Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Location
2.2. Genetic Material and Seedling Production
2.3. Crop Management
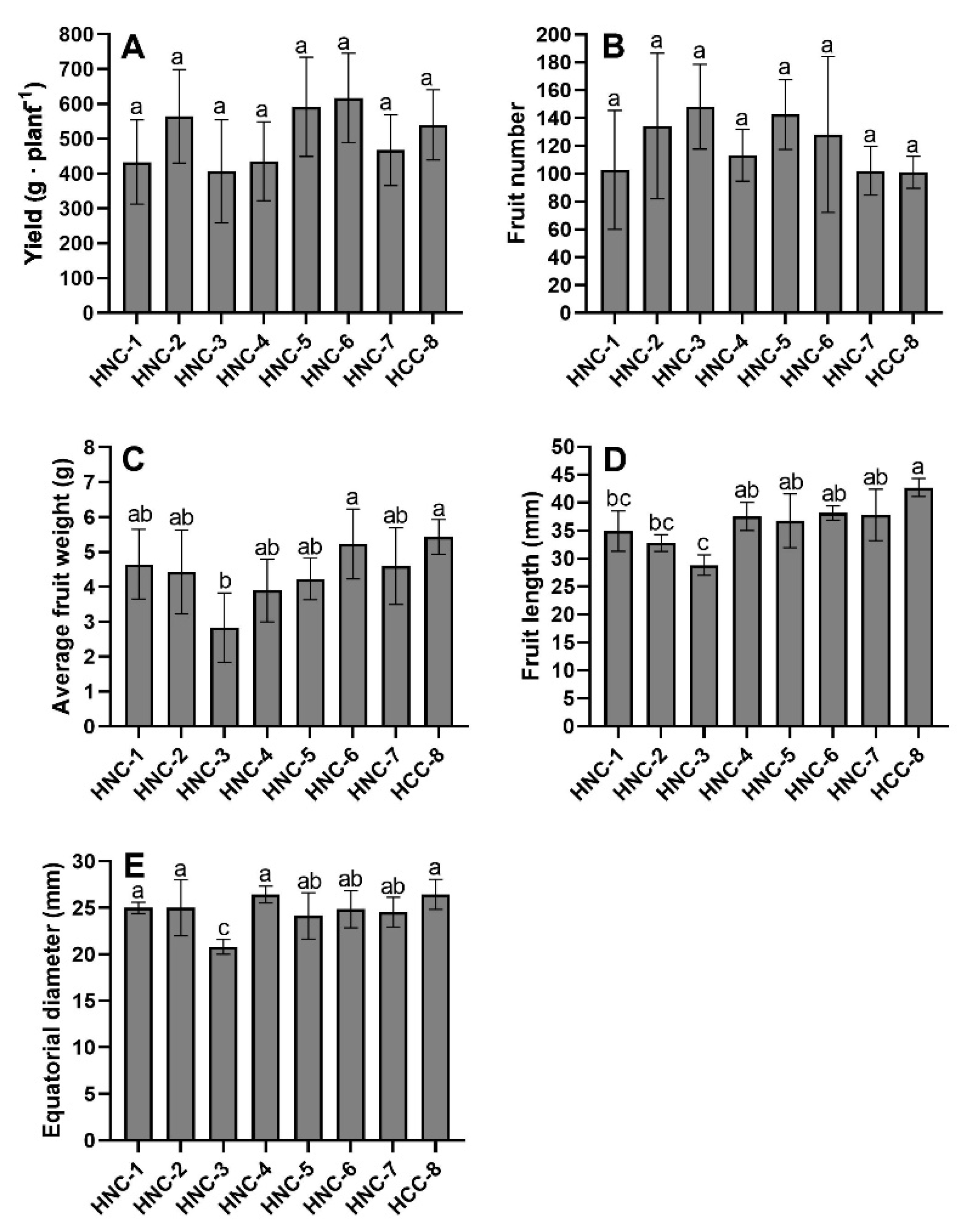
2.4. Fruit Yield Components
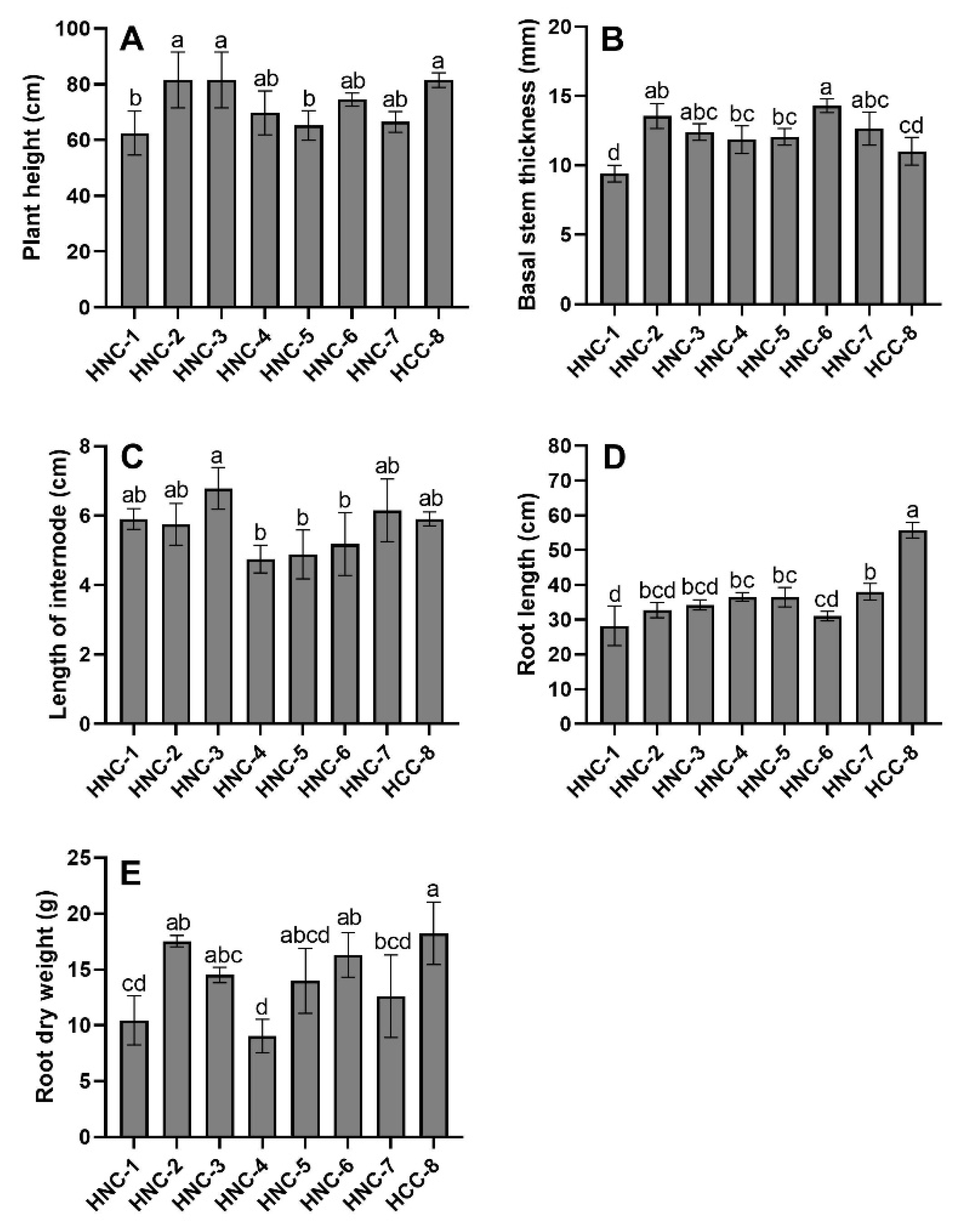
2.5. Agronomic Parameters
2.6. Physicochemical Analysis of Habanero Peppers
2.7. Fruit Color Tests
2.8. Functional Analysis
2.8.1. Capsaicinoid Extraction
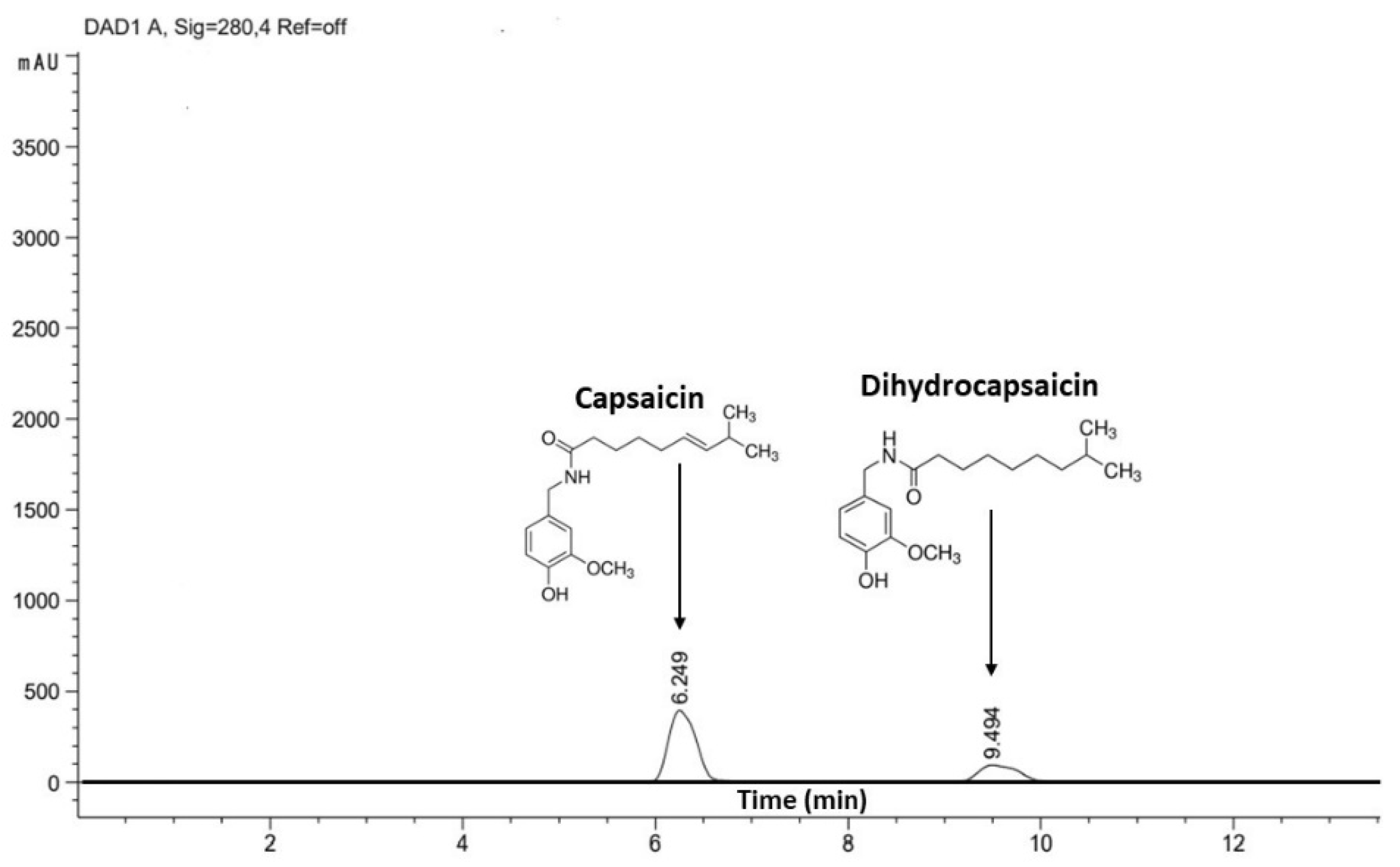
2.8.2. Quantification of Capsaicin and Dihydrocapsaicin by High Performance Liquid Chromatography (HPLC)
2.8.3. Determination of Scoville Units (SHU)
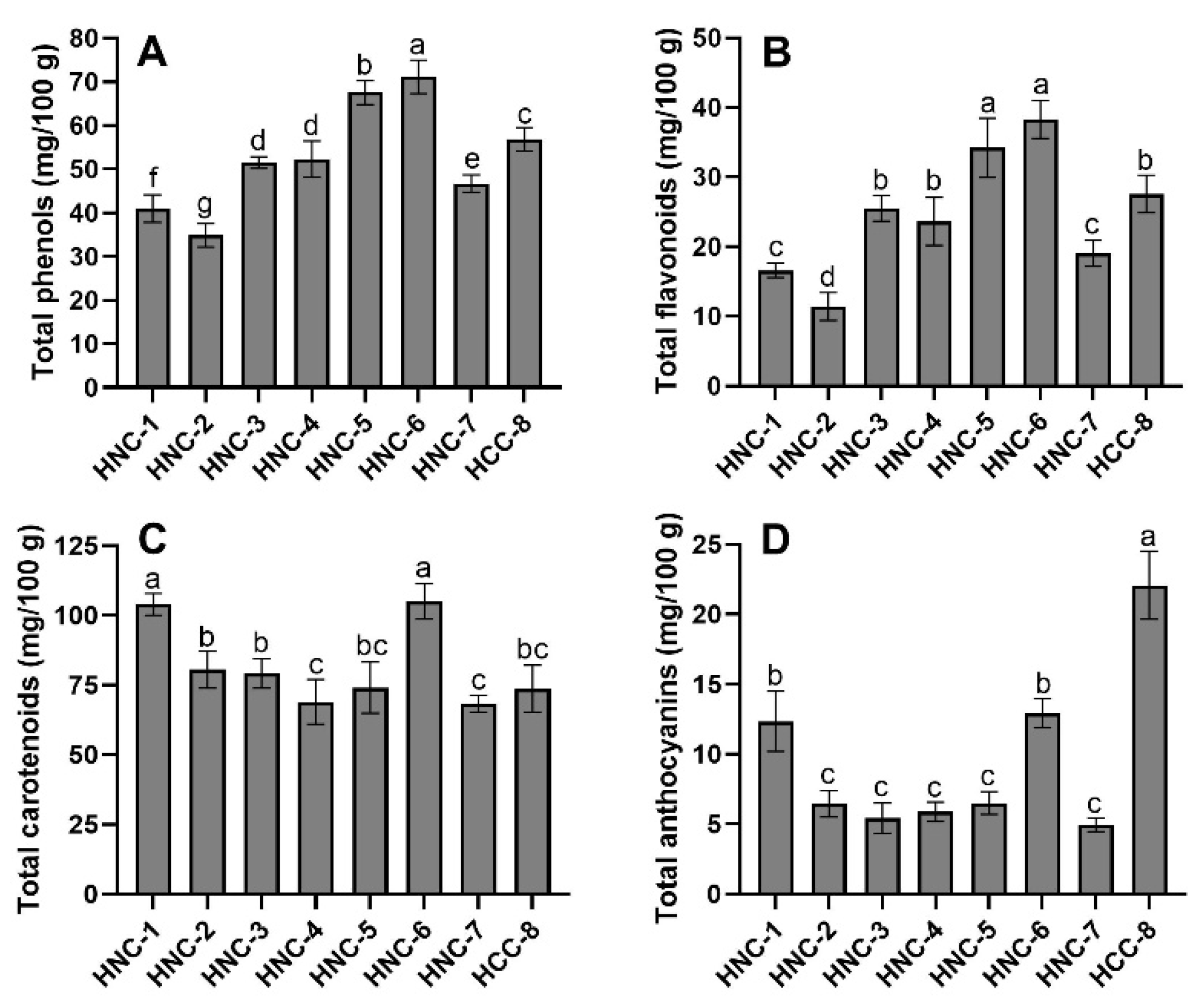
2.8.4. Extraction and Quantification of Phenolic Compounds
2.9. Antioxidant Capacity
2.10. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Fruit Yield Components
3.2. Agronomic Parameters
3.3. Fruit Color
3.4. Capsaicinoid Content
3.5. Polyphenol and Carotenoid Content
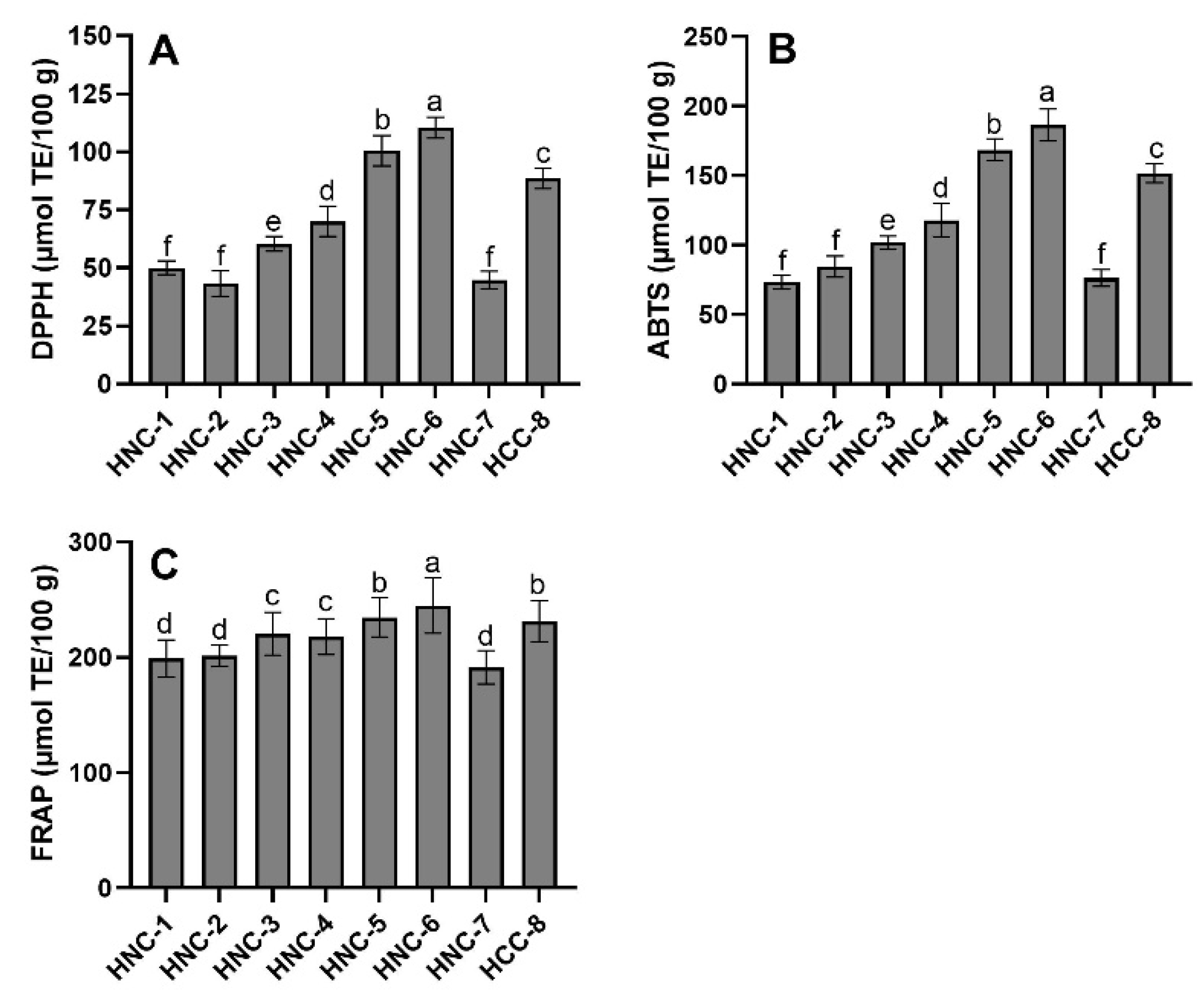
3.6. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; González, M.F.; Rodríguez-Jimenes, G.D.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Baba, V.Y.; Rocha, K.R.; Gomes, G.P.; Ruas, C.D.F.; Ruas, P.M.; Rodrigues, R.; Gonçalves, L.S.A. Genetic diversity of Capsicum chinense accessions based on fruit morphological characterization and AFLP markers. Genet. Resour. Crop. Evol. 2015, 63, 1371–1381. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, L.; Peña-Yam, L.; Álvarez-Gil, M.; Iglesias-Andreu, L.; Avilés-Viñas, S.; Canto-Flick, A.; Guzmán-Antonio, A.; Santana-Buzzy, N. Selection of Habanero Pepper F1 Hybrids (Capsicum chinense Jacq.) at the Yucatan Peninsula, Mexico with a High Potential for Different Markets. Agriculture 2020, 10, 478. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Castro, E.D.B.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno-Fernández, D.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2018, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.S.; Gómez, K.R.; Ordo~Nez, Y.M.; Ancona, D.B. Polyphenols, Ascorbic Acid and Carotenoids Contents and Antioxidant Properties of Habanero Pepper (Capsicum chinense) Fruit. Food Nutr. Sci. 2013, 4, 47–54. [Google Scholar] [CrossRef]
- Mendes, N.D.S.; Gonçalves, C.B.D.A. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Mudrić, S.; Gašić, U.; Dramićanin, A.K.; Ćirić, I.; Milojković-Opsenica, D.M.; Popović-Đorđević, J.B.; Momirović, N.M.; Tešić, L. The polyphenolics and carbohydrates as indicators of botanical and geographical origin of Serbian autochthonous clones of red spice paprika. Food Chem. 2017, 217, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Urbina, S.L.; Roberts, M.D.; Kephart, W.C.; Villa, K.B.; Santos, E.N.; Olivencia, A.M.; Bennett, H.M.; Lara, M.D.; Foster, C.A.; Purpura, M.; et al. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite 2017, 113, 264–273. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Bosland, P.W. Exogenous applications of capsaicin inhibits seed germination of Capsicum annuum. Sci. Hortic. 2016, 203, 29–31. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2019, 45, 16–28. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Fayos, O.; González-De-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-González, M.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages. Agronomy 2020, 10, 1337. [Google Scholar] [CrossRef]
- Food and Agriculture Information Service (SIAP). Statistical Yearbook of Agricultural Production 2020. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 10 May 2021).
- García-López, J.I.; Lira-Saldivar, R.H.; Zavala-García, F.; Olivares-Sáenz, E.; Niño-Medina, G.; Ruiz-Torres, N.A.; Méndez-Argüello, B.; Díaz-Barriga, E. Effects of zinc oxide nanoparticles on growth and antioxidant enzymes of Capsicum chinense. Toxicol. Environ. Chem. 2018, 100, 560–572. [Google Scholar] [CrossRef]
