Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions, Plant Material, and Irrigation Treatments
2.2. Biomass and Leaf Area
2.3. Stomata Characteristics
2.4. Leaf Gas Exchange, Chlorophyll a Fluorescence, and RWC
2.5. Determination of Chl and Carotenoid Content
Chl b = 34.09A652.4 − 15.28A665.2.
Carotenoids = (1000A470 − 1.63Chla − 104.96Chlb)/221.
2.6. Estimation of Proline Content
2.7. Estimation of MDA Content
2.8. Extraction and Assay of Antioxidant Enzymes
2.9. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.10. Statistical Analysis
3. Results
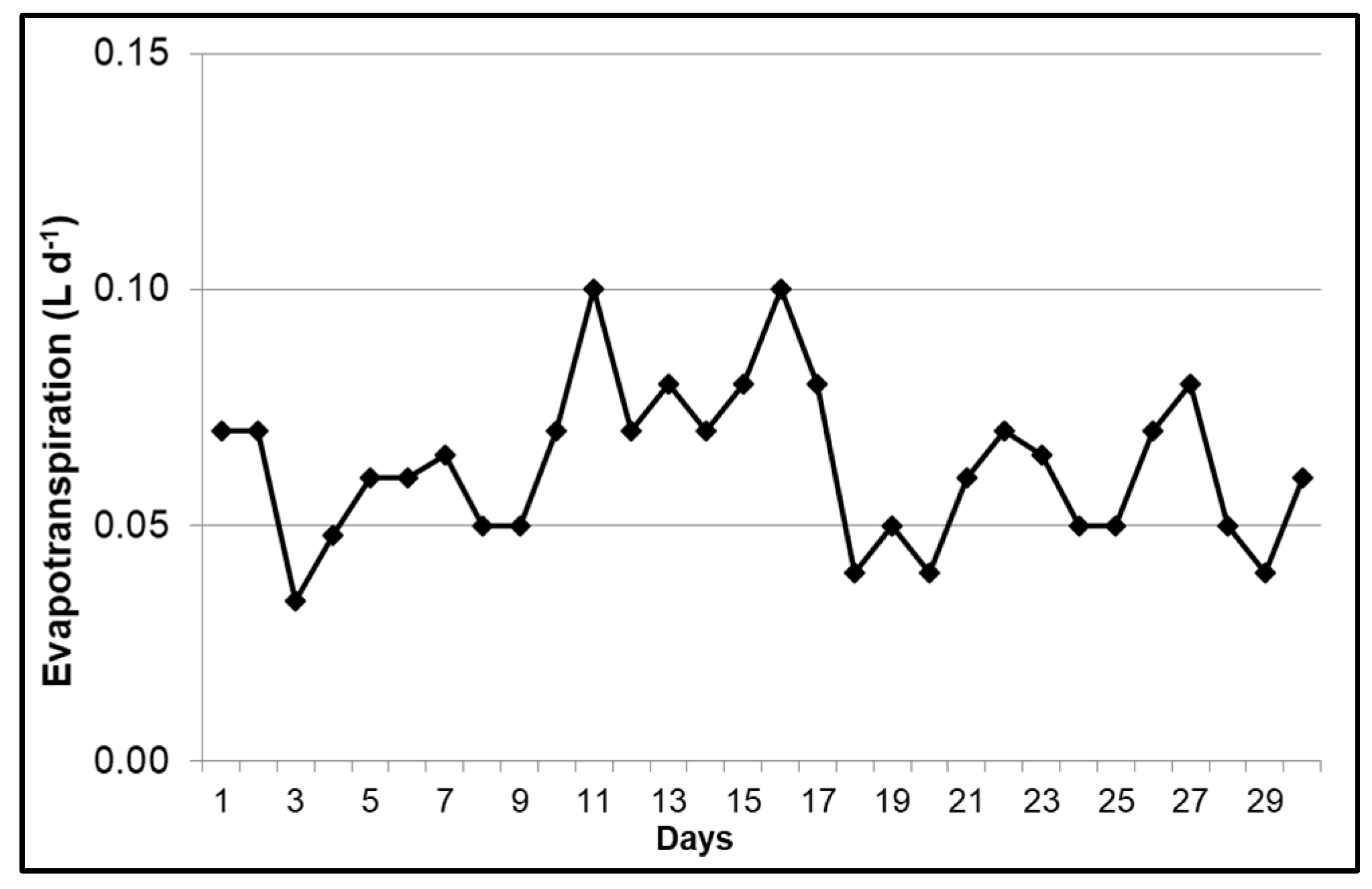
3.1. Evapotranspiration
3.2. Biomass and Leaf Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidari, Z.; Nazarideljou, M.J.; Rezaie Danesh, Y.; Khezrinejad, N. Morphophysiological and biochemical responses of Zinnia elegans to different irrigation regimes in symbiosis with Glomus mosseae. Int. J. Hortic. Sci. 2016, 3, 19–32. [Google Scholar] [CrossRef]
- Da Silva, F.F.; Wallach, R.; Chen, Y. Hydraulic properties of sphagnum peat moss and tuff (scoria) and their potential effects on water availability. In Optimization of Plant Nutrition; Springer: Berlin/Heidelberg, Germany, 1993; pp. 569–576. [Google Scholar]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the Saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Larkunthod, P.; Nounjan, N.; Siangliw, J.L.; Toojinda, T.; Sanitchon, J.; Jongdee, B.; Theerakulpisut, P. Physiological responses under drought stress of improved drought-tolerant rice lines and their parents. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 679–687. [Google Scholar] [CrossRef]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf morphology, rather than plant water status, underlies genetic variation of rice leaf rolling under drought. Plant. Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef]
- Jaramillo, R.E.; Nord, E.A.; Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Root cortical burden influences drought tolerance in maize. Ann. Bot. 2013, 112, 429–437. [Google Scholar] [CrossRef]
- Nemali, K.S.; Bonin, C.; Dohleman, F.G.; Stephens, M.; Reeves, W.R.; Nelson, D.E.; Castiglioni, P.; Whitsel, J.E.; Sammons, B.; Silady, R.A.; et al. Physiological responses related to increased grain yield under drought in the first biotechnology-derived drought tolerant maize. Plant Cell Environ. 2015, 38, 1866–1880. [Google Scholar] [CrossRef]
- Tezara, W.M.V.J.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Kim, J.; van Iersel, M.W. Slowly developing drought stress increases photosynthetic acclimation of Catharanthus roseus. Physiol. Plant. 2011, 143, 166–177. [Google Scholar] [CrossRef]
