Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Measurement of Chlorophyll Content and Photosystem II Quantum Yield
2.3. Measurement of Plant Growth
2.4. Measurement of Nitrate Content
2.5. Measurement of Total Phenol Content
2.6. Measurement of DPPH Radical Scavenging Capacity
2.7. Measurement of Ascorbic Acid
2.8. Measurement of Sugar Content
2.9. Measurement of Root Oxygen Consumption
2.10. Data Analysis
3. Results and Discussion
3.1. Effects of Nutrient Deficiency Timing on the Growth of Hydroponic Radish
3.2. Effects of Nutrient Deficiency on the Nutritional Components of Hydroponic Radish
3.3. Effects of Nutrient Deficiency on the Leaf and Fibrous Root Parameters of Hydroponically Grown Radish
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. J. Food Agric. Environ. 2003, 1, 80–86. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Jones Jr, J.B. Hydroponics: Its history and use in plant nutrition studies. J. Plant Nutr. 1982, 5, 1003–1030. [Google Scholar] [CrossRef]
- Yeh, D.M.; Lin, L.; Wright, C.J. Effects of mineral nutrient deficiencies on leaf development, visual symptoms and shoot–root ratio of Spathiphyllum. Sci. Hortic. 2000, 86, 223–233. [Google Scholar] [CrossRef]
- Abdel Mohsen, M.A.; Hassan, A.A.; El-Sewedy, S.M.; Aboul-Azm, T.; Magagnotti, C.; Fanelli, R.; Airoldi, L. Biomonitoring of n-nitroso compounds, nitrite and nitrate in the urine of Egyptian bladder cancer patients with or without Schistosoma haematobium infection. Int. J. Cancer 1999, 82, 789–794. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Speijers, G.J.; Meulenbelt, J. Health implications of exposure to environmental nitrogenous compounds. Toxicol. Rev. 2003, 22, 41–51. [Google Scholar] [CrossRef]
- Broadley, M.R.; Escobar-Gutiérrez, A.J.; Burns, A.; Burns, I.G. Nitrogen-limited growth of lettuce is associated with lower stomatal conductance. New Phytol. 2001, 152, 97–106. [Google Scholar] [CrossRef]
- Uribelarrea, M.; Crafts-Brandner, S.J.; Below, F.E. Physiological N response of field-grown maize hybrids (Zea mays L.) with divergent yield potential and grain protein concentration. Plant Soil 2009, 316, 151–160. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front. Plant 2016, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kim, H.I.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Curtis, I.S. The noble radish: Past, present and future. Trends Plant Sci. 2003, 8, 305–307. [Google Scholar] [CrossRef]
