Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Banana Blossom Sampling and Postharvest Treatments
2.3. Browning Score
2.4. Color Assessment
2.5. Total Phenolic Compounds
2.6. DPPH Radical Scavenging Assay
2.7. Phenylalanine Ammonia Lyase (PAL) Activity Assay
2.8. Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity Assays and Their Optimal pH Condition for Their Activities
2.9. Inhibition of PPO and POD Activities Using Different Organic Acids as Inhibitors
2.10. Antibrowning of Banana Blossoms by Different Organic Acids
2.11. Determination of Weight Loss Rate
2.12. Statistical Analysis
3. Results
3.1. Browning of Banana Blossom Cut-End Surfaces
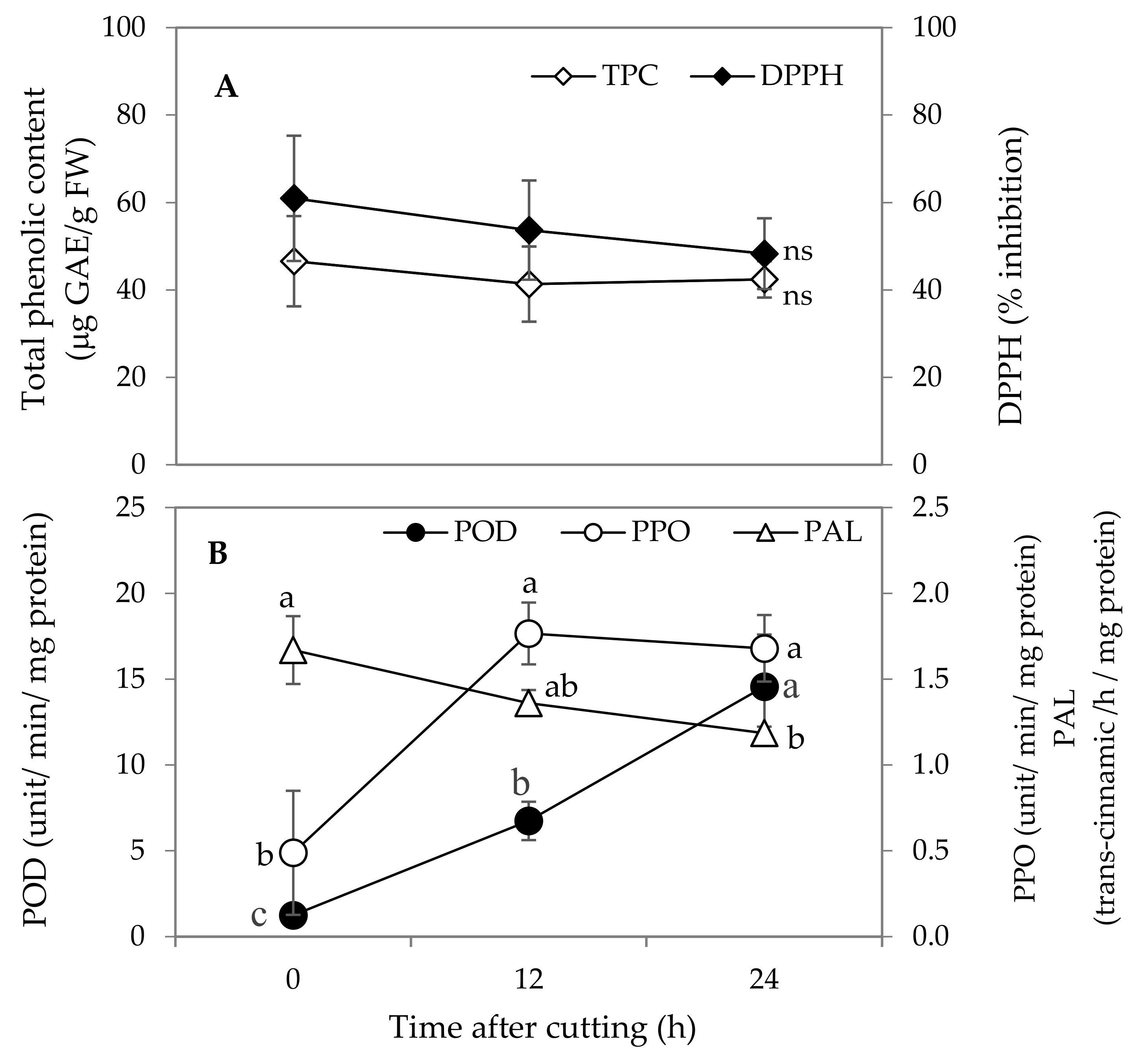
3.2. Total Phenolic Content (TPC) and DPPH
3.3. PAL, PPO and POD Activities
3.4. TPC, DPPH, PPO, POD and PAL Activities Related to Browning of Banana Blossoms after Cutting
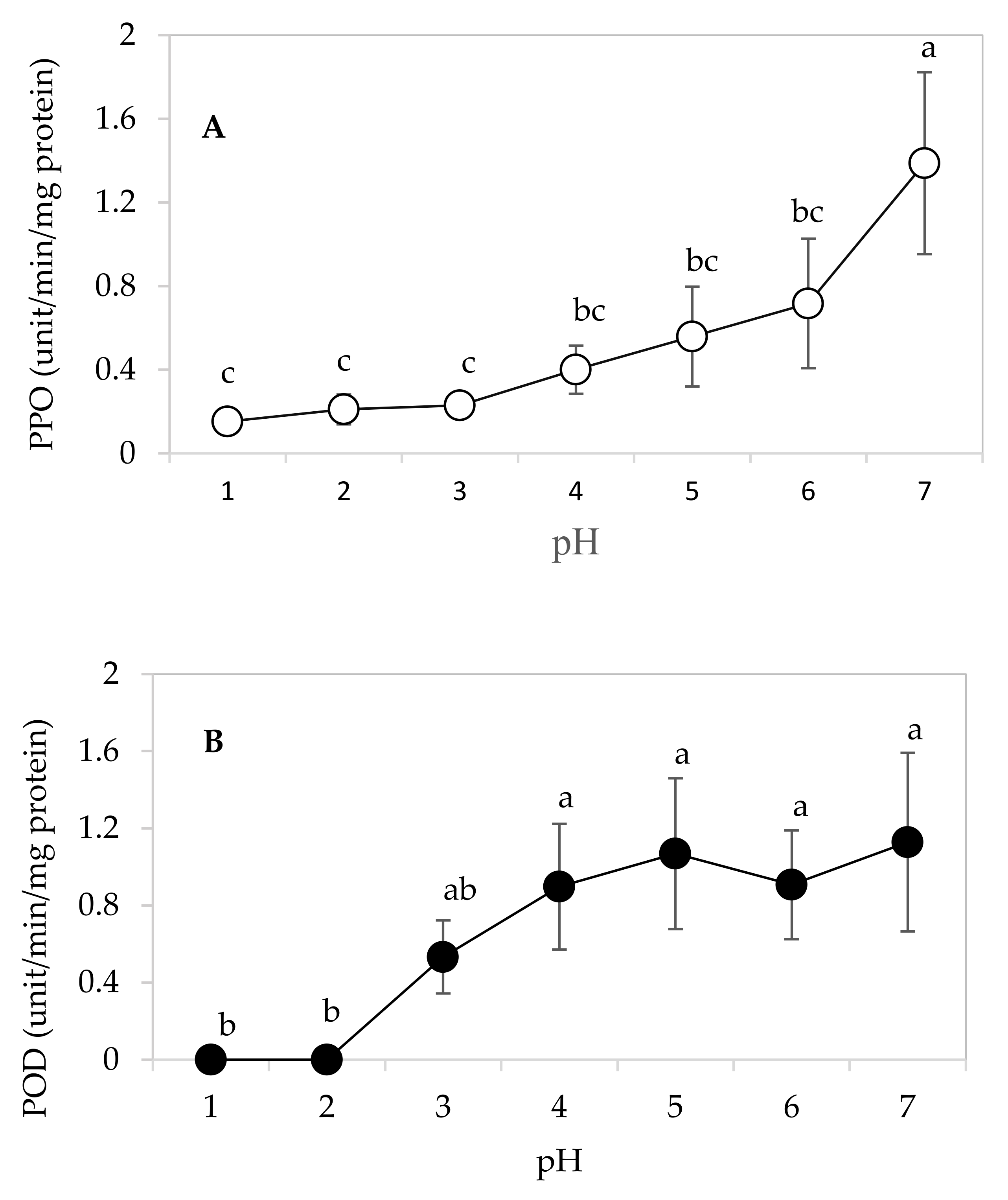
3.5. Optimal pH for PPO and POD Activities
3.6. Effects of Organic Acids as Inhibitors on PPO and POD Activities of Banana Blossoms
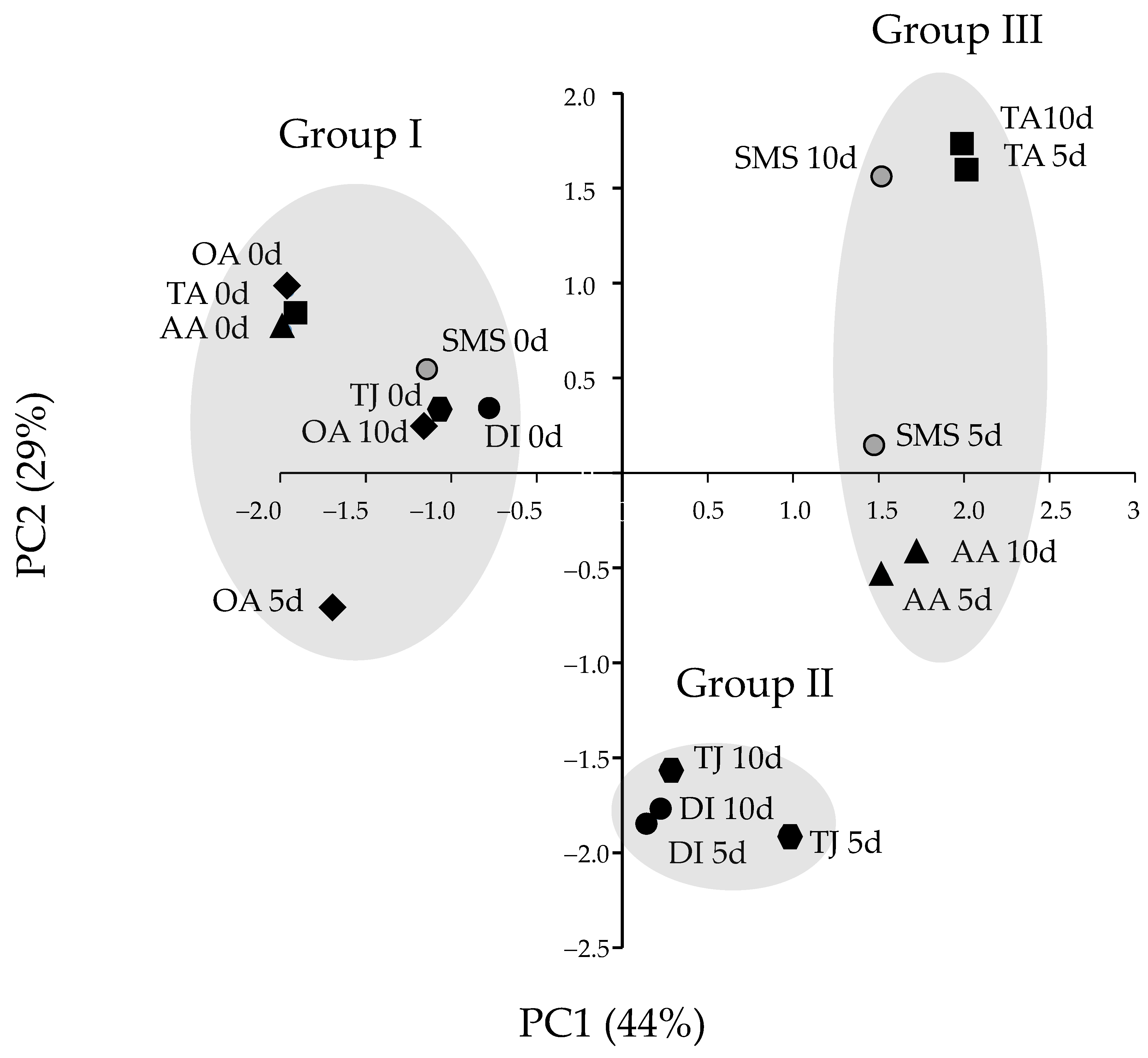
3.7. Effect of Antibrowning Agents on Browning and Weight Loss of Banana Blossoms
4. Discussion
4.1. Browning Mechanism at Cut-End Surface of Banana Blossoms
4.2. Oxalic Acid Treatment as the Most Effective Method to Prevent Browning after Cutting
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sheng, Z.W.; Ma, W.H.; Jin, Z.Q.; Bi, Y.; Sun, Z.G.; Dou, H.T.; Gao, J.H.; Li, J.Y.; Han, L.N. Investigation of dietary fiber, protein, vitamin E and other nutritional compounds of banana flower of two cultivars grown in China. Afr. J. Biotechnol. 2010, 9, 3888–3895. [Google Scholar] [CrossRef]
- Krishnan, A.; Sinija, V.R. Proximate Composition and Antioxidant Activity of Banana Blossom of Two Cultivars in India. Int. J. Agric. Food Sci. Technol. 2016, 7, 13–22. [Google Scholar]