- Nemali, K.; van Iersel, M.W. Relating whole-plant photosynthesis to physiological acclimations at leaf and cellular scales under drought stress in bedding plants. J. Am. Soc. Hortic. Sci. 2019, 144, 201–208. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Boyle, R.K.; McAinsh, M.; Dodd, I.C. Stomatal closure of Pelargonium× hortorum in response to soil water deficit is associated with decreased leaf water potential only under rapid soil drying. Physiol. Plant. 2016, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar] [CrossRef]
- Nemali, K.S.; Stephens, M. Plant abiotic stress: Water. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Elsevier: London, UK, 2014; pp. 335–342. [Google Scholar]
- Flexas, J.; Escalona, J.M.; Medrano, H. Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines. Plant Cell Environ. 1999, 22, 39–48. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll fluorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Evanylo, G.K.; Haering, K.C. Impact of biosolids on hormone metabolism in drought-stressed tall fescue. Crop. Sci. 2009, 49, 1893–1901. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenously applied proline as a tool to enhance water use efficiency: Case of fennel. Agric. Water Manag. 2018, 197, 138–146. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant. Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 93–136. [Google Scholar]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, L. Characterization of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline-earth hydroxides. Starch-Stärke 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Keles, Y.; Öncel, I. Growth and solute composition in two wheat species experiencing combined influence of stress conditions. Russ. J. Plant Physiol. 2004, 51, 203–209. [Google Scholar] [CrossRef]
- Dole, J.M.; Fanelli, F.L.; Fonteno, W.C.; Harden, B.; Blankenship, S.M. Post harvest handling of cut linaria, trachelium, and zinnia. HortScience 2005, 40, 1123B. [Google Scholar] [CrossRef]
- Pallavi, B.; Nivas, S.K.; D’souza, L.; Ganapathi, T.R.; Hegde, S. Gamma rays induced variations in seed germination, growth and phenotypic characteristics of Zinnia elegans var. Dreamland. Adv. Hortic. Sci. 2017, 31, 267–274. [Google Scholar] [CrossRef]
- Nau, J. Zinnia. In Ball Red Book Greenhouse Growing, 15th ed.; Ball, V., Ed.; J. Ball Publishing: West Chicago, IL, USA, 1991; pp. 785–787. [Google Scholar]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Lobell, D.B.; Gourdji, S.M. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–158. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Wang, Y.T. Impact of drought and temperature on growth and leaf gas exchange of six bedding plant species under greenhouse conditions. HortScience 2006, 41, 1408–1411. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392. [Google Scholar] [CrossRef]
