Abstract
Moringa oleifera Lam. is a fast-growing and drought-resistant tree of the Moringaceae family. The tree is known with some common names: miracle, ben oil, drumstick, horseradish or simply moringa. The plant grows in a variety of environments including harsh conditions. The plant has a high content of phytonutrients, being used as natural integrators to cure malnutrition. Phytochemical studies of plant organs showed the plant as a rich source of primary and secondary metabolites belonging to different classes of organic compounds. Pharmacological studies confirmed the use of the plant to cure several diseases and to possess nutraceutical properties. This review is aimed to contribute to the knowledge of M. oleifera by providing its plant description, phytochemistry and pharmacology.
1. Introduction
Moringa oleifera Lam. is a fast-growing and drought-resistant tree of the Moringaceae family. It is commonly known with several names including moringa, drumstick tree (for the long and slender seedpods), horseradish tree (for the roots taste resembling horseradish), ben oil tree (being rich in behenic acid), and miracle tree (for the medicinal properties) [1]. M. oleifera is the most popular of the thirteen species in the genus Moringa, the unique of the family Moringaceae which includes: M. concanensis, M. drouhardii, M. arborea, M. borziana, M. hildebrandtii, M. longituba, M. pygmaea, M. rivae, M. ruspoliana, M. ovalifolia, M. peregrine, and M. stenopetala. The species are native to Africa, Arabia, India, Southeast Asia, South America, the Pacific Islands, and the Caribbean [2]. It is distributed in Ethiopia, Florida, Philippines, and Sudan [2].
M. oleifera is one of the most popular plants that can grow in a variety of environments due to its ability to resist to harsh conditions such as high temperatures and limited water availability [3]. Therefore, it grows in a variety of soils including semi-dry, desert, or tropical soils and rainfall conditions [3]. The plant tolerates also different pH levels, ranging from 5.0 to 9.0. However, it prefers neutral pH and well-drained soils. It thrives in temperatures ranging from 25 to 40 °C, though it can withstand temperature swings of −1 to 3 °C and 38 to 48 °C during the coldest and warmest months, respectively [3]. The plant yield related to cutting period and planting density was studied and the data indicated that the highest planting density of 0.2 m × 0.2 m in combination with the intermediate cutting height of 30 cm produced the highest fresh matter and dry matter yield throughout the whole evaluation period [4].
M. oleifera is among the food plants richest in nutrients [5]. It has a high content in essential amino acids, proteins, minerals, vitamins, and polyphenols. It is a rich source of phytochemicals including flavonoids, anthocyanins, isothiocyanates, anthraquinone, alkaloids, essential oils, tannic acid, saponins, steroids, terpenoids, cardiac glycosides [6]. In addition, it is used to treat individuals with extreme malnutrition as well as for its pharmacological (hepatoprotective, antihypertensive, cholesterol-lowering, anti-urolithiasis, antifertility, antidiabetic, and antioxidant activity, nutraceutical properties, and antimicrobial) [6]. Moreover, M. oleifera is being used to help to breastfeed mothers improving postpartum milk production [7]. It is also used in Ayurvedic tradition specifically for cancer treatment [8]. M. oleifera leaves and buds were used against headache by rubbing them on the temples. Roots and root barks were used as anti-scorbutic [6]. The eye diseases were treated with the juice of the leaves added with honey. Dried seeds of M. oleifera were used in ophthalmic preparation, venereal affection anti-inflammatory and purgative and as tonic [6]. The use in ethnomedicine encouraged scientists to study this plant to determine its chemical composition and to investigate its pharmacological potential. This review is aimed to contribute to the knowledge of M. oleifera by providing plant description, phytochemistry and pharmacology.
2. Plant Description
M. oleifera is a short, slender, deciduous, perennial tree that grows to about 10 m tall, slender with drooping branches; branches and stem are brittle, with corky bark. Leaves are feathery, pale green, compound, tripinnate, (30–60 cm long), with many small leaflets, 1.3–2 cm long, 0.6–0.3 cm wide, lateral ones slightly elliptic, terminal ones obovate, and slightly larger (Figure 1). Flowers are fragrant, white, or creamy-white, 2.5 cm in diameter, and borne in sprays. The stamens are yellow, and the pods are pendulous, brown, triangular, splitting lengthwise into three parts when dry, and containing about 20 seeds embedded in the pith. The pod has nine ribs on both ends and the seeds are dark brown with three papery wings [1].

Figure 1.
Images of M. oleifera tree, leaves, seeds, and flowers.
The feathery leaves of the tripinnate complex have green curved leaflets that are 1–4 cm long (Figure 1). Because of its leaves, the tree is frequently mistaken for a leguminous plant. The alternate twice or thrice pinnate leaves appear at the branch tips in most cases. They have a long petiole with 8–10 pairs of pinnae, each bearing two sets of inverse elliptic leaflets and one at the apex and are 20–70 cm long when young [1].
The seeds have three papery wings and are oval with a tannish semi-permeable seed arrangement (Figure 1). Their arrangements are mostly brown to dark brown but can be white if portions are of low viability. It almost within a week, viable seeds sprout. The body has three white wings that run at 130 intervals from start to finish [1].