- Anand, S.; Sharma, M. Product Development from Banana Blossom Powder and Indian Gooseberry Powder for Anaemic Adolescent Girls. Int. J. Health Sci. Res. 2019, 9, 273–278. [Google Scholar]
- Wahyuningsih, D.; Hidayat, S.T.; Khafidhoh, N.; Suwondo, A.; Fatmasari, D.; Susiloretni, K.A. Effect of Musa Balbisiana Colla Extract on Breast Milk Production in Breastfeeding Mothers. Belitung Nurs. J. 2017, 3, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, R.; Gunasegaram, S.; Visvanathan, R.; Jayathilake, C.; Weththasinghe, P.; Jayawardana, B.C.; Vidanarachchi, J.K. Banana Blossom (Musa acuminate Colla) Incorporated Experimental Diets Modulate Serum Cholesterol and Serum Glucose Level in Wistar Rats Fed with Cholesterol. Cholesterol 2016, 2016, 9747412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrill, A. Banana blossom: The next vegan food star with the texture of fish. The Guardian, 16 March 2019. [Google Scholar]
- Jiang, Y.; Duan, X.; Joyce, D.; Zhang, Z.; Li, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Vámos-Vigyázó, L.; Haard, N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Luo, Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Pardossi, A.; Tognoni, F.; Guidi, L. Physiological basis of sensitivity to enzymatic browning in “lettuce”, “escarole” and “rocket salad” when stored as fresh-cut products. Food Chem. 2007, 104, 209–215. [Google Scholar] [CrossRef]
- Li-Qin, Z.; Jie, Z.; Shu-Hua, Z.; Lai-Hui, G. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Hisaminato, H.; Murata, M.; Homma, S. Relationship between the Enzymatic Browning and Phenylalanine Ammonia-lyase Activity of Cut Lettuce, and the Prevention of Browning by Inhibitors of Polyphenol Biosynthesis. Biosci. Biotechnol. Biochem. 2001, 65, 1016–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of Polyphenol Oxidase and Peroxidase and Influence on Browning of Cold Stored Strawberry Fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zheng, Y.; Tang, S.; Rui, H.; Wang, C.Y. A combination of hot air and methyl jasmonate vapor treatment alleviates chilling injury of peach fruit. Postharvest Biol. Technol. 2009, 52, 24–29. [Google Scholar] [CrossRef]
