, Fu H, Sun Z. Decoupling of P from C, N, and K Elements in Cucumber Leaves Caused by Nutrient Imbalance under a Greenhouse Continuous Cropping System. Horticulturae. 2021; 7(12):528. https://doi.org/10.3390/horticulturae7120528
Chicago/Turabian StyleZheng, Shiwei, Ting Bian, Shuang Wang, Xiaolan Zhang, Xiao Li, Yongyong Zhang, Hongdan Fu, and Zhouping Sun. 2021. "Decoupling of P from C, N, and K Elements in Cucumber Leaves Caused by Nutrient Imbalance under a Greenhouse Continuous Cropping System" Horticulturae 7, no. 12: 528. https://doi.org/10.3390/horticulturae7120528
APA StyleZheng, S., Bian, T., Wang, S., Zhang, X., Li, X., Zhang, Y., Fu, H., & Sun, Z. (2021). Decoupling of P from C, N, and K Elements in Cucumber Leaves Caused by Nutrient Imbalance under a Greenhouse Continuous Cropping System. Horticulturae, 7(12), 528. https://doi.org/10.3390/horticulturae7120528