- Hong, J.; Gruda, N.S. The potential of introduction of Asian vegetables in Europe. Horticulturae 2020, 6, 38. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The vertical farm: A review of developments and implications for the vertical city. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- Tabaglio, V.; Boselli, R.; Fiorini, A.; Ganimede, C.; Beccari, P.; Santelli, S.; Nervo, G. Reducing nitrate accumulation and fertilizer use in lettuce with modified intermittent Nutrient Film Technique (NFT) system. Agronomy 2020, 10, 1208. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Pannico, A.; El-Nakhel, C.; Fascella, G.; Duri, L.G.; Cristofano, F.; Gentile, B.R.; Giordano, M.; Rouphael, Y.; et al. Nutrient Solution Deprivation as a Tool to Improve Hydroponics Sustainability: Yield, Physiological, and Qualitative Response of Lettuce. Agronomy 2021, 11, 1469. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Nutrient Solution Concentration on the Growth of Hydroponic Sweetpotato. Agronomy 2020, 10, 1708. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hiyama, M.; Hikosaka, S.; Goto, E. Effects of concentration and temperature of nutrient solution on growth and camptothecin accumulation of Ophiorrhiza pumila. Plants 2020, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components–calculation of qP and Fv’/Fm’; without measuring Fo’. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Sakamoto, M.; Wada, M.; Suzuki, T. Effect of partial excision of early taproots on growth and components of hydroponic carrots. Horticulturae 2020, 6, 5. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl jasmonate and salinity increase anthocyanin accumulation in radish sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of root-zone temperature on growth and quality of hydroponically grown red leaf lettuce (Lactuca sativa L. cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350. [Google Scholar] [CrossRef]
- Eguchi, T.; Yoshida, S. A cultivation method to ensure tuberous root formation in sweetpotatoes (Ipomoea batatas (L.) Lam.). Environ. Control Biol. 2004, 42, 259–266. [Google Scholar] [CrossRef]