- Oba, K.; Uritani, I.; Iwatsuki, N.; Alvarez, A.M.; Garcia, V.V. Partial Purification and Characterization of Polyphenol Oxidase Isozymes in Banana Bud. Biosci. Biotechnol. Biochem. 1992, 56, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Chomkitichai, W.; Chaiyakhan, Y.; Petphinit, N.; Pudpang, P.; Chomkitichai, W. Effect of Tamarind Juice on Browning Reduction of Fresh-cut Banana Blossom during Storage. Agric. Sci. J. 2018, 4, 91–94. (In Thai) [Google Scholar]
- Saideswara Rao, Y.; Mary Mathew, K. Tamarind. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 2, pp. 512–533. [Google Scholar] [CrossRef]
- Tongdee, S. Postharvest Handling and Technology of Tropical Fruit. Acta Hortic. 1992, 713–717. [Google Scholar] [CrossRef]
- Whangchai, K.; Saengnil, K.; Uthaibutra, J. Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Prot. 2006, 25, 821–825. [Google Scholar] [CrossRef]
- Venkatachalam, K. The Different Concentrations of Citric Acid on Inhibition of Longkong Pericarp Browning during Low Temperature Storage. Int. J. Fruit Sci. 2015, 15, 353–368. [Google Scholar] [CrossRef]
- Vasconcelos, C.M.; de Oliveira, E.B.; Rossi, S.N.; Arantes, L.F.; Puschmann, R.; Chaves, J.B.P. Evaluating Strategies to Control Enzymatic Browning of Minimally Processed Yacon (Smallanthus sonchifolius). Food Bioprocess Technol. 2015, 8, 1982–1994. [Google Scholar] [CrossRef]
- Huang, H.; Jian, Q.; Jiang, Y.; Duan, X.; Qu, H. Enhanced chilling tolerance of banana fruit treated with malic acid prior to low-temperature storage. Postharvest Biol. Technol. 2016, 111, 209–213. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C.; Wongs-Aree, C. Effect of Silver Particle-longkong Peel Extract Coating on Postharvest Decay and Browning in Longkong Fruit. Hortic. J. 2020, 89, 328–336. [Google Scholar] [CrossRef]
