Spectral Blocking of Solar Radiation in High Tunnels by Poly Covers: Its Impact on Nutritional Quality Regarding Essential Nutrients and Health-Promoting Phytochemicals in Lettuce and Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Light and UV Measurements
2.3. Lettuce and Tomato Fruit Sampling
2.4. Anthocyanin, Chlorophyll and Total Carotenoids
2.5. Total Phenolic Compounds and Antioxidant Capacity
2.6. Individual Phenolic Compounds
2.7. Individual Carotenoids
2.8. Essential Nutrients
2.9. Statistical Analyses
3. Results and Discussion
3.1. Spectral Transmission Characteristics of Poly Covers and Shade Cloth
3.2. Anthocyanins, Total Chlorophyll and Carotenoids
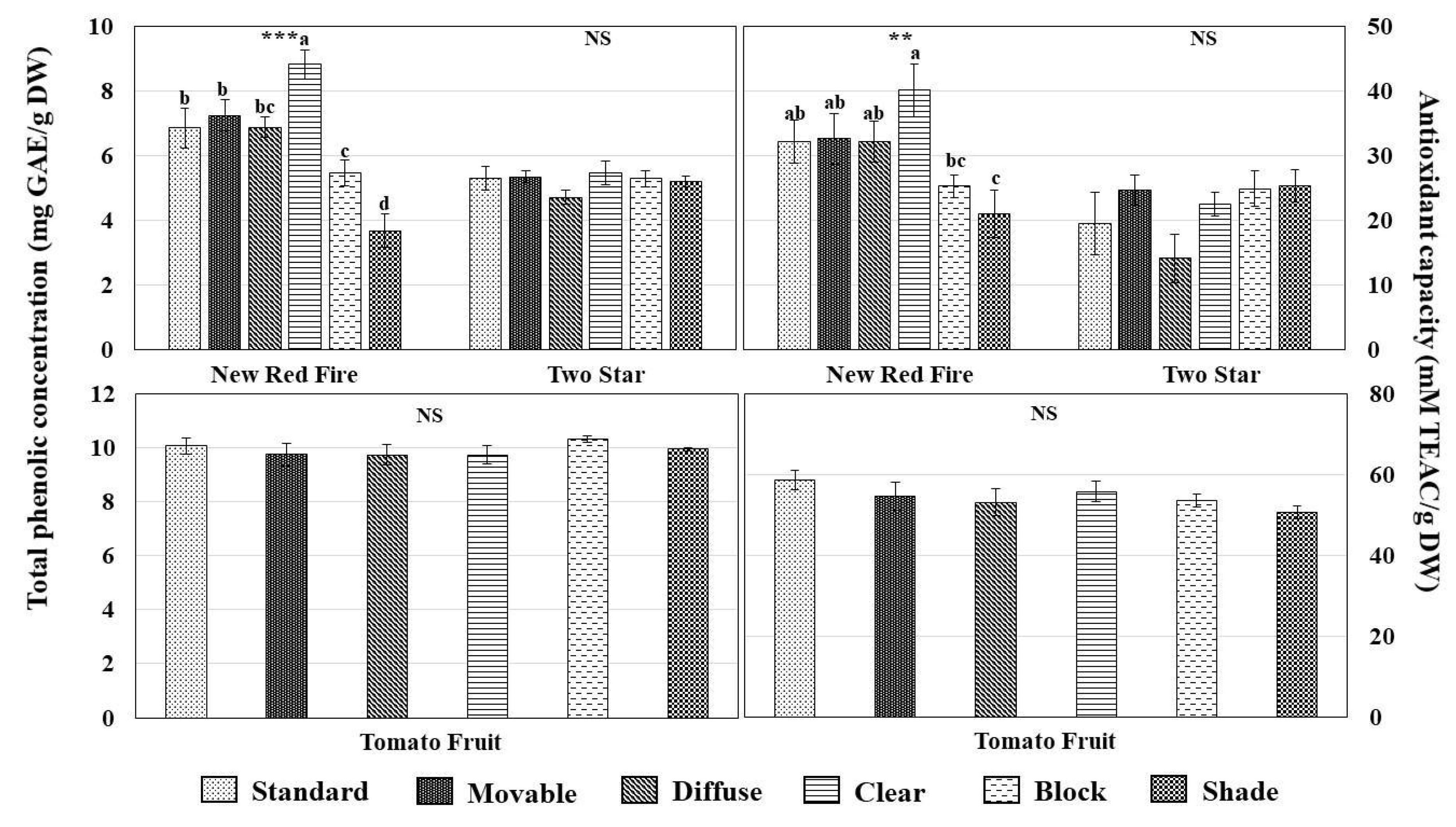
3.3. Total Phenolic Concentration and Antioxidant Capacity
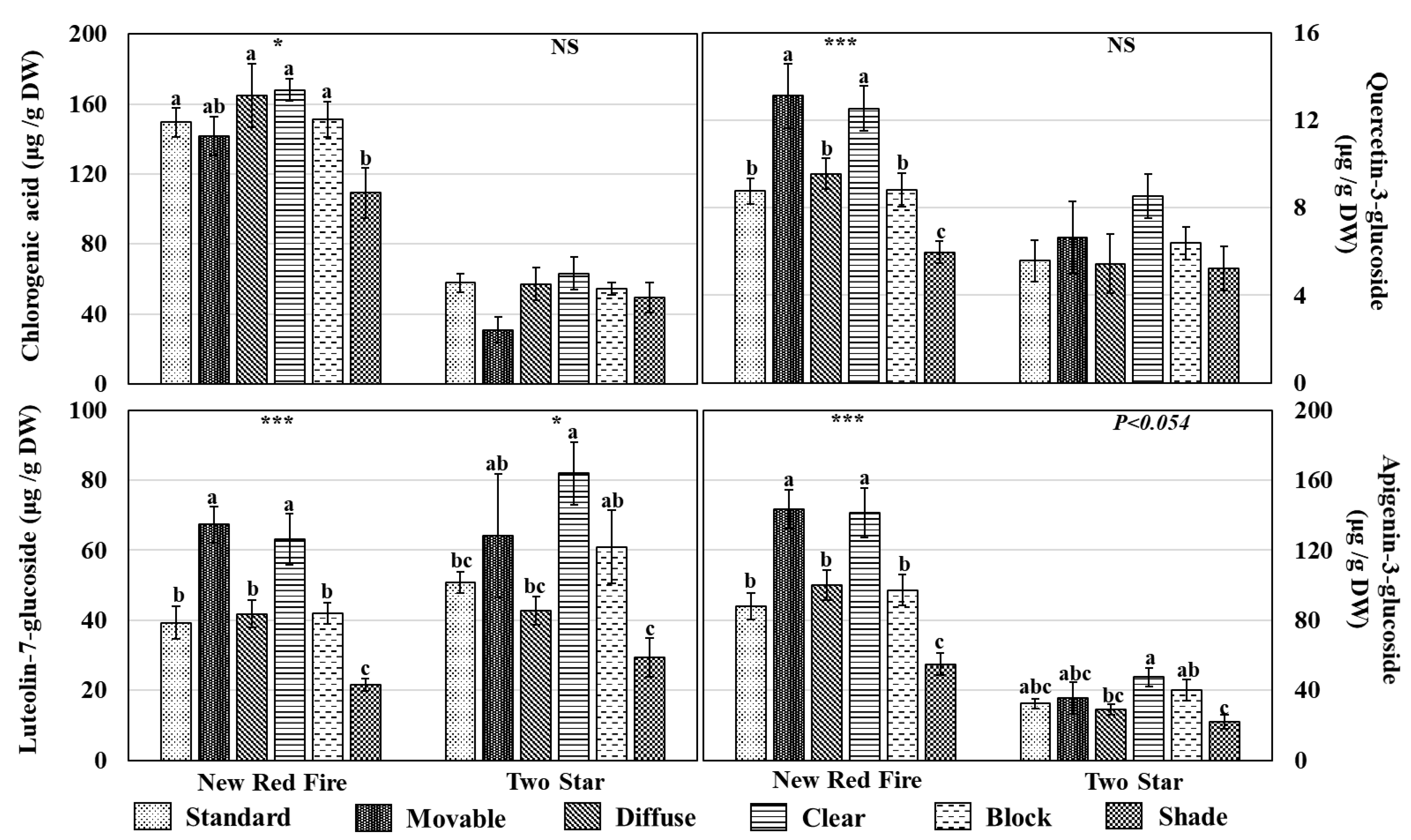
3.4. Individual Phenolic Compounds and Carotenoids