Flowers are prominent, softly fragrant, and are borne on inflorescences 15–25 cm long. They are mostly white to cream in color, 2.5 cm in diameter, and tinged pink in a few varieties (Figure 1). However, the flowers are fragrant and 2.5 cm wide, and they bloom profusely in auxiliary, dropping panicles that are 10–25 cm long. They have a dotted base and are white in color. The direct lanceolate sepals are five-reflexed. The five petals are rumored to be thin. Except for the lowest stamen, they are reflexed and consist of five stamens and five staminodes [1].
3. Phytochemistry
Table 1 lists all the metabolites that have been found in M. oleifera extracts. Many studies have focused their efforts in analyzing the polar and non-polar extracts of M. oleifera leaves and seeds to characterize the chemical composition of this plant and try to explain the beneficial characteristics demonstrated by lots of works in recent years. Selected characteristic compounds of the plant are reported in Figure 2.

Table 1.
List of compounds extracted from Moringa oleifera leaves and seeds.
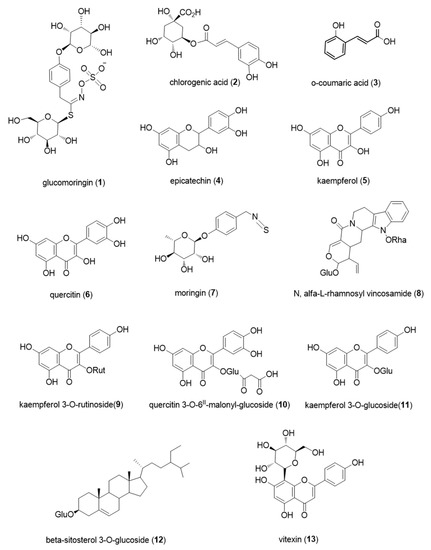
Figure 2.
Bioactive metabolites isolated from M. oleifera.
3.1. Non-Polar Compounds
Fatty acids are present in very high quantities. In their work, Lee et al. [9] performed an extraction on M. oleifera leaves powder using 70% methanol and then partitioning the outcome with hexane, dichloromethane, ethyl acetate, butanol, and water. Finally, the last part of extraction was conducted by supercritical fluid extraction (SFE). The most abundant fatty acid found in the resulting extracts were cis-11-Eicosenoic acid (also called gondoic acid), a monounsaturated acid contained in many oils extracted from plants, followed by palmitic acid (23.65%), and then linoleic acid and oleic acid (6.84% and 5.92%), and apart from them, the other ones are present in quantities lower than 5%. In seeds extracts, the highest content of fatty acid was registered for oleic acid (74.5%), then palmitic acid (7.92%), stearic acid (7.10%), and then minor fatty acids present in smaller quantities [9]. These fatty acids are important because they take part in metabolic processes that lead to the synthesis of ω-3 fatty acid, which have important roles in regulation processes of cellular development and immune system [14]. As showed by the same group, cis-11-eicosenoic acid, linoleic acid, and oleic acid exhibited an antimicrobial effect on Staphylococcus aureus, avoiding biofilm formation by inhibition of cell growth [9,10]. The oily fraction of M. oleifera leaves is also very important for cosmetic usage, as they are used as a fundamental part of skin and hair products [10].
Moreover, alkanes and alkanes derivatives are another abundant class of molecules in moringa extracts. Chelliah et al. [10] conducted a complete analysis on M. oleifera, performing different extractions with hexane, methanol and water. The extracts were then analyzed with GC-MS, and several compounds were found, the most abundant of which were 1,30-triacontanediol (14.98%) and octacosane (8.57%), in leaves from Madurai variety, and nonacosane (15.55%) and γ-Sitosterol (9.56%) for Chennai variety. Conversely, in seeds they found 6-octadecenoic acid (52.24%), n-hexadecanoic acid (palmitic acid) (6.17%), oleic acid (5.12%) as major compounds in Madurai variety, and (Z)-13-docosenamide (13.62%), propionamide (4.48%), ethyl oleate (4.33%) in Chennai variety [10]. Most of them are alkanes or their derivatives, and they are synthesized by plants to build up an external waxy layer (also called epicuticular wax) to protect leaves from surface wetting and water evaporation. 6-octadecenoic acid, also called petroselinic acid, and palmitic acid are two fatty acids regularly present in plant oils. γ-Sitosterol belongs to the class of the phytosterols, and it is an isomer of β-sitosterol, well known because there is scientific evidence that proved the antidiabetic properties of γ-Sitosterol in vivo against type II diabetes [17,18]. Similar compounds were found by Zhao & Zhang [12] that carried on a Soxhlet extraction with hexane. The procedure was conducted for 8 h, and when the dried extract was analyzed by GC-MS analysis, they stated that the most abundant compounds in the extract were nonacosane (18.65%), 1, 2, 4-trimethylbenzene (16.96%), heptacosane (7.45%) [12].