- Moline, H.E.; Buta, J.G.; Newman, I.M. Prevention of browning of banana slices using natural products and their derivatives. J. Food Qual. 1999, 22, 499–511. [Google Scholar] [CrossRef]
- Nkhata, S.G. Total color change (ΔE∗) is a poor estimator of total carotenoids lost during post-harvest storage of biofortified maize grains. Heliyon 2020, 6, e05173. [Google Scholar] [CrossRef] [PubMed]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Havananda, T.; Luengwilai, K. Variation in floral antioxidant activities and phytochemical properties among butterfly pea (Clitoria ternatea L.) germplasm. Genet. Resour. Crop. Evol. 2019, 66, 645–658. [Google Scholar] [CrossRef]
- Salar, R.K.; Sharma, P.; Purewal, S.S. In Vitro antioxidant and free radical scavenging activities of stem extract of Euphorbia trigona Miller. Tang Humanit. Med. 2015, 5, e14. [Google Scholar] [CrossRef] [Green Version]
- Faragher, J.; Chalmers, D. Regulation of Anthocyanin Synthesis in Apple Skin. III. Involvement of Phenylalanine Ammonia-lyase. Funct. Plant Biol. 1977, 4, 133–141. [Google Scholar] [CrossRef]
- Benjamin, N.D.; Montgomery, M.W. Polyphenol Oxidase of Royal Ann Cherries: Purification and Characterization. J. Food Sci. 1973, 38, 799–806. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, X. Characterisation of polyphenol oxidase and peroxidase and the role in browning of loquat fruit. Czech J. Food Sci. 2015, 33, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ahvenainen, R. New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends Food Sci. Technol. 1996, 7, 179–187. [Google Scholar] [CrossRef]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; van der Waals, J.E. Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Nishimura, M.; Murai, N.; Haruta, M.; Homma, S.; Itoh, Y. A Transgenic Apple Callus Showing Reduced Polyphenol Oxidase Activity and Lower Browning Potential. Biosci. Biotechnol. Biochem. 