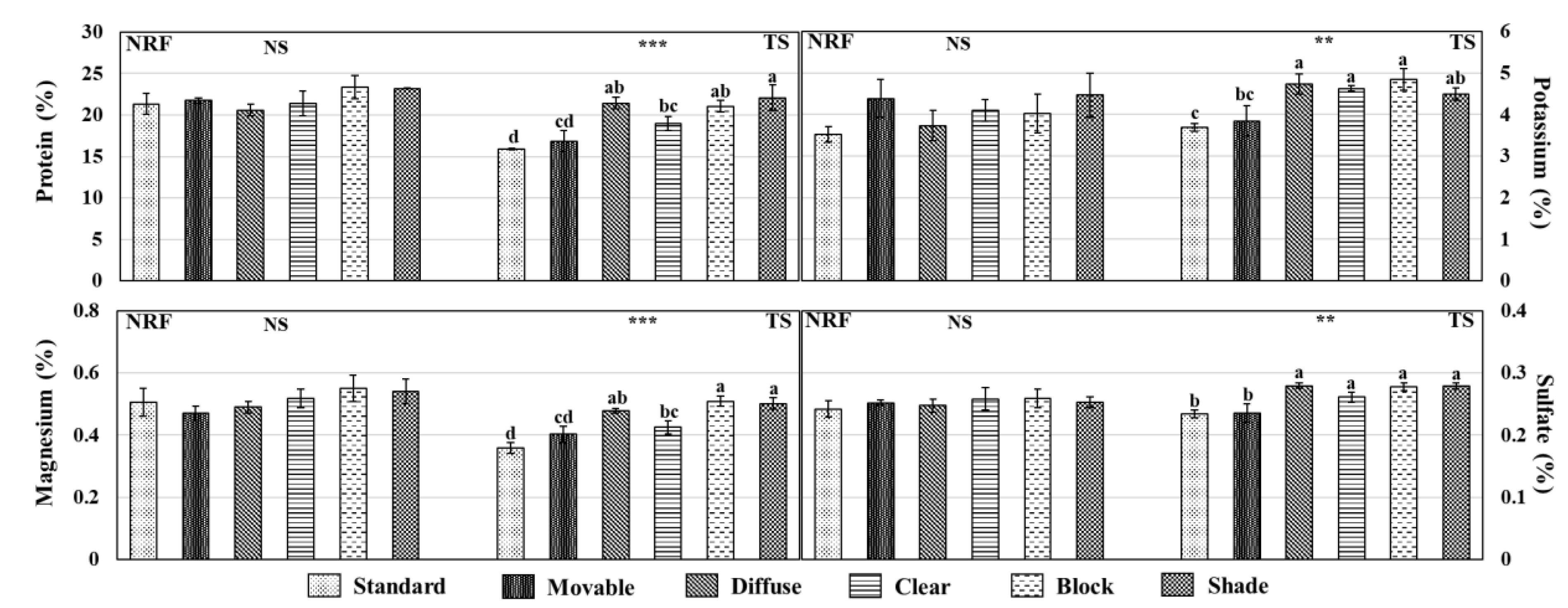
3.5. Essential Nutrients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamont, W.J. Overview of the use of high tunnels worldwide. HortTechnology 2009, 19, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Bruce, A.B.; Maynard, E.T.; Farmer, J.R. Farmers’ perspective on challenges and opportunities associated with using high tunnels for specialty crops. HortTechnology 2019, 29, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Carey, E.E.; Jett, L.; Lamont, W.J.; Nennich, T.T.; Orzolek, M.D.; Williams, K.A. Horticultural crop production in high tunnels in the United States: A snapshot. HortTechnology 2009, 19, 37–43. [Google Scholar] [CrossRef]
- Knewtson, S.J.B.; Carey, E.E.; Kirkham, M.B. Management practices of growers using high tunnels in the central great plains of the United States. HortTechnology 2010, 20, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Beecher, G.R. Nutrient content of tomatoes and tomato products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT-Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Antioxidant phytochemicals in lettuce grown in high tunnels and open field. Hortic. Environ. Biotechnol. 2011, 52, 133–139. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food. Compost. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer 1992, 18, 1–29. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Muller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef] [Green Version]
- USDA, Food and Nutrition Service, Dietary guidelines for Americans. 2020. Available online: http://www.fns.usda.gov/cnpp/dietaryguidelines-americans (accessed on 1 July 2021).