Essential oils are mixtures of dozens or hundreds of molecules, mainly volatiles compounds belonging to the classes of terpenes, alcohols, aldehydes, ketones, esters, phenols and others, often extracted with hydrodistillation, solvent-free microwave extraction (SFME) or supercritical fluid extraction using supercritical carbon dioxide. Despite a wide utilization of essential oils in alternative medicine and aromatherapy, they possess some important properties, such as: antibacterial activity against gram-positive species, probably because of external membrane disruption due to phenolic compounds activity; antioxidant activity, probably due to secondary metabolites with conjugated double bonds; anti-inflammatory activity, triggered by a wide variety of compounds, including antioxidants. An interesting analysis on essential oils was conducted by Chuang et al. [11] that extracted essential oils from M. oleifera leaves using hydrodistillation and tested the extract on fungi cultures finding out that both essential oils and seeds extracts had a positive effect against fungi development, probably exerting anti-fungal activity by disrupting the cellular membrane. The non-polar extract obtained were run through GC-MS instrument, and the main compounds found were mostly alkanes and alkenes, including hexacosane, pentacosane, tetracosane making up almost 30% of total extracts. Moreover, phytol was identified (7.66% based on peak area) [11]. Phytol is a diterpene alcohol very abundant in plants because it is one of the two components of chlorophyll. Phytol is used as a food additive, and a group of scientists have proposed that it can be useful to treat schistosomiasis, a disease caused by a parasite [19]. Lastly, a small quantity of terpenes and terpene derivative were found, with hexahydrofarnesylacetone being the most abundant one (1.30%) and then smaller quantities of linalool oxide, farnsylacetone, isolongifolene α-ionene, and α- and β-ionone, barely present in traces [11].
3.2. Polar Compounds
M. oleifera leaves are very high in protein content, and thus, they are highly rich in amino acids. All the essential amino acids can be found in M. oleifera leaves and they contribute to more than 50% of total amino acids content. Amino acids presence in leaves was represented by high quantities of glutamic acid. Chelliah et al. [10] found 15.86 g over 16 g of nitrogen (g/16 gN) of glutamic acid in Madurai variety and 15.33 g/16 gN in Chennai variety, while Lalas et al. [14] reported 268.7 mg/100 g of glutamic acid. According to Chelliah et al. [10], methionine was the least abundant in Madurai variety, which was found in quantity of 1.90 g/16 gN, and histidine was the lowest in Chennai variety, present in 3.12 g/16 gN. Conversely, according to Lalas et al. [14] the least abundant was cysteine which was present in 29.6 mg/100 g of dried leaves. In seeds, the most present amino acid was glutamic acid (14.23 g/16 gN in Madurai, 14.74 g/16 gN in Chennai), whereas the least abundant was threonine for Madurai variety (3.20 g/16 gN) and valine for Chennai variety (2.37 g/16 gN) [10,14]. Regarding glucosinolates, Lopez-Rodriguez et al. [15] have collected few interesting studies that performed extractions with 70% and 80% methanol, and by using HPLC and UPLC they found different isomers of glucomoringin (4-[(α-L-rhamnosyloxy)-benzyl]-glucosinolate) (Figure 2(1)) in M. oleifera leaves and seeds [15]. These compounds belong to the class of the glucosinolates, natural compounds synthesized by plants responsible for pungent flavor and smell. Their basic structure is formed by a sugar (thioglucose group), bound to a central carbon atom, and the carbon is then bound to a side chain, a nitrogen and a sulphate group, derived from an amino acid. The side chain varies depending on the glucosinolate, determining their biological activity. The main role of glucosinolates is plant defense against infections and diseases. There can be three types of glucosinolates depending on the amino acid that participates to the synthesis of them: aliphatic, aromatic or indolic glucosinolates. Aromatic glucosinolates seems to be the main ones that can be found in M. oleifera leaf extracts [15]. Moreover, glucosinolates are precursors of isothiocyanates, molecules containing the -N=C=S group that have showed biological activity in vitro, in particular, they showed hypoglycemic effects, antioxidant and anti-inflammatory effects, anticancer and chemopreventive effects, and it seems that isothiocyanates from M. oleifera are more stable than the ones found in other crucifers, because they contain rhamnose, which makes the structure more stable [15]. The review written by Leone et al. [16] shows a series of tables with many phenolic acids, molecules that belong to the class of polyphenols. They are formed by a monohydroxybenzoic acid unit and different side groups bound the phenolic ring depending on the compound and their importance is due to the documented antioxidant, anti-inflammatory, antimutagenic and anticancer activities [16]. Phenolic acids were extracted with polar solvents, using different percentages of methanol, water, ethanol, and analyzed via HPLC and LC-MS. The major compounds belonging to this category were caffeic acid, chlorogenic acid (Figure 2(2)), o-coumaric acid (Figure 2(3)), ellagic acid, ferulic acid and gallic acid, all of them present in quantities between 0.018 mg/g of dried leaves (ellagic acid) and 6.457 mg/g of dried leaves (coumaric acid). More polyphenols are listed in the following tables of the same study. The main polyphenols found are epicatechin (Figure 2(4)), kaempferol (Figure 2(5)), myricetin, quercetin (Figure 2(6)). Myricetin, quercetin and kaempferol are three natural polyphenols that belong to the class of flavonoids, molecules that have a common 15-carbon backbone made by two phenyl rings and a heterocyclic ring between them. The difference between these three molecules lies in the different numbers of hydroxyl groups bound to the basic structure, and thus they exert similar effects on human health. Previous studies listed in the review of Salvamani et al. have shown that quercetin could have several beneficial effects on human health, such as vasorelaxation to lower blood pressure, positive effects on dyslipidemia, on obesity by reducing accumulation of fats and promoting beta oxidation, anti-inflammatory, anti-atherogenic and anti-atherosclerotic effects in in vivo experiments [20]. Antimicrobial activity of quercetin was also reported. In a recent work, Montone et al. [21] demonstrated the importance of quercetin combined with lactoferrin and hydroxyapatite (LF-HA)—whose beneficial biological effects were widely investigated [22,23,24]—as antimicrobial agent in food industry. Kaempferol shows a similar impact on health, being a powerful agent against cardiovascular diseases endothelial damage, oxidative stress and against arteriosclerosis. Myricetin effects are explained by the same group, who listed several beneficial effects of this flavonoid, such as antioxidant, antiviral, anticarcinogenic, antiplatelet, hypoglycemic, and cytoprotective properties. Similarly to quercetin, it also showed positive effects on hypertension and accumulation of fats in the body of laboratory rats [20]. Quercetin was the most abundant flavonoid in M. oleifera extracts. The highest quantity of quercetin found among the studies is 16.64 mg/g of dried weight (dw), whereas for myricetin is 5.804 mg/g dw, and for kaempferol is 4.59 mg/g dw. The last relevant molecules considered in Leone et al. [16] are the glucosinolates, in particular they listed 4-(α-L-acetylrhamnopyranosyloxy)-benzyl-isothiocyanate (commonly known as moringin) (Figure 2(7)) and three isomers of 4-O-(α-Lacetylrhamnopyranosyloxy)-benzyl-isothiocyanate. Totally, they ranged from 21.84 mg/g dw to 59.4 mg/g dw among the two works considered in the above-mentioned review [16]. Moreover, some studies have reported cancer chemoprotective activity, allelopathic activity and repellent/insecticidal activity [25].