- Ismail, S.M. Influence of deficit irrigation on water use efficiency and bird pepper production (Capsicum annuum L.). Meteor. Environ. Arid Land Agric. Sci. 2010, 21, 21–22. [Google Scholar] [CrossRef]
- Romero-Lozada, M.D.P.; Murillo, C.F.E.; Garcia, S.M.S.M.; Guerrero, J.J.W.; Puentes-Páramo, Y.J.; Menjivar-Flores, J.C. Eficiencia de uso de nutrientes en ají tabasco (Capsicum frutescens L.) y habanero (Capsicum chínense Jacq.). Rev. Investig. Agra. Amb. 2016, 7, 121. [Google Scholar] [CrossRef][Green Version]
- Meneses-Lazo, R.E.; Garruña-Hernández, R.; Latournerie-Moreno, L.; Andrade-Torres, J.L.; Pérez-Gutiérrez, A. Caracterización fenológica y fisiológica de variedades experimentales de chile habanero con alto potencial agronómico. Rev. Fitotec. Mex. 2018, 41, 67–74. [Google Scholar] [CrossRef]
- López-Espinosa, S.T.; Latournerie-Moreno, L.; Castañón-Nájera, G.; Ruiz-Sánchez, E.; Gómez-Leyva, J.F.; Andueza-Noh, R.H.; Mijangos-Cortés, J.O. diversidad genética de chile habanero (Capsicum chinense Jacq.) mediante ISSR. Rev. Fitotec. Mex. 2018, 41, 227–236. [Google Scholar] [CrossRef][Green Version]
- Meraz, M.R.; Cavazos, G.A.; Aguilar, R.M. Jaguar: Cultivar de chile habanero para México. Rev. Mex. Cienc. Agric. 2018, 9, 487–492. [Google Scholar] [CrossRef][Green Version]