- Kusakawa, T.; Inoue, M. Damage to pot-cultured carrot growth due to a temporarily raised groundwater level and flooding period. Hortic. Res. 2010, 9, 495–500. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of pot volume on the growth of sweetpotato cultivated in the new hydroponic system. Sustain. Agric. Res. 2018, 7, 137–145. [Google Scholar] [CrossRef]
- Asao, T.; Kitazawa, H.; Washizu, K.; Ban, T.; Pramanik, M.H.R. Effect of different nutrient levels on anthocyanin and nitrate-N contents in turnip grown in hydroponics. J. Appl. Hortic. 2005, 72, 87–89. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop. Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Black, B.L.; Fuchigami, L.H.; Coleman, G.D. Partitioning of nitrate assimilation among leaves, stems and roots of poplar. Tree Physiol. 2002, 22, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Razgallah, N.; Abid, G.; Chikh-Rouhou, H.; Hassen, A.; M’hamdi, M. Nitrate content and expression of putative nitrate transporter genes in lettuce fertilized with nitrogen fertilizers. Int. J. Veg. Sci. 2017, 23, 173–184. [Google Scholar] [CrossRef]
- Jia, L.; Qin, Y.; Chen, Y.; Fan, M. Fertigation improves potato production in Inner Mongolia (China). J. Crop. Improv. 2018, 32, 648–656. [Google Scholar] [CrossRef]
- Katsuhara, M.; Shibasaka, M. Cell death and growth recovery of barley after transient salt stress. J. Plant Res. 2000, 113, 239. [Google Scholar] [CrossRef]
- de Lacerda, C.F.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A. Changes in growth and in solute concentrations in sorghum leaves and roots during salt stress recovery. Environ. Exp. Bot. 2005, 54, 69–76. [Google Scholar] [CrossRef]
- Techawongstien, S.; Nawata, E.; Shigenaga, S. Responses of chili pepper cultivars to transient water stress. J. Jpn. Soc. Hortic. Sci. 1992, 61, 85–92. [Google Scholar] [CrossRef]
- Suralta, R.R.; Inukai, Y.; Yamauchi, A. Dry matter production in relation to root plastic development, oxygen transport, and water uptake of rice under transient soil moisture stresses. Plant Soil 2010, 332, 87–104. [Google Scholar] [CrossRef]
- Monti, A.; Brugnoli, E.; Scartazza, A.; Amaducci, M.T. The effect of transient and continuous drought on yield, photosynthesis and carbon isotope discrimination in sugar beet (Beta vulgaris L.). J. Exp. Bot. 2006, 57, 1253–1262. [Google Scholar] [CrossRef]