Finally, Bhalla et al. [13] conducted identification on two different extracts of M. oleifera. They found out that carbonic acid, butyl 2-pentyl ester (20.64%), 2-Isopropoxyethyl propionate (16.87%), 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (8.99%), 1,3-Dioxolan-2-one, 4,5-dimethyl- (6.16%) were the compounds present in highest peak area in the aqueous extract. A methanolic extraction was performed as well, and because of the different polarity, more compounds were identified in the methanolic extraction compared to the aqueous. In methanolic extracts, 1,3-propanediol, 2-ethyl-2-(hydroxymethyl)- (21.19%), propionic acid, 2-methyl-, octyl ester (15.02%), ethanamine, N-ethyl-N-nitroso- (5.21%), and 9,12,15-octadecatrienoic acid were the most abundant. These extracts were also tested to examine their antioxidant activity on free radicals, with the methanolic extract showing a higher capacity of scavenging free radicals than the aqueous extracts [13]. As stated by the authors, this is probably due to higher polyphenolic molecules found in methanolic extract. A high antioxidant activity can be useful because of a high request both by food and cosmetic industry, as they are used for skin treatment and for food enrichment.
4. Pharmacology
The pharmacological studies on M. oleifera are listed in Table 2. Different plant organs were extracted with a series of organic solvents and the obtained extracts were tested to determine and confirm the bioactivity known by the ethnomedicine. Pure compounds were also tested and showed interesting bioactivities (Table 2 and Figure 2).

Table 2.
Pharmacological activity of organic compounds or extracts of Moringa oleifera.
A recent review by Vergara-Jimenez et al. [25] reports the major studies dealing with the use of M. oleifera in prevention and alleviation of several chronic conditions, including hypercholesterolemia, high blood pressure, diabetes, insulin resistance, non-alcoholic liver disease, cancer, and overall inflammation.
4.1. Leaves: Cardiovascular Activity
Going into details, plant leaves were extracted mainly with hydroalcoholic solvents by different research groups and the extract were tested to evaluate their potential in cardiovascular diseases. Panda et al. [38] isolated from the extracts the alkaloid N, α-L-rhamnosyl vincosamide (Figure 2(8)) and studied the protective effect in isoproterenol (ISO)-induced cardiac toxicity in rats. Oral administration of the alkaloid at 40 mg/kg for seven days markedly reduced the ISO-induced increase in the levels of serum cardiac markers such as troponin-T, creatine kinase-MB, lactate dehydrogenase, and glutamate pyruvate transaminase, as well as cardiac lipid peroxidation. A parallel increase in the cellular antioxidants suggested its cardio-protective and free radical scavenging potential, which was later confirmed by in vitro study [38]. Rats treated with test compound also improved the ISO-induced abnormal changes in ECG, as well as in cardiac histology. A reduction in myocardial necrosis was further evidenced by the tri-phenyl tetrazolium chloride (TTC) stain in isolated test drug pre-treated rats. The work of Halaby et al. [34] aimed to study the potential effect of fortified bread with M. oleifera leaves powder under 5%, 10%, and 15% concentrations to give more protection against hyperlipidemia. Thirty-two male albino rats were used in this experiment. Results indicated that bread fortified with the extract at 10% and even better at 15% when given to rats for 45 days caused reduction of total cholesterol, triglycerides, low-density lipoprotein, and very-low-density lipoprotein. Kidney function was improved and there was significant reduction in uric acid serum, urea, and creatinine compared to the positive control group [34]. The study of Adisakwattana et al. [42] investigated the effect of leaf water extract of M. oleifera on inhibition of alpha-glucosidase and pancreatic alpha-amylase related to diabetes mellitus. Moreover, the study also determined in vitro bile acid binding capacity as well as inhibition of cholesterol micellization, pancreatic lipase, and cholesterol esterase activity. Specific inhibition to intestinal sucrase was found (IC50 value of 0.78 ± 0.21 mg/mL) along with a markedly inhibition (40.22% ± 2.64%) of cholesterol micelle formation [42].