2001, 65, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Richard-Forget, F.C.; Gauillard, F.A. Oxidation of Chlorogenic Acid, Catechins, and 4-Methylcatechol in Model Solutions by Combinations of Pear (Pyrus communis Cv. Williams) Polyphenol Oxidase and Peroxidase: A Possible Involvement of Peroxidase in Enzymatic Browning. J. Agric. Food Chem. 1997, 45, 2472–2476. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Kinetic Study of Oxalic Acid Inhibition on Enzymatic Browning. J. Agric. Food Chem. 2000, 48, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Yoruk, R.; Marshall, M.R. A Survey on the Potential Mode of Inhibition for Oxalic Acid on Polyphenol Oxidase. J. Food Sci. 2003, 68, 2479–2485. [Google Scholar] [CrossRef]
- Prenen, J.A.; Boer, P.; Dorhout Mees, E.J. Absorption kinetics of oxalate from oxalate-rich food in man. Am. J. Clin. Nutr. 1984, 40, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Ioniţă, E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G.; Bahrim, G. pH-induced structural changes of tyrosinase from Agaricus bisporus using fluorescence and in silico methods. J. Sci. Food Agric. 2014, 94, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
| Storage Time (h) | L* | a* | b* | ∆E* | Browning Score | |
|---|---|---|---|---|---|---|
| 0 |  | 75.5 ± 0.6 a | −2.7 ± 0.2 b | 20.9 ± 1.7 NS | - | 1.2 ± 0.4 b |
| 12 |  | 55.7 ± 8.6 b | 4.2 ± 3.2 a | 22.9 ± 1.8 NS | 21.2 ± 2.8 NS | 4.2 ± 0.8 a |
| 24 |  | 55.9 ± 10.1 b | 4.8 ± 3.5 a | 23.1 ± 1.1 NS | 21.6 ± 3.2 NS | 4.3 ± 0.6 a |
| PAL | PPO | POD | L* Value | Browning Score | Phenolic Content | DPPH Assay | |
|---|---|---|---|---|---|---|---|
| PAL | 1.000 | ||||||
| PPO | −0.829 ** | 1.000 | |||||
| POD | −0.870 ** | 0.660 | 1.000 | ||||
| L* value | 0.826 ** | −0.957 ** | −0.752 * | 1.000 | |||
| Browning score | −0.840 ** | 0.954 ** | 0.782 * | −0.977 | 1.000 | ||
| Phenolic content | 0.430 | −0.547 | −0.187 | 0.317 | −0.402 | 1.000 | |