- Elibox, W.; Meynard, C.P.; Umaharan, P. Morphological Changes Associated with Postharvest Fruit Deterioration and Physical Parameters for Early Determination of Shelf Life in Capsicum chinense Jacq. HortScience 2015, 50, 1537–1541. [Google Scholar] [CrossRef]
- Simonovska, J.; Škerget, M.; Knez, Ž.; Srbinoska, M.; Kavrakovski, Z.; Grozdanov, A.; Rafajlovska, V. Physicochemical characterization and bioactive compounds of stalk from hot fruits of Capsicum annuum L. Maced. J. Chem. Chem. Eng. 2016, 35, 199. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.H.; Hernández-Fuentes, A.D.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A.A.; Juárez-Maldonado, A. Cu Nanoparticles in Hydrogels of Chitosan-PVA Affects the Characteristics of Post-Harvest and Bioactive Compounds of Jalapeño Pepper. Molecules 2017, 22, 926. [Google Scholar] [CrossRef]
- Commission Internationale De L’ecleirage. Cie 15: Technical Report: Colorimetry, Commission Internationale De L’ecleirage, 3rd ed.; CIE: Vienna, Austria, 2004. [Google Scholar]
- ColorHexa. Color Encyclopedia: Information and Conversion. Computer Software. 2020. Available online: https://www.colorhexa.com/ (accessed on 21 January 2021).