- Alvarez, S.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in different phenological stages of potted geranium plants: Water consumption, water relations and ornamental quality. Acta Physiol. Plant. 2013, 35, 1257–1267. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant Prod. 2008, 2, 353–366. [Google Scholar]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Bian, S.; and Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar] [CrossRef]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V. Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol. Biochem. 2004, 42, 899–906. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Jones, M.M.; Turner, N.C.; Osmond, C.B. Mechanisms of drought resistance. In The Physiology and Biochemistry of Drought Resistance in Plants; Paleg, L.G., Aspinal, D., Eds.; Academic Press: Sydney, Australia, 1981; pp. 15–35. [Google Scholar]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Heschel, M.S.; Sultan, S.E.; Glover, S.; Sloan, D. Population differentiation and plastic responses to drought stress in the generalist annual Polygonum persicaria. Int. J. Plant Sci. 2004, 165, 817–824. [Google Scholar] [CrossRef]
- Azhar, N.; Hussain, B.; Ashraf, M.Y.; Abbasi, K.Y. Water stress mediated changes in growth: Physiology and secondary metabolites of desi ajwain (Trachyspermum ammi L.). Pak. J. Bot. 2011, 43, 15–19. [Google Scholar]
- Khalid, K.A. Influence of water stress on growth essential oil and chemical composition of herbs (Ocimum sp.). Int. Agrophys. 2006, 20, 289–296. [Google Scholar]
- Gao, S.; Wang, Y.; Yu, S.; Huang, Y.; Liu, H.; Chen, W.; He, X. Effects of drought stress on growth, physiology and secondary metabolites of two Adonis species in Northeast China. Sci. Hortic. 2020, 259, 108795. [Google Scholar] [CrossRef]
- Borges, I.B.; Cardoso, B.K.; Silva, E.I.S.; de Oliveira, J.E.S.; da Silva, R.F.; de Rezende, C.A.M.; Gonçalves, J.E.; Junior, R.P.; de Souza, S.G.H.; Gazim, Z.C. Evaluation of performance and chemical composition of Petroselinum crispum essential oil under different conditions of water deficit. Afr. J. Agric. Res. 2016, 11, 480–486. [Google Scholar] [CrossRef][Green Version]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-anatomical adaptations of two Tagetes erecta L. cultivars with contrasting response to drought stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Álvarez, S.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Root system response to drought and salinity: Root distribution and water transport. In Root Engineering; Soil Biology Series; Morte, A., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 40, pp. 325–352. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef]
- Babaei, K.; Moghaddam, M.; Farhadi, N.; Pirbalouti, A.G. Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hortic. 2021, 284, 110116. [Google Scholar] [CrossRef]