4.2. Leaves: Anti-Inflammatory Activity
Kooltheat et al. [30] prepared an ethyl acetate fraction of M. oleifera from fresh leaves that showed high levels of phenolic and antioxidant activities. Since macrophages, TNF and related cytokines play an important pathophysiologic role in lung damage induced by cigarette smoke, the authors examined the effects of MOEF on cigarette smoke extract-induced cytokine production by human macrophages. Human monocyte derived macrophages (MDM) pre-treated with varying concentrations of M. oleifera showed decreased production of TNF, IL-6, and IL-8 in response to LPS and cigarette smoke extract. The decrease was evident at both cytokine protein and mRNA levels. Furthermore, the extract inhibited the expression of RelA, a gene implicated in the NF-κB p65 signaling in inflammation. The findings highlight the ability of M. oleifera to inhibit cytokines (IL-8) which promote the infiltration of neutrophils into the lungs and others (TNF, IL-6) which mediate tissue disease and damage [30].
4.3. Leaves: Antihypertensive Activity
Dangi et al. [39] studied the antihypertensive activity of the leaves extract. Preliminary studies indicated that a leaves water extract is efficacious in reducing the chronotropic and inotropic effects on the isolated frog heart. The alkaloids obtained by extract fractionation converted into their salt form, were tested for their activity on the isolated frog heart. The total alkaloidal salts were found to have a negative inotropic effect on the frog heart [39]. This activity was further characterized by test on the isolated guinea pig ileum. In their study Bais et al. [37] evaluated the anti-obesity activity of methanolic leaves extract in rats. Chronic administration of high fat diet (HFD) in rats produced hypercholesterolemia leading to an increase in the body weight total cholesterol, triglycerides, and attenuation in the levels of HDL. Treatment of obese rats with the extract for 49 days resulted in a significant decrease in the level of liver biomarkers, organ weight, and blood glucose level [37].
4.4. Leaves: Radical Scavenging and Antioxidant Activity
Siddhuraju et al. [27] studied the radical scavenging capacities and antioxidant activities. All leaf extracts were capable of scavenging peroxyl and superoxyl radicals. Among the three different moringa samples, both methanol and ethanol extracts of Indian origins showed the highest antioxidant activities, 65.1% and 66.8%, respectively, in the beta-carotene-linoleic acid system. Nonetheless, increasing concentration of all the extracts had significantly (p < 0.05) increased reducing power, which may in part be responsible for their antioxidant activity. The major bioactive compounds of phenolics were found to be flavonoid groups such as quercetin and kaempferol [39]. After this paper several other authors reported studied on the antioxidant activity of the leaves extract [25,29]. Xu et al. [28] correlated the antioxidant activity to some flavonoid glycosides (Figure 2(9–11)) while Wahyuni et al. [41] to the tannin content [28,41]. This last paper reported a new approach based on HPLC, tandem MS spectrometry (MS-MS), and chemometrics tallowing to correlate specific flavonoid glycosides in the extract with the antioxidant activity tested. Tiloke et al. [40] studied the antioxidant potential of the extract by the synthesis of phytonanoparticles and the fundamental role as a potential antiproliferative agents against cancer. The resultant gold phytonanoparticles are useful in cancer therapies with improved survival rates and quality of life.
4.5. Leaves: Anticancer Activity
Khalafalla et al. [31] tested different leaves extracts against leukemia and hepatocarcinoma cells in vitro. The extracts could kill majority (70–86%) of the abnormal cells among primary cells harvested from 10 patients with acute lymphoblastic leukemia and 15 with acute myeloid leukemia, as well as a culture of hepatocarcinoma cells (75% death), but most significantly by the hot water and ethanol extracts.
4.6. Leaves: Hepatoprotective and Nutraceutical Activity
Consumption of high-fat diet (HFD) induces nonalcoholic fatty liver disease) and may lead to multiple complications affecting human health. In the study of Das et al. [32] the preventive as also curative hepatoprotective activity effect of M. oleifera leaf extract in alleviating HFD induced liver injury in mice has been reported. Results suggested that M. oleifera leaf extract treatment protected HFD-induced liver damage as indicated by histopathology and liver enzyme activity compared to only-HFD fed group (p < 0.05). Interestingly, early signs of HFD-induced fatty liver were also alleviated by M. oleifera leaf extract. Moreover, significant increase in endogenous antioxidant parameters and lower lipid peroxidation were found in liver of all M. oleifera leaf extract treated groups [32].
Almatrafi et al. [33,35] studied the mechanisms by which M. oleifera leaves modulate hepatic lipids on guinea pigs. Low moringa or 15% high moringa diets with 0.25% dietary cholesterol to induce hepatic steatosis. This study demonstrates that M. oleifera leaves may prevent hepatic steatosis by affecting gene expression related to hepatic lipids synthesis resulting in lower concentrations of cholesterol and triglycerides and reduced inflammation in the liver.