- Romina, P.; Abeledo, L.G.; Mantese, A.I.; Miralles, D.J. Differential root and shoot biomass recovery in wheat and barley with transient waterlogging during preflowering. Plant Soil 2017, 417, 481–498. [Google Scholar]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A review of environment effects on nitrate accumulation in leafy vegetables grown in controlled environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Khan, K.A.; Yan, Z.; He, D. Impact of light intensity and nitrogen of nutrient solution on nitrate content in three lettuce cultivars prior to harvest. J. Agric. Sci. 2018, 10, 99–109. [Google Scholar] [CrossRef]
- Khan, K.A.; Hussain, N.; Ahmad, M.; ul Haq, M.I.; Ali, S.; Hameed, M.S.; Hussain, M.I.; Ahmad, M.; Noureen, A.; Maqbool, M.; et al. Increased Light Intensity and Diluted NPK solution Significantly Influence Lettuce Nitrate Content. J. Environ. Agric. Sci. 2019, 20, 28–35. [Google Scholar]
- Banihani, S.A. Radish (Raphanus sativus) and diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.F.; Gorni, P.H.; Vitolo, H.F.; Carvalho, M.E.A.; Pacheco, A.C. Sweetpotato tolerance to drought is associated to leaf concentration of total chlorophylls and polyphenols. Theor. Exp. Plant Physiol. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Juszczuk, I.M.; Wiktorowska, A.; Malusá, E.; Rychter, A.M. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant Soil 2004, 267, 41–49. [Google Scholar] [CrossRef]
- Hassanpour, H.; Khavari-Nejad, R.A.; Niknam, V.; Najafi, F.; Razavi, K. Effects of penconazole and water deficit stress on physiological and antioxidative responses in pennyroyal (Mentha pulegium L.). Acta Physiol. Plant. 2012, 34, 1537–1549. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; De Gara, L. Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef] [PubMed]
- Mogren, L.; Reade, J.; Monaghan, J. Effects of environmental stress on ascorbic acid content in baby leaf spinach (Spinacia oleracea). In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), Lisbon, Portugal, 22 August 2010; Volume 939, pp. 205–208. [Google Scholar]
- Shafiq, S.; Akram, N.A.; Ashraf, M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015, 185, 68–75. [Google Scholar] [CrossRef]