- Ueda, A.; Kanechi, M.; Uno, Y.; Inagaki, N. Photosynthetic limitations of a halophyte sea aster (Aster tripolium L.) under water stress and NaCl stress. J. Plant Res. 2003, 116, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Merwad, A.M.A.; Desoky, E.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Lakshmanana, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303. [Google Scholar] [CrossRef]
- Kiani, S.P.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water stressed conditions. Plant. Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Asrar, A.W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Rosales-Serna, R.; Kohashi-Shibata, J.; Acosta-Gallegos, J.A.; Trejo-López, C.; Ortiz Cereceres, J.; Kelly, J.D. Biomass distribution, maturity acceleration and yield in drought stressed common bean cultivars. Field Crop. Res. 2004, 85, 203–211. [Google Scholar] [CrossRef]
- Lu, F.; Bu, Z.; Lu, S. Estimating chlorophyll content of leafy green vegetables from adaxial and abaxial reflectance. Sensors 2019, 19, 4059. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, P.; Tafreshi, S.A.H.; Ebrahimi, M.A.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A.; Nezami, A.; Shoor, M. Effects of drought stress on cold hardiness of non-acclimated viola (Viola× wittrockiana ‘Iona Gold with Blotch’) in controlled conditions. Sci. Hortic. 2018, 238, 98–106. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant. Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef]
- Devi, M.A.; Giridhar, P. Variations in physiological response, lipid peroxidation, antioxidant enzyme activities, proline and isoflavones content in soybean varieties subjected to drought stress. Proc. Natl. Acad. Sci. USA 2015, 85, 35–44. [Google Scholar] [CrossRef]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Expt. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. (Eds.) Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer International Publishing: Berlin/Heidenberg, Germany, 2016; p. 386. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, F.; Zhang, W.; Liu, C.; Zhao, X. Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hortic. Environ. Biotechnol. 2012, 53, 183–192. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Chaeikar, S.S.; Marzvan, S.; Khiavi, S.J.; Rahimi, M. Changes in growth, biochemical, and chemical characteristics and alteration of the antioxidant defense system in the leaves of tea clones (Camellia sinensis L.) under drought stress. Sci. Hortic. 2020, 265, 109257. [Google Scholar] [CrossRef]
- Espinoza, A.; Martína, A.S.; Lopez-Climentb, M.; Ruiz-Laraa, S.; Gomez-Cadenasb, A.; Casarettoa, J. Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J. Plant Physiol. 2013, 170, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Catola, S.; Marino, G.; Emiliani, G.; Huseynova, T.; Musayev, M.; Akparov, Z.; Maserti, B.E. Physiological and metabolomic analysis of Punica granatum (L.) under drought stress. Planta 2016, 243, 441–449. [Google Scholar] [CrossRef] [PubMed]