Richter et al. [36] evaluated the suitability of freeze-dried moringa leaf meal as alternative protein source for Nile tilapia. Three experimental diets were formulated substituting 10%, 20%, and 30% of the total dietary protein of fish with plant leaves. Diets with higher % of moringa leaves significantly depressed growth performance of the fish compared the other diets. Total phenolics, non-hemolytic saponins, phytic acid, neutral detergent fiber, and acid detergent fiber in the 30% diet may have contributed to the poorer growth performance in these groups thus suggesting the 10% substituting the best option.
4.7. Leaves: Antimicrobial Activity
Oluduro et al. [26] investigated the antimicrobial activities of the leaf extract of on a series of enteropathogenic and orthopaedics’ wounds bacteria and fungi using paper disc diffusion method. All the leaf extracts showed little inhibitory effect on the enteropathogens, whereas aqueous and methanolic extracts showed appreciable inhibitory effects on the orthopaedic’s wounds bacteria at 30 mg/mL. Ethanolic extract did not show any zone of growth inhibition on the wound bacteria. Minimum inhibitory concentration was 20 mg/mL on all the enteropathogens and ranged from 3.75 to 30 µg/mL on the orthopaedics’ wounds organisms. The study showed that leaves possess inhibitory properties thus can serve as an alternative therapy for wounds and certain fungal infections.
4.8. Leaves, Stems, Pods: Anti-Allergic Activity
The anti-allergic activity of the extracts of leaves, seeds, and pods and of the isolated compounds (β-sitosterol-3-O-glucoside (Figure 2(12)), glucomoringin and quercitin) was evaluated by Abd Rani et al. [47] using rat basophilic leukaemia (RBL-2H3) cells for early and late phases of allergic reactions using as positive control the drug, ketotifen fumarate. The early phase was determined based on the inhibition of beta-hexosaminidase and histamine release, while the late phase was based on the inhibition of interleukin (IL-4) and tumor necrosis factor (TNF-α) release. Both extract and pure compounds showed inhibitions (Table 2) thus indicating a potential use as anti-allergic drug candidate.
4.9. Seeds: Anti-Inflammatory Activity
Wolff et al. [48] reported from the ethanolic seed extract high concentrations of a glucosinolate with isothiocyanate functional group (MIC-1, 1) a compound possessing anti-inflammatory and antidiabetic properties. Because it was not characterized metabolically, the authors studied its bioaccessibility using a human intestinal model and bioavailability using serum from treated rats. The results suggest that the compound remains largely unmodified during uptake, unlike other isothiocyanates, and has favorable bioaccessibility and bioavailability characteristics for a potential therapeutic agent.
The extracts of moringa seeds are used in rural areas of developing countries to treat drinking water because the seeds contain lectins, carbohydrate-binding proteins able to reduce water turbidity because of their coagulant activity. The study by Araújo et al. [43] evaluated the cytotoxic and anti-inflammatory properties of the aqueous seed extract on NCI-H292, HT-29, and HEp-2 cancer cell lines, and on murine erythrocytes (Table 2). In particular, the extracts exhibited anti-inflammatory activity on lipopolysaccharide-stimulated murine macrophages by regulating the production of nitric oxide, TNF-α and IL-1β. The aqueous seed extract reduced leukocyte migration in a mouse model of carrageenan-induced pleurisy; the myeloperoxidase activity and nitric oxide, TNF-α and IL-1β levels were similarly reduced. Histological analysis of the lungs showed that the extract reduced the number of leukocytes.
4.10. Seeds: Antiviral Activity
Xiong et al. [44] isolated eleven compounds from methanol seeds extract, including two previously unknown and nine known compounds. These compounds were authenticated as a carbamate, three phenylglycosides, four phenol glycosides, two nucleosides, and one flavonoid. Antivirus activity analyses revealed that Moringa A, glucomoringin, and Vitexin (Figure 2(13)) possessed strong inhibitory effects against the H1N1 virus, having IC50 values in the range of IC50 = 0.26 ± 0.03, 0.98 ± 0.17, and 3.42 ± 0.37 μg/mL, respectively. Furthermore, these three compounds could decrease the levels of TNF-α, IL-6, and IL-1β, which occur in hosts because of H1N1 infections.
4.11. Seeds: Anticancer Activity
Five-Fluorouracil (5-FU) is a strong anticancer agent commonly used for the treatment of various malignancies. Famurewa et al. [49] explored whether moringa seed oil could protect against 5-FU-induced nephrotoxicity and its mechanism of action in Wistar rats. Rats were subjected to prophylactic oral treatment of moringa seed oil (Table 2). The data obtained suggested that 5-FU-induced nephrotoxicity by oxidative stress, exacerbation of pro-inflammation and apoptosis. The inhibition of the alterations by moringa seed oil is relevant in the clinical management of 5-FU nephrotoxicity in cancer patients.
4.12. Seeds: Antioxidant Activity
Phenolic compounds, antioxidant and antibacterial activities of defatted seed flour were investigated by Singh et al. [45]. The results showed that extractability of phenolic compounds was significantly higher (p < 0.05) in bound phenolic extract (4173.00 ± 32.22 mg gallic acid equivalents (GAE)/100 g) than in free phenolic extract (780.00 ± 14.2 mg GAE/100 g) and it showed higher antioxidant and antimicrobial activities. The IC50 value for DPPH radical scavenging activity was 0.9 ± 0.05 and 14.9 ± 0.07 mg/mL for bound phenolic and free phenolic extracts, respectively. Bound phenolic extract was more effective (minimum inhibitory concentration (MIC), 0.06–0.157%) than free phenolic extract (MIC, 0.117–0.191%) against tested bacteria. The data indicated that moringa seeds could be a good source of antioxidants and antibacterials for food and pharmaceutical industries.