- Ryu, W.-K.; Kim, H.-W.; Kim, G.-D.; Rhee, H.-I. Rapid determination of capsaicinoids by colorimetric method. J. Food Drug Anal. 2017, 25, 798–803. [Google Scholar] [CrossRef]
- Escobedo, L.O.; Amezquita, L.E.G.; Olivas, G.I.; Paz, J.O.; Sepúlveda, D.R. Capsaicinoids content and proximate composition of Mexican chili peppers (Capsicum spp.) cultivated in the State of Chihuahua. CYTA-J. Food. 2013, 11, 179–184. [Google Scholar] [CrossRef]
- Todd, P.H.; Bensinger, M.G.; Biftu, T. Determination of pungency due to capsicum by gas-liquid chromatography. J. Food Sci. 1977, 42, 660–665. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. J. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- López-Contreras, J.J.; Zavala-García, F.; Urías-Orona, V.; Martínez-Ávila, G.C.G.; Rojas, R.; Niño-Medina, G. Chromatic, Phenolic and Antioxidant Properties of Sorghum bicolor Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 366–370. [Google Scholar] [CrossRef]
- Latournerie, M.L.; López, V.J.S.; Castañón, N.G.; Mijangos, C.J.O.; Espada, V.G.; Pérez, G.A.; Ruiz, S.E. Evaluación agronómica de germoplasma de chile habanero (Capsicum chinense Jacq.). Agroproductividad 2015, 8, 25–29. [Google Scholar]
- Pech May, A.M.; Castañón Nájera, G.; Tun Suárez, J.M.; Mendoza Elos, M.; Mijangos Cortés, J.O.; Pérez Gutiérrez, A.; Latournerie Moreno, L. Efectos heteróticos y aptitud combinatoria en poblaciones de chile dulce (Capsicum annuum L.). Rev. Fitotec. Mex. 2010, 33, 353–360. [Google Scholar] [CrossRef]
- Tucuch-Haas, C.J.; Alcántar-González, G.; Ordaz-Chaparro, V.M.; Santizo-Rincón, J.A.; Larqué-Saavedra, A. Producción y calidad de chile habanero (Capsicum chinense Jacq.) con diferentes relaciones NH4+/NO3- y tamaño de partícula de sustratos. Terra Latinoam 2012, 30, 9–15. [Google Scholar]
- Peña, Y.L.P. Selection of Progenitors of Habanero Pepper (Capsicum chinense Jacq.) to Obtain Hybrids with High Productive Po-tential. Ph.D. Thesis, Centro de Investigación Científica de Yucatán, Mérida, Yucatán, México, January 2020. Available online: https://cicy.repositorioinstitucional.mx/jspui/bitstream/1003/1740/1/PCB_D_2020_Laura_Pena.pdf (accessed on 14 July 2021).
- Ramírez-Luna, L.E.; Castillo, A.C.C.; Aceves, N.E.; Carrillo, A.E. Efecto de productos con reguladores de crecimiento sobre la floración y amarre de fruto en chile ‘Habanero’. Rev. Chapingo Ser. Hortic. 2005, 11, 93–98. [Google Scholar] [CrossRef]
- Tapia, V.M.; Larios, G.A.; Díaz, S.D.D.; Ramírez, O.G.; Hernández, P.A.; Vidales, F.I. Guillén, A.H. Producción hidropónica de chile habanero negro (Capsicum chinense Jacq.). Rev. Fitotec. Mex. 2016, 39, 241–245. [Google Scholar]
- López Arcos, A.M.; Poot, M.J.E.; Mijangos, C.M.A. Response of habanero pepper (Capsicum chinense L. Jacq.) organic fertilizer supply in Tabasco, Mexico. UDO Ag. 2012, 12, 307–312. [Google Scholar]
- López-Gómez, J.D.; Villegas-Torres, O.G.; Sotelo Nava, H.; Andrade Rodríguez, M.; Juárez López, P.; Martínez Fernández, E. Yield and quality of habanero chili (Capsicum chinense Jacq.) by effect of nutritional regimen. Rev. Mex. Cienc. Agric. 2017, 8, 1747–1758. [Google Scholar]
- Zewdie, Y.; Bosland, P.W. Evaluation of genotype, environment, and genotype-by-environment interaction for capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]
- Manju, P.R.; Sreelathakumary, I. Genetic variability, heritability and genetic advance in hot chilli (Capsicum chinense Jacq.). J. Trop. Agric. 2002, 40, 4–6. [Google Scholar]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Heritability and Genetic Advance among Chili Pepper Genotypes for Heat Tolerance and Morphophysiological Characteristics. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.; Umaharan, P. Genetic Structure and Phylogenetic Relationships of Capsicum chinense. J. Am. Soc. Hortic. Sci. 2012, 137, 250–262. [Google Scholar] [CrossRef]
- Gómez, H.G.; Godina, F.R.; Ortiz, H.O.; Mendoza, A.B.; Torres, V.R.; De La Fuente, M.C. Use of Chitosan-PVA Hydrogels with Copper Nanoparticles to Improve the Growth of Grafted Watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef]