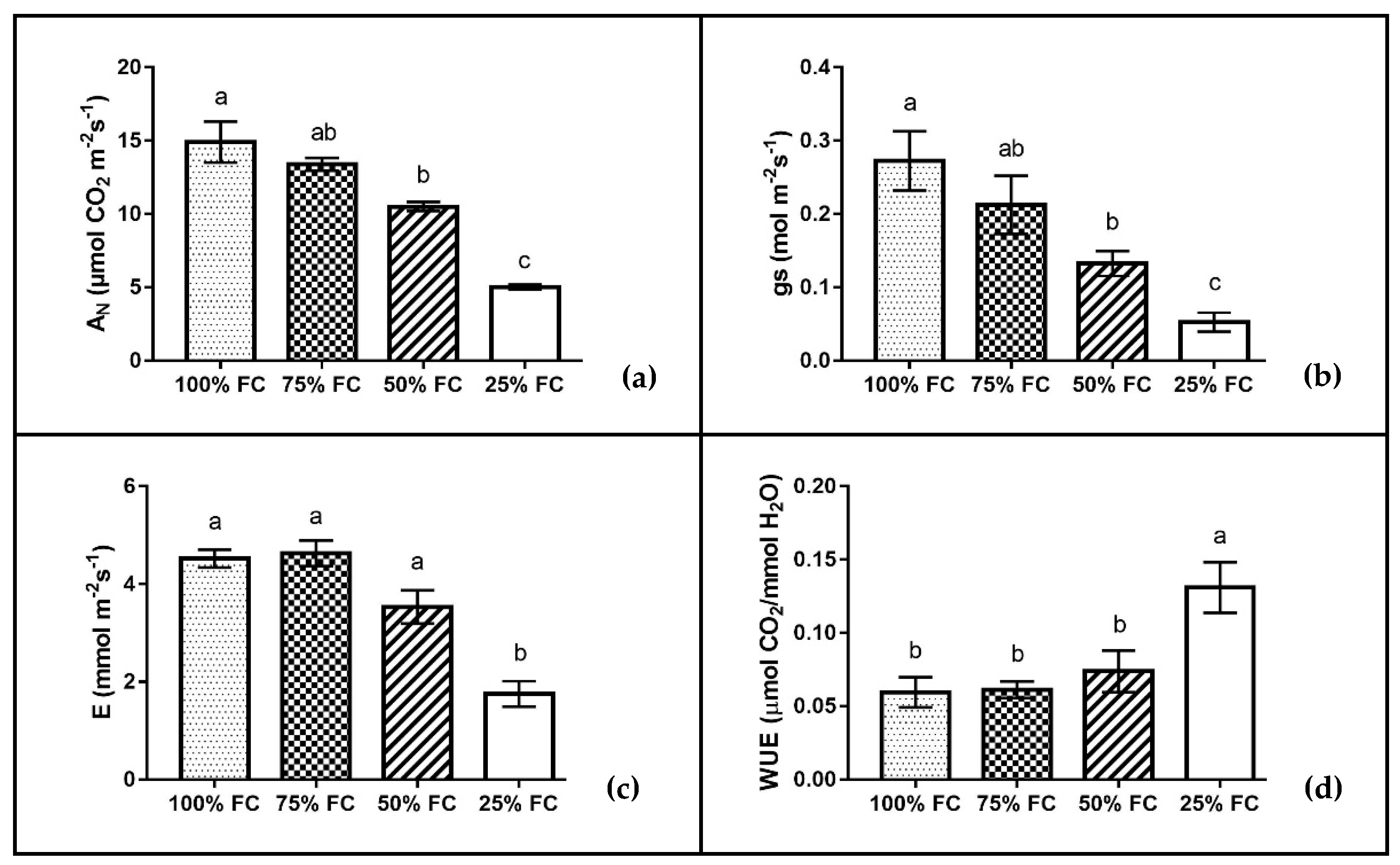




| Drought Stress | Plant Height (cm) | Stem Diameter (mm) | Leaf Number (n.) | Total Leaf Area (cm2) | Leaf Fresh Biomass (g plant−1) | Stem Fresh Biomass (g plant−1) | Root Fresh Biomass (g plant−1) | Total Dry Biomass (g plant−1) | R/S |
|---|---|---|---|---|---|---|---|---|---|
| 100% FC | 17.2 ± 0.3 a | 4.6 ± 0.1 a | 15.8 ± 0.4 a | 210.4 ± 4.5 a | 7.6 ± 0.2 a | 2.7 ± 0.2 a | 7.8 ± 0.6 a | 2.1 ± 0.1 a | 0.5 ± 0.0 b |
| 75% FC | 15.8 ± 0.8 ab | 4.6 ± 0.0 a | 14.8 ± 0.4 a | 218.5 ± 6.4 a | 8.1 ± 0.7 a | 2.8 ± 0.2 a | 7.7 ± 0.6 a | 2.6 ± 0.0 a | 0.7 ± 0.0 b |
| 50% FC | 14.2 ± 0.3 b | 3.6 ± 0.2 b | 13.8 ± 0.6 a | 166.2 ± 9.2 b | 5.5 ± 0.3 b | 1.7 ± 0.1 b | 6.0 ± 0.3 a | 1.9 ± 0.3 a | 1.1 ± 0.2 a |
| 25% FC | 10.7 ± 0.2 c | 2.9 ± 0.2 b | 9.7 ± 0.4 b | 104.4 ± 5.5 c | 3.6 ± 0.3 c | 1.1 ± 0.1 c | 3.0 ± 0.5 b | 1.0 ± 0.1 b | 0.4 ± 0.0 b |
| Significance | *** | *** | *** | *** | *** | ** | *** | ** | * |
| Drought Stress | Stomata | |
|---|---|---|
| Density (n mm−2) | Size (µm) | |
| 100% FC | 321.8 ± 25.2 b | 84.8 ± 1.7 a |
| 75% FC | 253.8 ± 23.9 b | 83.8 ± 4.8 a |
| 50% FC | 376.2 ± 9.7 a | 72.4 ± 0.6 ab |
| 25% FC | 396.2 ± 8.0 a | 55.7 ± 0.1 b |
| Significance | * | ** |
| Drought Stress | RWC (%) | Fv/Fm |
|---|---|---|
| 100% FC | 73.8 ± 3.1 a | 0.74 ± 0.01 a |
| 75% FC | 66.7 ± 2.7 a | 0.73 ± 0.00 a |
| 50% FC | 66.7 ± 2.9 a | 0.72 ± 0.02 ab |
| 25% FC | 50.7 ± 1.5 b | 0.68 ± 0.02 b |
| Significance | ** | * |
| Drought Stress | Enzyme Activity | |||
|---|---|---|---|---|
| CAT (U mg−1 Protein) | GPX (U mg−1 Protein) | SOD (U mg−1 Protein) | DPPH (mg TE g−1 FW) | |
| 100% FC | 0.0040 ± 0.0005 b | 5.6 ± 0.6 b | 29.5 ± 0.2 b | 6.7 ± 0.1 b |
| 75% FC | 0.0042 ± 0.0008 b | 5.9 ± 0.4 b | 23.4 ± 0.1 b | 6.9 ± 0.1 b |
| 50% FC | 0.0069 ± 0.0003 a | 8.0 ± 0.9 a | 45.8 ± 3.3 a | 6.7 ± 0.2 b |
| 25% FC | 0.0072 ± 0.0001 a | 9.2 ± 0.5 a | 44.0 ± 1.5 a | 8.6 ± 0.4 a |
| Significance | ** | ** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; Romano, D. Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress. Horticulturae 2021, 7, 362. https://doi.org/10.3390/horticulturae7100362
Toscano S, Romano D. Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress. Horticulturae. 2021; 7(10):362. https://doi.org/10.3390/horticulturae7100362
Chicago/Turabian StyleToscano, Stefania, and Daniela Romano. 2021. "Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress" Horticulturae 7, no. 10: 362. https://doi.org/10.3390/horticulturae7100362
APA StyleToscano, S., & Romano, D. (2021). Morphological, Physiological, and Biochemical Responses of Zinnia to Drought Stress. Horticulturae, 7(10), 362. https://doi.org/10.3390/horticulturae7100362