Finally, Liao et al. [46] identified β-sitosterol isolated from M. oleifera stems as an anti-inflammatory compound on two cell lines, keratinocytes and macrophages induces by PGN, TNF-α, or LPS. β-sitosterol over a dose range of 7.5 to 30 μM, dispersed in the medium of the well as nanoparticles with diameters of 50 ± 5 nm, was able to suppress the secretion of the above-mentioned inflammatory factors.
5. Conclusions
M. oleifera is an ancient medicinal plant growing in a variety of environments and climates, able to resist harsh environments that recently received an increased interest in the food industry and as phytopharmaceuticals.
The phytochemical analysis of the plant’s organs identified metabolites belonging to different classes, including flavonoids, anthocyanins, isothiocyanates, anthraquinones, alkaloids, essential oils, tannic acid, saponins, steroids, terpenoids, and cardiac glycosides. Pharmacological studies confirmed the use of the plant as traditional medicine, showing bioactivity including hepatoprotective, antihypertensive, cholesterol-lowering, anti-urolithiasis, antifertility, antidiabetic, and antioxidant activity, nutraceutical and antimicrobial properties. In addition, most published papers reported biological studies on the plant extracts while only few studies tested pure compounds. Interestingly, these compounds belong to the class of phenolics, including phenylpropanoids, flavonoids, flavonoid O-glycosides, flavonoid C-glycosides, and glucosinolates. Thus, the reported paper reviewing the literature on the phytochemistry and pharmacology of the plant indicates that more research is needed to investigate the chemical and biological properties on a larger scale and to clarify the mechanism of action. This aspect will be crucial for its use in phytopharmaceutical, nutraceutical, and food industries.
Author Contributions
Conceptualization, A.A., M.A., M.P., L.G., M.S., R.C., V.L.; data curation, A.A., M.A., M.P., L.G.; writing—original draft preparation, A.A., M.A., M.P., V.L.; writing—review and editing, A.A., M.S., R.C., V.L.; supervision, R.C., V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olson, M.E.; Fahey, J.W. Moringa oleifera: Un árbol multiusos para las zonas tropicales secas. Rev. Mex. Biodivers 2011, 82, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.E. Moringaceae. In Flora of North America North of Mexico. Flora N. Am. Assoc. 2010, 7, 167–169. [Google Scholar]
- Yadava, U.L. Exotic horticultural plants with commercial potential in the UnitedStates market: Introduction to the Workshop. HortScience 1996, 31, 764–765. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Y.; Wu, J. Yield and quality of Moringa oleifera under different planting densities and cutting heights in southwest China. Ind. Crops Prod. 2016, 91, 88–96. [Google Scholar] [CrossRef]
- Alli, E.; And, R.; Arumugam, T. Moringa oleifera (Lam)-A nutritional powerhouse. J. Crop Weed 2017, 13, 238–246. [Google Scholar]
- Fahey, J.W. Moringa oleifera: A review of the medicinal potential. Acta Hortic. 2017, 1158, 209–224. [Google Scholar] [CrossRef]
- Renityas, N.N. The Effectiveness of Moringa Leaves Extract and Cancunpoint Massage Towards Breast Milk Volume on Breastfeeding Mothers. J. Ners Kebidanan (J. Ners Midwifery) 2018, 5, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.R. Microbiological monitoring of laboratory mice. Genet. Eng. Mice Handb. 2016, 157–164. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Chelliah, R.; Ramakrishnan, S.; Antony, U. Nutritional quality of Moringa oleifera for its bioactivity and antibacterial properties. Int. Food Res. J. 2017, 24, 825–833. [Google Scholar]
- Chuang, P.H.; Lee, C.W.; Chou, J.Y.; Murugan, M.; Shieh, B.J.; Chen, H.M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013, 118, 497–502. [Google Scholar] [CrossRef]
- Bhalla, N.; Ingle, N.; Patri, S.V.; Haranath, D. Phytochemical analysis of Moringa oleifera leaves extracts by GC-MS and free radical scavenging potency for industrial applications. Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Karageorgou, I.; Batra, G.; Nanos, G.D.; Makris, D.P. Nutritional Characterization of Leaves and Herbal Tea of Moringa oleifera Cultivated in Greece. J. Herbs Spices Med. Plants 2017, 23, 320–333. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and Isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef]