- Martínez-Gaitán, C.; Granados, M.R.; Fernández, M.D.; Gallardo, M.; Thompson, R.B. Recovery of 15N Labeled Nitrogen Fertilizer by Fertigated and Drip Irrigated Greenhouse Vegetable Crops. Agronomy 2020, 10, 741. [Google Scholar] [CrossRef]
- Vera-Guzman, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; López, M.G. Phytochemical Evaluation of Wild and Cultivated Pepper (Capsicum annuum L. and C. pubescens Ruiz & Pav.) from Oaxaca, Mexico. Chil. J. Agric. Res. 2011, 71, 578–585. [Google Scholar] [CrossRef]
- Rodríguez-Maturino, A. Antioxidant activity and bioactive compounds of Chiltepin (Capsicum annuum var. glabriusculum) and Habanero (Capsicum chinense): A comparative study. J. Med. Plants Res. 2012, 6, 1758–1763. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef]
- Herrera-Hernández, I.M.; Armendáriz-Fernández, K.V.; Muñoz-Márquez, E.; Sida-Arreola, J.P.; Sánchez, E. Characterization of Bioactive Compounds, Mineral Content and Antioxidant Capacity in Bean Varieties Grown in Semi-Arid Conditions in Zacatecas, Mexico. Foods 2018, 7, 199. [Google Scholar] [CrossRef]
- Fonseca, R.; Lopes, R.; Barros, W.; Lopes, M.; Ferreira, F. Morphologic characterization and genetic diversity of Capsicum chinense Jacq. accessions along the upper Rio Negro-Amazonas. Crop. Breed. Appl. Biotechnol. 2008, 8, 187–194. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Cirlini, M.; Luzzini, G.; Morini, E.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. Evaluation of the volatile fraction, pungency and extractable color of different Italian Capsicum annuum cultivars designed for food industry. Eur. Food Res. Technol. 2019, 245, 2669–2678. [Google Scholar] [CrossRef]
- Butcher, J.D.; Crosby, K.M.; Yoo, K.S.; Patil, B.; Ibrahim, A.; Leskovar, D.I.; Jifon, J.L. Environmental and Genotypic Variation of Capsaicinoid and Flavonoid Concentrations in Habanero (Capsicum chinense) Peppers. HortScience 2012, 47, 574–579. [Google Scholar] [CrossRef]
- Canto-Flick, A.; Balam-Uc, E.; Bello, J.B.; Guzman, C.A.L.; Solís-Marroquín, D.; Avilés-Viñas, S.; Gómez-Uc, E.; López-Puc, G.; Santana-Buzzy, N.; Iglesias-Andreu, L.G. Capsaicinoids Content in Habanero Pepper (Capsicum chinense Jacq.): Hottest Known Cultivars. HortScience 2008, 43, 1344–1349. [Google Scholar] [CrossRef]
- Pérez-Ambrocio, A.; Guerrero-Beltrán, J.; Aparicio-Fernández, X.; Sosa, R.A.; Hernández-Carranza, P.; Cid-Pérez, S.; Ochoa-Velasco, C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018, 135, 19–26. [Google Scholar] [CrossRef]
- Iqbal, Q.; Amjad, M.; Asi, M.R.; Arino, A.; Ziaf, K.; Nawaz, A.; Ahmad, T. Stability of Capsaicinoids and Antioxidants in Dry Hot Peppers under Different Packaging and Storage Temperatures. Foods 2015, 4, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Q.; Amjad, M.; Asi, M.R.; Nawaz, A.; Khan, S.M.; Ariño, A.; Ahmad, T. Irradiation Maintains Functional Components of Dry Hot Peppers (Capsicum annuum L.) under Ambient Storage. Foods 2016, 5, 63. [Google Scholar] [CrossRef]
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vazquez-Flota, F.A.; Miranda-Ham, M.L. Antioxidant Capacity and Total Phenolic Content in Fruit Tissues from Accessions of Capsicum chinense Jacq. (Habanero Pepper) at Different Stages of Ripening. Sci. World J. 2014, 195, 809073. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to organic cultivation of heirloom Capsicum peppers: Variation in the level of bioactive compounds and effect of ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Arnnok, P.; Ruangviriyachai, C.; Mahachai, R.; Techawongstien, S.; Chanthai, S. Determination of total phenolics and an-thocyanin contents in the pericarp of hot chilli pepper (Capsicum annuum L.). Int. Food Res. J. 2012, 19, 235–243. [Google Scholar]
- Rodriguez-Uribe, L.; Hernandez, L.; Kilcrease, J.P.; Walker, S.; O’Connell, M. Capsaicinoid and Carotenoid Composition and Genetic Diversity of Kas I and Ccs in New Mexican Capsicum annuum L. Landraces. HortScience 2014, 49, 1370–1375. [Google Scholar] [CrossRef]