- Hayata, Y.; Suzuki, Y. The relationships of plant hormones, sugars and nitrogen to the early development of radish root. J. Jpn. Soc. Hortic. Sci. 1982, 51, 56–61. [Google Scholar] [CrossRef][Green Version]
- Hara, M.; Oki, K.; Hoshino, K.; Kuboi, T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]
- Simko, I. Genetic variation in response to N, P, or K deprivation in baby leaf lettuce. Horticulturae 2020, 6, 15. [Google Scholar] [CrossRef]
- Muchecheti, F.; Madakadze, C.; Soundy, P. Leaf chlorophyll readings as an indicator of nitrogen status and yield of spinach (Spinacia oleracea L.) grown in soils amended with Luecaena leucocephala prunings. J. Plant Nutr. 2016, 39, 539–561. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Giordano, M.; Pannico, A.; Carillo, P.; Fusco, G.M.; De Pascale, S.; Rouphael, Y. Cultivar-specific performance and qualitative descriptors for butterhead Salanova lettuce produced in closed soilless cultivation as a candidate salad crop for human life support in space. Life 2019, 9, 61. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. Daily macronutrient uptake patterns in relation to plant age in hydroponic lettuce. J. Plant Nutr. 2016, 39, 1357–1364. [Google Scholar] [CrossRef]
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. C. R. Biol. 2010, 333, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, H.; Chen, Y.; Zheng, Q.; Li, B.; Li, Z. TaSCL14, a novel wheat (Triticum aestivum L.) GRAS gene, regulates plant growth, photosynthesis, tolerance to photooxidative stress, and senescence. J. Genet. Genom. 2015, 42, 21–32. [Google Scholar] [CrossRef]
- Chishaki, N.; Horiguchi, T. Responses of secondary metabolism in plants to nutrient deficiency. Soil Sci. Plant Nutr. 1997, 43, 987–991. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Zhang, H.; Wang, J.; Chen, Z.; Luo, L.; Liu, G.; Liu, P. Metabolic alterations provide insights into Stylosanthes roots responding to phosphorus deficiency. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Valdés-López, O.; Yang, S.S.; Aparicio-Fabre, R.; Graham, P.H.; Reyes, J.L.; Vance, C.P.; Hernández, G. MicroRNA expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol. 2010, 187, 805–818. [Google Scholar] [CrossRef]