| DPPH assay | 0.638 | −0.590 | −0.481 | 0.423 | −0.532 | 0.923 ** | 1.000 |
| Organic Acid Agents | Organic Acid Concentration | pH | Inhibition of Enzyme Activity (%) | |
|---|---|---|---|---|
| PPO | POD | |||
| Control 1/ | 0% | 7.0 ± 0.0 | 0 ± 0 d | 0 ± 0 d |
| 5.0 ± 0.0 | 39 ± 2 c | 4 ± 7 d | ||
| 3.0 ± 0.0 | 82 ± 7 a | 52 ± 7 bc | ||
| 1.0 ± 0.0 | 88 ± 3 a | 100 ± 0 a | ||
| TA | 1% | 3.3 ± 0.4 b | 68 ± 8 ab | 25 ± 18 c |
| 3% | 2.5 ± 0.3 c | 86 ± 4 a | 77 ± 9 b | |
| 5% | 1.7 ± 0.6 c | 83 ± 3 a | 99 ± 2 a | |
| CA | 1% | 4.0 ± 0.0 b | 46 ± 11 b | 0 ± 0 d |
| 3% | 2.8 ± 0.0 c | 76 ± 8 a | 0 ± 0 d | |
| 5% | 2.5 ± 0.0 c | 83 ± 4 a | 75 ± 6 b | |
| MA | 1% | 3.6 ± 0.0 b | 79 ± 11 a | 0 ± 0 d |
| 3% | 2.8 ± 0.0 c | 79 ± 1 a | 36 ± 7 c | |
| 5% | 2.5 ± 0.0 c | 78 ± 3 a | 83 ± 2 b | |
| OA | 1% | 2.6 ± 0.0 c | 74 ± 14 a | 0 ± 0 d |
| 3% | 0.9 ± 0.3 d | 78 ± 10 a | 100 ± 0 a | |
| 5% | 0.7 ± 0.1 d | 90 ± 1 a | 100 ± 0 a | |
| AA | 1% | 5.6 ± 0.1 a | 83 ± 2 a | 0 ± 0 d |
| 3% | 2.7 ± 0.9 c | 86 ± 0 a | 100 ± 0 a | |
| 5% | 2.6 ± 0.8 c | 87 ± 7 a | 100 ± 0 a | |
| Inhibitor | L* | ||
|---|---|---|---|
| 0 Day | 5 Days | 10 Days | |
| DI water | 68.6 ± 4.6 B,a | 60.6 ± 5.8 B,b | 61.5 ± 5.5 BC,b |
| 2.5% TJ | 71.4 ± 3.0 B,a | 60.2 ± 3.3 B,b | 60.1 ± 2.5 BC,b |
| 1% SMS | 75.5 + 0.0 A,a | 54.3 + 6.8 C,b | 53.4 + 6.8 C,b |
| 5% TA | 76.9 ± 0.5 A,a | 55.4 ± 3.2 BC,b | 55.9 ± 3.4 BC,b |
| 3% AA | 77.7 ± 0.7 A,a | 59.6 ± 2.1 B,b | 58.5 ± 2.5 B,b |
| 3% OA | 77.3 ± 1.4 A,a | 66.8 ± 2.8 A,b | 66.4 ± 2.4 A,b |
| Inhibitor | a* | ||
| 0 Day | 5 Days | 10 Days | |
| DI water | −0.3 ± 1.9 AB,b | 4.9 ± 2.5 AB,a | 5.2 ± 2.4 AB,a |
| 2.5% TJ | −1.0 ± 1.7 AB,b | 5.3 ± 1.8 AB,a | 6.3 ± 1.6 AB,a |
| 1% SMS | 0.8 + 0.0 A,b | 7.6 + 1.4 A,a | 4.3 + 1.4 A,a |
| 5% TA | −3.4 ± 0.3 B,b | 5.2 ± 1.8 B,a | 5.0 ± 1.8 B,a |
| 3% AA | −3.5 ± 0.3 B,b | 6.3 ± 1.0 AB,a | 7.0 ± 0.5 AB,a |
| 3% OA | −3.4 ± 0.6 B,b | 0.0 ± 0.7 C,a | 0.0 ± 0.7 C,a |
| Inhibitor | ∆E* | ||
| 0 Day | 5 Days | 10 Days | |