- Heber, D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J. Postgrad Med. 2004, 50, 145–149. [Google Scholar]
- Woolley, A.; Sumpter, S.; Lee, M.; Xu, J.; Barry, S.; Wang, W.; Rajashekar, C.B. Accumulation of mineral nutrients and phytochemicals in lettuce and tomato grown in high tunnel and open field. Am. J. Plant Sci. 2019, 10, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Iwamoto, T.; Carey, E.E. Antioxidant capacity of leafy vegetables as affected by high tunnel environment, fertilisation and growth stage. J. Sci. Food. Agri. 2007, 87, 2692–2699. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, D. Effects of light on secondary metabolites in selected leafy greens: A Rev. Front. Plant Sci. 2020, 11, 11–497. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proitti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED Systems. J. Plant Growth Reg. 2021. [Google Scholar] [CrossRef]
- Krizek, D.T.; Britz, S.J.; Mirecki, R.M. Inhibitory effects of ambient levels of solar UV-A and UV- B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant. 1998, 103, 1–7. [Google Scholar] [CrossRef]
- Hemming, S.; Dueck, T.; Janse, F.; van Noort, F. The effect of diffuse light on crops. Acta Hortic. 2008, 801, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Lang, K.M.; Nair, A.; Moore, K.J. Cultivar selection and placement of shadecloth on Midwest high tunnels affects colored bell pepper yield, fruit quality, and plant growth. HortScience 2020, 55, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food. Compost. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Gude, K.M. Altering solar light with high tunnel coverings to improve nutrition of lettuce and tomato. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2020. [Google Scholar]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The effect of supplemental blue, red and farred light on the growth and the nutritional quality of red and green leaf lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef] [Green Version]
- Gude, K.M.; Rajashekar, C.B.; Cunningham, B.; Kang, Q.; Wang, W.; Lee, M.; Rivard, C.L.; Pliakoni, E.D. Effect of high tunnel coverings on antioxidants of breaker and light red tomatoes at harvest and during ripening. Agronomy 2020, 10, 1639. [Google Scholar] [CrossRef]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type transcription factor, ABA-inducible bHLH-type transcription factor/JA-associated MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis. Plant Cell. 2013, 25, 1641–1656. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rpt. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Cox., S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia x hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar] [CrossRef]
- Milton, K.; Dintzis, F.R. Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica 1981, 13, 177–181. [Google Scholar] [CrossRef]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agri. Food. Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef]
- Lee, M.; Rivard, C.; Pliakoni, E.; Wang, W.; Rajashekar, C.B. Supplemental UV-A and UV-B affect the nutritional quality of lettuce and tomato: Health-promoting phytochemicals and essential nutrients. Am. J. Plant Sci. 2021, 12, 104–126. [Google Scholar] [CrossRef]
- Hipol, R.L.B.; Dionisio-Sese, M.L. Impact of light variation on the antioxidant properties of red lettuce. Electron. J. Biol. 2014, 10, 28–34. [Google Scholar]



| Season | Temperature °C | |||
|---|---|---|---|---|
| Max 2 | DOF 1 | Min 2 | DOF | |
| Spring | 25.6 0 ± 1.30 | 0.98 ± 0.82 | 10.5 ± 1.15 | 0.83 ±o.70 |
| Summer | 29.80 ± 0.77 | 0.61 ± 0.46 | 19.50 ± 0.69 | 0.55 ± 0.34 |
| Fall | 17.75 ± 1.00 | 0.73 ± 0.67 | 10.50 ± 1.15 | 0.83 ± 0.70 |