- Sirohi, G.; Pandey, B.K.; Deveshwar, P.; Giri, J. Emerging trends in epigenetic regulation of nutrient deficiency response in plants. Mol. Biotechnol. 2016, 58, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, H.; Wang, B.; Sun, X.; Lu, X.; Yu, W. High quality yield in lettuce in response to low nitrate content can be achieved by reduced nitrogen application. Int. J. Agric. Biol. 2018, 20, 2243–2250. [Google Scholar]
- He, J.; Qin, L. Impacts of Reduced Nitrate Supply on Nitrogen Metabolism, Photosynthetic Light-Use Efficiency, and Nutritional Values of Edible Mesembryanthemum crystallinum. Front. Plant Sci. 2021, 12, 686910. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Soteriou, G.A.; Graziani, G.; Kyratzis, A.; Antoniou, C.; Ritieni, A.; Pascale, S.D.; Rouphael, Y. Preharvest nutrient deprivation reconfigures nitrate, mineral, and phytochemical content of microgreens. Foods 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]


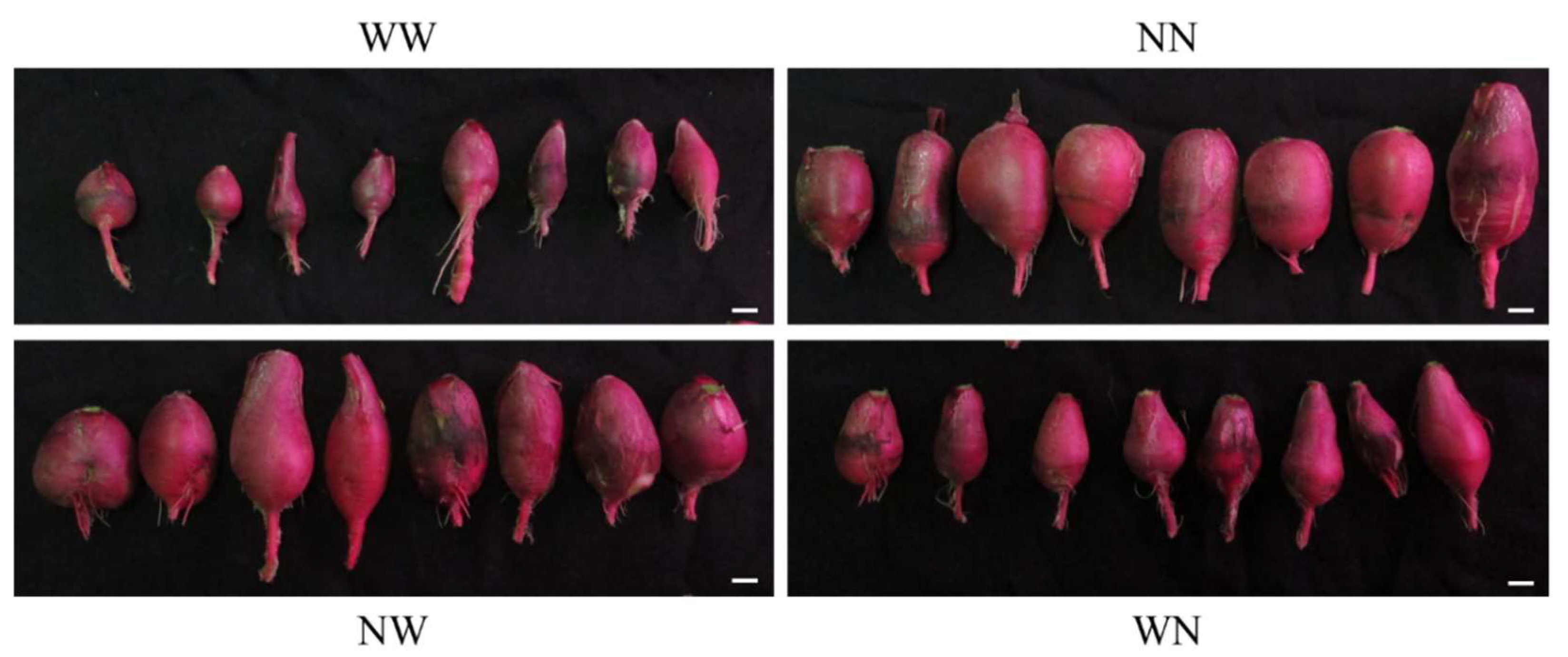
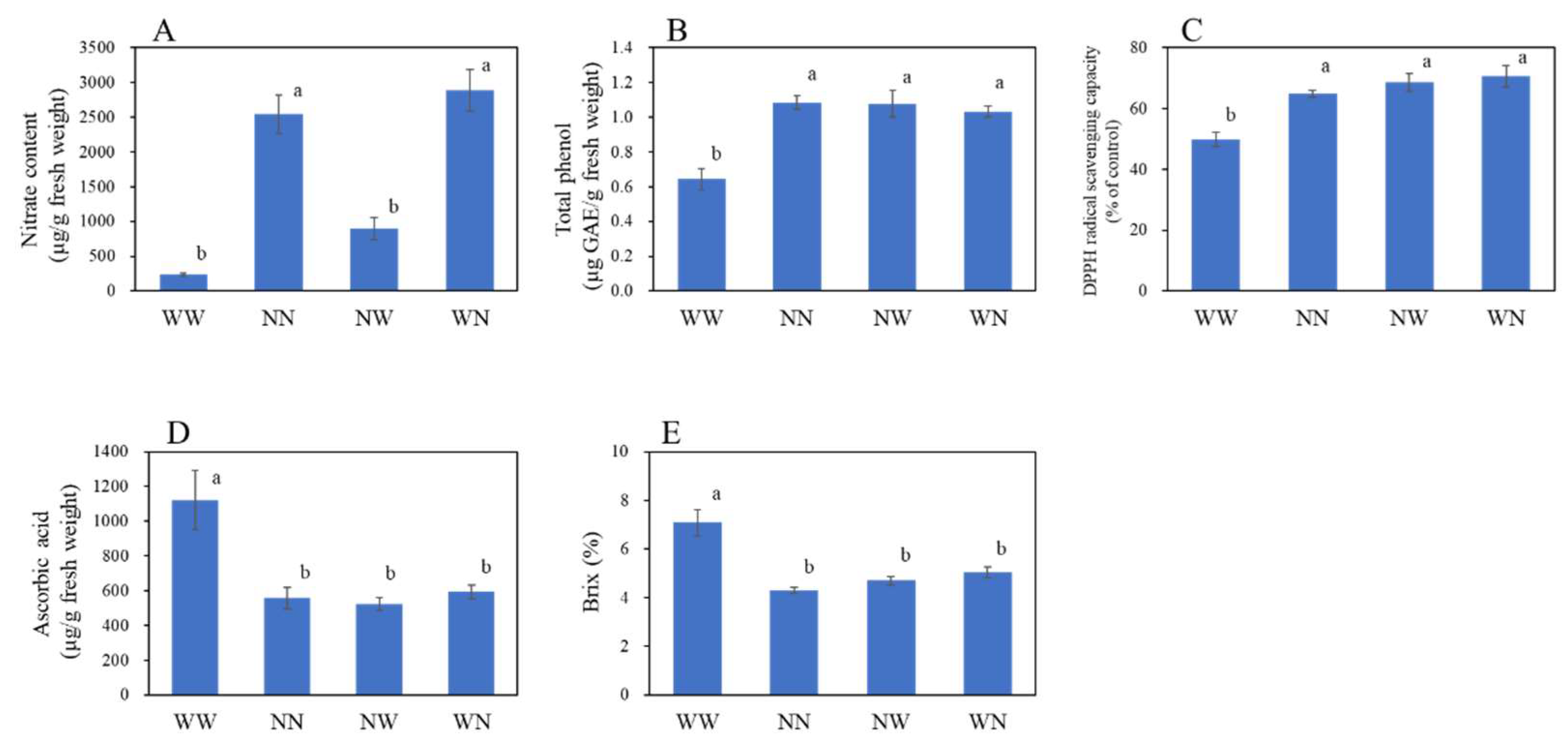
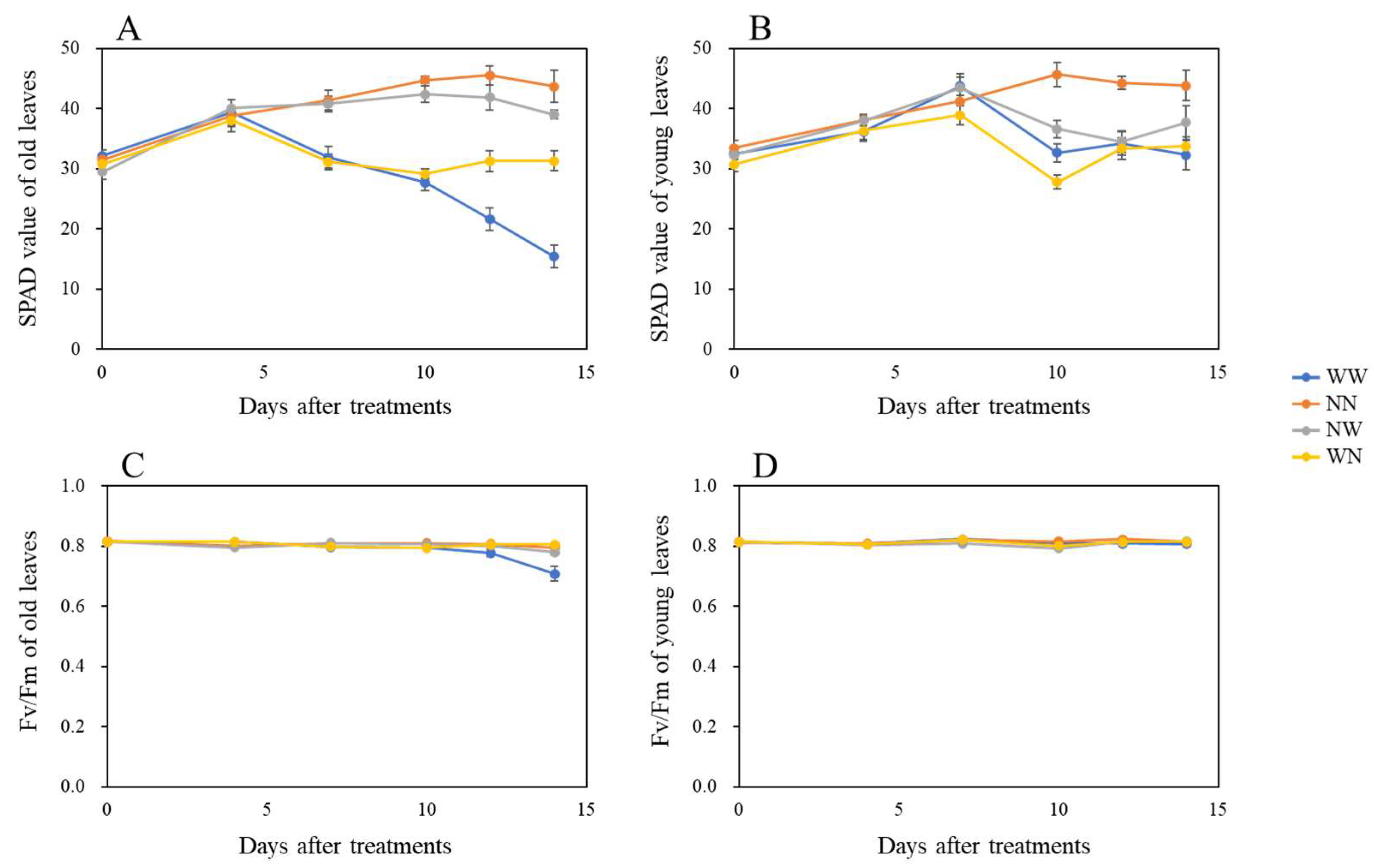

| Treatment 1 | Leaf Number | Top/Taproot Ratio |
|---|---|---|
| WW | 6.9 b 2 | 0.6 b |
| NN | 9.0 a | 1.1 ab |
| NW | 8.9 a | 0.6 b |
| WN | 8.6 a | 2.0 a |
| Treatment 1 | Fresh Weight (g) | Dry Weight (g) | Water Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Top | Taproot | Fibrous Root | Top | Taproot | Fibrous Root | Top | Taproot | Fibrous Root | |
| WW | 1.9 c 2 | 4.2 b | 0.6 b | 0.3 c | 0.5 b | 0.06 b | 83.7 b | 86.8 b | 90.8 b |
| NN | 16.9 a | 19.7 a | 0.9 ab | 1.3 a | 1.1 a | 0.07 ab | 92.7 a | 94.4 a | 92.2 a |
| NW | 7.3 bc | 16.5 a | 0.9 ab | 0.6 bc | 1.0 a | 0.07 ab | 91.6 a | 93.5 a | 92.2 a |
| WN | 10.7 ab | 6.1 b | 1.4 a | 0.7 b | 0.4b | 0.11 a | 93.5 a | 93.1 a | 92.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae 2021, 7, 525. https://doi.org/10.3390/horticulturae7120525
Sakamoto M, Komatsu Y, Suzuki T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae. 2021; 7(12):525. https://doi.org/10.3390/horticulturae7120525
Chicago/Turabian StyleSakamoto, Masaru, Yoshiki Komatsu, and Takahiro Suzuki. 2021. "Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish" Horticulturae 7, no. 12: 525. https://doi.org/10.3390/horticulturae7120525
APA StyleSakamoto, M., Komatsu, Y., & Suzuki, T. (2021). Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae, 7(12), 525. https://doi.org/10.3390/horticulturae7120525