- Balamurugan, R.; Stalin, A.; Ignacimuthu, S. Molecular docking of γ-sitosterol with some targets related to diabetes. Eur. J. Med. Chem. 2012, 47, 38–43. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, N.; Fulgione, A.; Iannaccone, M.; Tomasetta, L.; Ianniello, F.; Martora, F.; Lelli, M.; Roveri, N.; Capuano, F.; Capparelli, R. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int. J. Nanomed. 2014, 9, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, P.; Papaianni, M.; Fulgione, A.; Guerra, F.; Capparelli, R.; Medaglia, C. An innovative approach to control H. Pylori-induced persistent inflammation and colonization. Microorganisms 2020, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin adsorbed onto biomimetic hydroxyapatite nanocrystals controlling—In vivo—The Helicobacter pylori infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Oluduro, A.O. Evaluation of Antimicrobial properties and nutritional potentials of Moringa oleifera Lam.leaf in South-Western Nigeria. Malays. J. Microbiol. 2012, 8, 59–67. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, G.; Guo, M. Correlations between phytochemical fingerprints of Moringa oleifera leaf extracts and their antioxidant activities revealed by chemometric analysis. Phytochem. Anal. 2021, 32, 698–709. [Google Scholar] [CrossRef]
- Hossain, M.A.; Disha, N.K.; Shourove, J.H.; Dey, P. Determination of Antioxidant Activity and Total Tannin from Drumstick (Moringa oleifera Lam.) Leaves Using Different Solvent Extraction Methods. Turk. J. Agric.—Food Sci. Technol. 2020, 8, 2749–2755. [Google Scholar] [CrossRef]
- Kooltheat, N.; Pankla Sranujit, R.; Chumark, P.; Potup, P.; Laytragoon-Lewin, N.; Usuwanthim, K. An ethyl acetate fraction of Moringa oleifera Lam. inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients 2014, 6, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Khalafalla, M.M.; Abdellatef, E.; Dafalla, H.M.; Nassrallah, A.A.; Aboul-Enein, K.M.; Lightfoot, D.A.; El-Deeb, F.E.; El-Shemy, H.A. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr. J. Biotechnol. 2010, 9, 8467–8471. [Google Scholar] [CrossRef]
- Das, N.; Sikder, K.; Ghosh, S.; Fromenty, B.; Dey, S. Moringa oleifera lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J. Exp. Biol. 2012, 50, 404–412. [Google Scholar]
- Almatrafi, M.M.; Vergara-Jimenez, M.; Smyth, J.A.; Medina-Vera, I.; Fernandez, M.L. Moringa oleifera leaves do not alter adi-pose tissue colesterol accumulation or inflammation in guinea pigs fed a hypercholesterolemic diet. EC Nutr. 2017, 18, 1330. [Google Scholar]
- Halaby, M.S.; Elmetwaly, E.M.; Omar, A.A.A. Effect of Moringa oleifera on serum lipids and kidney function of hyperlipidemic rats. J. Appl. Sci. Res. 2013, 9, 5189–5198. [Google Scholar]
- Almatrafi, M.M.; Vergara-Jimenez, M.; Murillo, A.G.; Norris, G.H.; Blesso, C.N.; Fernandez, M.L. Moringa leaves prevent hepatic lipid accumulation and inflammation in guinea pigs by reducing the expression of genes involved in lipid metabolism. Int. J. Mol. Sci. 2017, 18, 1330. [Google Scholar] [CrossRef] [PubMed]
- Richter, N.; Siddhuraju, P.; Becker, K. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.). Aquaculture 2003, 217, 599–611. [Google Scholar] [CrossRef]
- Bais, S.; Singh, G.S.; Sharma, R. Antiobesity and Hypolipidemic Activity of Moringa oleifera Leaves against High Fat Diet-Induced Obesity in Rats. Adv. Biol. 2014, 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In Vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Dangi, S.Y.; Jolly, C.I.; Narayanan, S. Antihypertensive activity of the total alkaloids from the leaves of Moringa oleifera. Pharm. Biol. 2002, 40, 144–148. [Google Scholar] [CrossRef]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharmacother. 2018, 108, 457–466. [Google Scholar] [CrossRef]
- Wahyuni, R.; Wignyanto, W.; Wijana, S.; Sucipto, S. Optimization of protein and tannin extraction in Moringa oleifera leaf as antioxidant source. Food Res. 2020, 4, 2224–2232. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chanathong, B. A-Glucosidase Inhibitory Activity and Lipid-Lowering. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 803–808. [Google Scholar]
- Araújo, L.C.C.; Aguiar, J.S.; Napoleão, T.H.; Mota, F.V.B.; Barros, A.L.S.; Moura, M.C.; Coriolano, M.C.; Coelho, L.C.B.B.; Silva, T.G.; Paiva, P.M.G. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS ONE 2013, 8, e81973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Riaz Rajoka, M.S.; Zhang, M.X.; He, Z. Isolation and identification of two new compounds from the seeds of Moringa oleifera and their antiviral and anti-inflammatory activities. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Govardhan Singh, R.S.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K.P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.Z.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In Vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wolff, K.; Jaja-Chimedza, A.; Kim, Y.; Waterman, C.; Poulev, A.; Raskin, I.; Ribnicky, D. Moringa isothiocyanate-1 is bioaccessible and bioavailable as a stable unmodified compound. Phytochem. Lett. 2020, 38, 33–38. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Asogwa, N.T.; Aja, P.M.; Akunna, G.G.; Awoke, J.N.; Ekeleme-Egedigwe, C.A.; Maduagwuna, E.K.; Folawiyo, A.M.; Besong, E.E.; Ekpono, E.U.; et al. Moringa oleifera seed oil modulates redox imbalance and iNOS/NF-ĸB/caspase-3 signaling pathway to exert antioxidant, anti-inflammatory and antiapoptotic mechanisms against anticancer drug 5-fluorouracil-induced nephrotoxicity in rats. S. Afr. J. Bot. 2019, 127, 96–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).