- Gómez-García, M.D.R.; Ochoa-Alejo, N. Biochemistry and Molecular Biology of Carotenoid Biosynthesis in Chili Peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.P.; Flores-Cordova, M.A. Bioactive Compounds and Antioxidant Activity in Different Grafted Varieties of Bell Pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Padilha, H.K.M.; Pereira, E.D.S.; Munhoz, P.C.; Vizzotto, M.; Valgas, R.A.; Barbieri, R.L. Genetic variability for synthesis of bioactive compounds in peppers (Capsicum annuum) from Brazil. Food Sci. Technol. 2015, 35, 516–523. [Google Scholar] [CrossRef]
- Turturică, M.; Oancea, A.M.; Râpeanu, G.; Bahrim, G. Anthocyanins: Naturally occuring fruit pigments with functional properties. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2015, 39, 9–24. [Google Scholar]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Bashyal, U.; Lee, Y.-S. Variations in proximate nutrients, phytochemicals, and antioxidant activity of field-cultivated red pepper fruits at different harvest times. Hortic. Environ. Biotechnol. 2016, 57, 493–503. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Avilés-Betanzos, K.A.; Ramírez-Rivera, E.D.J.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants 2020, 9, 1394. [Google Scholar] [CrossRef] [PubMed]

| Genotype | Color | Origin | Type | Selection Cycle in Coahuila | Picture |
|---|---|---|---|---|---|
| HNC-1 | Orange | Yucatán | Creole | 2 |  |
| HNC-2 | Orange | Jaguar | Selection | 3 |  |
| HNC-3 | Orange | Jaguar | Selection | 3 |  |
| HNC-4 | Orange | Jaguar | Selection | 3 |  |
| HNC-5 | Orange | Yucatán | Creole | 2 |  |
| HNC-6 | Orange | Yucatán | Creole | 2 |  |
| HNC-7 | Orange | Jaguar * | Selection | 3 |  |
| HCC-8 | Chocolate | Coahuila | Creole | 7 |  |
| NS% | NO3− | H2PO4− | SO42− | Cl− | HCO3− and CO32− | NH4+ | K+ | Mg2+ | Ca2+ | Na+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Fructification-100 | 12 | 2.0 | 7.0 | 3.2 | 1.0 | 2.0 | 7.0 | 4.2 | 9.0 | 3.1 |
| Flowering-75 | 9.0 | 1.5 | 5.3 | 3.2 | 1.0 | 1.5 | 5.3 | 3.2 | 6.8 | 3.1 |
| Trasplant-50 | 6.0 | 1.0 | 3.5 | 3.2 | 1.0 | 1.0 | 3.5 | 2.1 | 4.5 | 3.1 |
| Genotypes | Color Parameters | |||
|---|---|---|---|---|
| L * | C * | h * | View | |
| HNC-1 | 42.40 ± 0.56 c | 55.32 ± 0.99 a | 44.86 ± 0.63 c |  |
| HNC-2 | 47.64 ± 1.35 b | 54.66 ± 1.76 a | 45.52 ± 1.46 c |  |
| HNC-3 | 48.38 ± 0.92 b | 53.06 ± 1.50 a | 45.66 ± 1.69 bc |  |
| HNC-4 | 48.36 ± 2.21 b | 54.46 ± 1.59 a | 45.06 ± 2.03 c |  |
| HNC-5 | 51.06 ± 1.55 a | 52.08 ± 2.84 a | 51.90 ± 3.10 a |  |
| HNC-6 | 40.28 ± 0.75 c | 54.12 ± 3.20 a | 51.15 ± 1.99 a |  |
| HNC-7 | 51.56 ± 1.19 a | 53.14 ± 1.70 a | 50.64 ± 1.28 ab |  |
| HCC-8 | 26.88 ± 0.63 d | 6.30 ± 1.81 b | 32.04 ± 4.81 d |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camposeco-Montejo, N.; Flores-Naveda, A.; Ruiz-Torres, N.; Álvarez-Vázquez, P.; Niño-Medina, G.; Ruelas-Chacón, X.; Torres-Tapia, M.A.; Rodríguez-Salinas, P.; Villanueva-Coronado, V.; García-López, J.I. Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico. Horticulturae 2021, 7, 372. https://doi.org/10.3390/horticulturae7100372
Camposeco-Montejo N, Flores-Naveda A, Ruiz-Torres N, Álvarez-Vázquez P, Niño-Medina G, Ruelas-Chacón X, Torres-Tapia MA, Rodríguez-Salinas P, Villanueva-Coronado V, García-López JI. Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico. Horticulturae. 2021; 7(10):372. https://doi.org/10.3390/horticulturae7100372
Chicago/Turabian StyleCamposeco-Montejo, Neymar, Antonio Flores-Naveda, Norma Ruiz-Torres, Perpetuo Álvarez-Vázquez, Guillermo Niño-Medina, Xochitl Ruelas-Chacón, María Alejandra Torres-Tapia, Pablo Rodríguez-Salinas, Victor Villanueva-Coronado, and Josué I. García-López. 2021. "Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico" Horticulturae 7, no. 10: 372. https://doi.org/10.3390/horticulturae7100372
APA StyleCamposeco-Montejo, N., Flores-Naveda, A., Ruiz-Torres, N., Álvarez-Vázquez, P., Niño-Medina, G., Ruelas-Chacón, X., Torres-Tapia, M. A., Rodríguez-Salinas, P., Villanueva-Coronado, V., & García-López, J. I. (2021). Agronomic Performance, Capsaicinoids, Polyphenols and Antioxidant Capacity in Genotypes of Habanero Pepper Grown in the Southeast of Coahuila, Mexico. Horticulturae, 7(10), 372. https://doi.org/10.3390/horticulturae7100372