| DI water | 12.4 ± 4.3 NS | 11.8 ± 1.0 NS | |
| 2.5% TJ | 13.8 ± 2.9 NS | 15.5 ± 3.5 NS | |
| 1% SMS | 23.4 ± 2.5 NS | 24.3 ± 5.5 NS | |
| 5% TA | 23.4 ± 2.6 NS | 22.9 ± 2.8 NS | |
| 3% AA | 22.4 ± 2.0 NS | 23.2 ± 2.3 NS | |
| 3% OA | 11.6 ± 2.2 NS | 12.0 ± 1.7 NS | |
| Inhibitor | Browning score | ||
| 0 Day | 5 Days | 10 Days | |
| DI water | 1.0 ± 0.0 A,b | 3.6 ± 0.9 B,a | 3.6 ± 0.9 B,a |
| 2.5% TJ | 1.0 ± 0.0 A,b | 3.4 ± 0.5 B,a | 3.4 ± 0.5 B,a |
| 1% SMS | 1.0 + 0.5 A,b | 2.8 + 0.8 B,a | 3.8 + 0.8 B,a |
| 5% TA | 1.0 ± 0.0 A,b | 4.8 ± 0.4 A,a | 5.0 ± 0.0 A,a |
| 3% AA | 1.0 ± 0.0 A,b | 3.8 ± 0.8 B,a | 3.8 ± 0.8 B,a |
| 3% OA | 1.0 ± 0.0 A,b | 2.8 ± 0.8 B,a | 3.8 ± 1.3 B,a |
| Inhibitor | Weight loss (% of initial FW) | ||
| 0 Day | 5 Days | 10 Days | |
| DI water | 0.3 ± 0.4 AB,a | 0.8 ± 0.9 A,a | |
| 2.5% TJ | 0.5 ± 0.3 A,a | 0.6 ± 0.3 A,a | |
| 1% SMS | 0.4 ± 0.2 A,a | 0.2 ± 0.0 AB,a | |
| 5% TA | 0.2 ± 0.1 AB,a | 0.3 ± 0.3 AB,a | |
| 3% AA | 0.3 ± 0.2 AB,a | 0.5 ± 0.2 A,a | |
| 3% OA | 0.2 ± 0.2 AB,a | 0.1 ± 0.1 AB,a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewjumpol, G.; Srisamlee, S.; Beckles, D.M.; Luengwilai, K. Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction. Horticulturae 2021, 7, 373. https://doi.org/10.3390/horticulturae7100373
Kaewjumpol G, Srisamlee S, Beckles DM, Luengwilai K. Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction. Horticulturae. 2021; 7(10):373. https://doi.org/10.3390/horticulturae7100373
Chicago/Turabian StyleKaewjumpol, Geerada, Surasak Srisamlee, Diane M. Beckles, and Kietsuda Luengwilai. 2021. "Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction" Horticulturae 7, no. 10: 373. https://doi.org/10.3390/horticulturae7100373
APA StyleKaewjumpol, G., Srisamlee, S., Beckles, D. M., & Luengwilai, K. (2021). Enzymatic Browning in Banana Blossoms and Techniques for Its Reduction. Horticulturae, 7(10), 373. https://doi.org/10.3390/horticulturae7100373