| Phytochemicals Compounds (μg/g DW) | |||||
|---|---|---|---|---|---|
| Lettuce | Gallic Acid | Chicoric Acid | Rutin | Kaempferol-3-Glucoside | |
| New Red Fire | Standard | 16.41 | 215.47 | 2.25 | 7.11 |
| Movable | 15.96 | 249.74 | 1.88 | 6.22 | |
| Diffuse | 13.25 | 237.39 | 2.00 | 6.92 | |
| Clear | 16.38 | 248.30 | 2.03 | 6.85 | |
| Block | 16.78 | 224.11 | 1.89 | 5.62 | |
| Shade | 13.12 | 210.91 | 1.71 | 5.41 | |
| Significance | NS | NS | NS | NS | |
| Two Star | Standard | 12.09 | 250.07 | 2.65 | 3.85 |
| Movable | 9.92 | 169.81 | 1.76 | 3.01 | |
| Diffuse | 15.72 | 266.56 | 2.33 | 3.91 | |
| Clear | 16.25 | 290.83 | 2.10 | 4.25 | |
| Block | 13.34 | 273.27 | 2.07 | 3.88 | |
| Shade | 13.81 | 290.46 | 2.48 | 4.54 | |
| Significance | NS | NS | NS | NS | |
| Lettuce | Ca % | Cu ppm | Fe ppm | Mn ppm | Zn ppm | |
|---|---|---|---|---|---|---|
| New Red Fire | Standard | 1.01 | 6.2 | 512.2 | 71.3 | 39.5 |
| Movable | 1.12 | 6.1 | 786.6 | 84.2 | 36.8 | |
| Diffuse | 1.03 | 6.1 | 607.0 | 74.0 | 38.7 | |
| Clear | 1.11 | 5.6 | 384.2 | 62.6 | 35.4 | |
| Block | 1.17 | 5.9 | 523.8 | 80.6 | 40.1 | |
| Shade | 1.16 | 5.4 | 458.5 | 76.8 | 37.8 | |
| Significance | NS | NS | NS | NS | NS | |
| Two Star | Standard | 1.26 | 5.7 | 523.2 | 67.3 | 37.0 |
| Movable | 1.35 | 5.5 | 336.0 | 78.1 | 33.2 | |
| Diffuse | 1.42 | 4.8 | 399.5 | 71.9 | 34.5 | |
| Clear | 1.34 | 5.1 | 325.2 | 64.0 | 33.2 | |
| Block | 1.43 | 5.2 | 422.4 | 74.6 | 36.5 | |
| Shade | 1.39 | 4.3 | 276.3 | 65.6 | 33.8 | |
| Significance | NS | NS | NS | NS | NS |
| Essential Nutrients for Tomato Fruits | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein % | C % | P % | K % | Ca % | Mg % | SO4-S% | Cu ppm | Fe ppm | Mn ppm | Zn ppm | |
| Standard | 14.4 | 37.7 | 0.3 | 3.1 | 0.16 | 0.1 | 0.12 | 8.0 | 52.0 | 12.5 | 19.3 |
| Movable | 14.7 | 37.4 | 0.3 | 3.3 | 0.15 | 0.2 | 0.12 | 7.4 | 71.5 | 14.5 | 19.8 |
| Diffuse | 14.7 | 37.1 | 0.3 | 3.3 | 0.15 | 0.1 | 0.20 | 43.9 | 68.4 | 13.1 | 19.7 |
| Clear | 13.8 | 37.6 | 0.3 | 3.2 | 0.16 | 0.2 | 0.12 | 7.5 | 52.6 | 11.9 | 19.4 |
| Block | 15.1 | 37.4 | 0.3 | 3.2 | 0.17 | 0.2 | 0.13 | 7.5 | 102.5 | 14.5 | 20.7 |
| Shade | 16.4 | 37.1 | 0.4 | 3.7 | 0.12 | 0.2 | 0.14 | 8.1 | 67.6 | 12.4 | 23.9 |
| Significance | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Rivard, C.; Wang, W.; Pliakoni, E.; Gude, K.; Rajashekar, C.B. Spectral Blocking of Solar Radiation in High Tunnels by Poly Covers: Its Impact on Nutritional Quality Regarding Essential Nutrients and Health-Promoting Phytochemicals in Lettuce and Tomato. Horticulturae 2021, 7, 524. https://doi.org/10.3390/horticulturae7120524
Lee M, Rivard C, Wang W, Pliakoni E, Gude K, Rajashekar CB. Spectral Blocking of Solar Radiation in High Tunnels by Poly Covers: Its Impact on Nutritional Quality Regarding Essential Nutrients and Health-Promoting Phytochemicals in Lettuce and Tomato. Horticulturae. 2021; 7(12):524. https://doi.org/10.3390/horticulturae7120524
Chicago/Turabian StyleLee, Myungjin, Cary Rivard, Weiqun Wang, Eleni Pliakoni, Kelly Gude, and Channa B. Rajashekar. 2021. "Spectral Blocking of Solar Radiation in High Tunnels by Poly Covers: Its Impact on Nutritional Quality Regarding Essential Nutrients and Health-Promoting Phytochemicals in Lettuce and Tomato" Horticulturae 7, no. 12: 524. https://doi.org/10.3390/horticulturae7120524
APA StyleLee, M., Rivard, C., Wang, W., Pliakoni, E., Gude, K., & Rajashekar, C. B. (2021). Spectral Blocking of Solar Radiation in High Tunnels by Poly Covers: Its Impact on Nutritional Quality Regarding Essential Nutrients and Health-Promoting Phytochemicals in Lettuce and Tomato. Horticulturae, 7(12), 524. https://doi.org/10.3390/horticulturae7120524









