Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Samples
2.2. Physicochemical Analyses
2.2.1. Soluble Solids Content, Titratable Acidity and pH
2.2.2. Colour
2.2.3. Water Activity
2.2.4. Potassium Content
2.3. Bioactive Compounds Content
2.3.1. Folin–Ciocalteu Reagent (FCR) Reducing Capacity
2.3.2. Chlorophylls
2.3.3. Total Carotenoids
2.3.4. Vitamin C
2.4. Total Antioxidant Activity
2.5. Data Analyses
3. Results and Discussion
3.1. Physicochemical Characteristics
3.2. Bioactive Compounds
3.3. Total Antioxidant Activity
3.4. Weight Distribution of Some Characteristics by Melon Parts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crizel, T.; Jablonski, A.; Rios, A.; Rech, R.; Flôres, S. Dietary fiber from orange byproducts as potential fat replacer. LWT Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Duda-Chodak, A.D.A.; Tarko, T. Antioxidant properties of different fruit seeds and peels. Acta Sci. Pol. Technol. Aliment. 2007, 6, 29–36. [Google Scholar]
- Kim, M.; Kim, E.J.; Kim, Y.-N.; Choi, C.; Lee, B.-H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, S.; Sutar, N. Banana and its by-product utilisation: An overview. J. Sci. Ind. Res. 2010, 69, 323–329. [Google Scholar]
- Sundaram, S.; Anjum, S.; Dwivedi, P.; Rai, G.K. Antioxidant Activity and Protective effect of Banana Peel against Oxidative Hemolysis of Human Erythrocyte at Different Stages of Ripening. Appl. Biochem. Biotechnol. 2011, 164, 1192–1206. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Gloria Lobo, M.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Xu, G.H.; Chen, J.C.; Liu, D.H.; Zhang, Y.H.; Jiang, P.; Ye, X.Q. Minerals, Phenolic Compounds, and Antioxidant Capacity of Citrus Peel Extract by Hot Water. J. Food Sci. 2008, 73, C11–C18. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compos. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.M.; Brandão, T.R.S. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of Cantaloupe melon. J. Food Meas. Charact. 2018, 12, 292–300. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Zapata, S.; Dufour, J.-P. Ascorbic, Dehydroascorbic and Isoascorbic Acid Simultaneous Determinations by Reverse Phase Ion Interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- Guillén-Ríaos, P.; Burló, F.; Martíanez-Sánchez, F.; Carbonell-Barrachina, A. Effects of Processing on the Quality of Preserved Quartered Artichokes Hearts. J. Food Sci. 2006, 71, S176–S180. [Google Scholar] [CrossRef]
- Fundo, J.F.; Amaro, A.L.; Madureira, A.R.; Carvalho, A.; Feio, G.; Silva, C.L.M.; Quintas, M.A.C. Fresh-cut melon quality during storage: An NMR study of water transverse relaxation time. J. Food Eng. 2015, 167, 71–76. [Google Scholar] [CrossRef]
- Albuquerque, B.; Lidon, F.C.; Barreiro, M.G. A case study on the flavor properties of melon (Cucumis melo L.) cultivars. Fruits 2006, 61, 333–339. [Google Scholar] [CrossRef]
- Flores, F.B.; Martínez-Madrid, M.C.; Sánchez-Hidalgo, F.J.; Romojaro, F. Differential rind and pulp ripening of transgenic antisenseACC oxidase melon. Plant Physiol. Biochem. 2001, 39, 37–43. [Google Scholar] [CrossRef]
- Navarro, J.M.; Botía, P.; Pérez-Pérez, J.G. Influence of deficit irrigation timing on the fruit quality of grapefruit (Citrus paradisi Mac.). Food Chem. 2015, 175, 329–336. [Google Scholar] [CrossRef]
- Kohn, R.A.G.; Mauch, C.R.; Morselli, T.B.G.A.; Rombaldi, C.V.; Barros, W.S.; Sorato, V. Physical and chemical characteristics of melon in organic farming. Rev. Bras. Eng. Agríc. Ambient. 2015, 19, 656–662. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.; Fernández-Trujillo, J.P. Identification of melon fruit quality quantitative trait loci using near-isogenic lines. J. Am. Soc. Horticult. Sci. 2008, 133, 139–151. [Google Scholar] [CrossRef]
- Sigge, G.O.; Hansmann, C.F.; Joubert, E. Effect of storage conditions, packaging material and metabisulphite treatment on the color of dehydrated green bell peppers (Capsicum Annum L.). J. Food Qual. 2001, 24, 205–218. [Google Scholar] [CrossRef]
- USDA. USDA Nutrient Database for Standard Reference, Release 28. Available online: http://fnic.nal.usda.gov/food-composition/usda-nutrient-data-laboratory (accessed on 16 May 2016).
- Sabino, L.; Gonzaga, M.L.C.; Soares, D.J.; Lima, A.C.S.; Lima, J.S.S.; Almeida, M.M.B.; Sousa, P.; Figueiredo, R. Bioactive compounds, antioxidant activity, and minerals in flours prepared with tropical fruit peels. Acta Aliment. 2015, 44, 520–526. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef]
- Whelton, P.K.; He, J.; Cutler, J.A.; Brancati, F.L.; Appel, L.J.; Follmann, D.; Klag, M.J. Effects of Oral Potassium on Blood Pressure: Meta-analysis of Randomized Controlled Clinical Trials. JAMA 1997, 277, 1624–1632. [Google Scholar] [CrossRef]
- Manzoor, M.; Anwar, F.; Bhatti, I.; Jamil, A. Variation of phenolics and antioxidant activity between peel and pulp parts of pear (Pyrus communis L.) fruit. Pak. J. Bot. 2013, 45, 1521–1525. [Google Scholar]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Plaza, L.; Altisent, R.; Alegre, I.; Viñas, I.; Abadias, M. Changes in the quality and antioxidant properties of fresh-cut melon treated with the biopreservative culture Pseudomonas graminis CPA-7 during refrigerated storage. Postharvest Biol. Technol. 2016, 111, 25–30. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.M.S.; Vieira, M.C.; Silva, C.L.M. Modelling kinetics of watercress (Nasturtium officinale) colour changes due to heat and thermosonication treatments. Innov. Food Sci. Emerg. Technol. 2007, 8, 244–252. [Google Scholar] [CrossRef]
- Tadmor, Y.; Burger, J.; Yaakov, I.; Feder, A.; Libhaber, S.E.; Portnoy, V.; Meir, A.; Tzuri, G.; Sa’ar, U.; Rogachev, I.; et al. Genetics of Flavonoid, Carotenoid, and Chlorophyll Pigments in Melon Fruit Rinds. J. Agric. Food Chem. 2010, 58, 10722–10728. [Google Scholar] [CrossRef] [PubMed]
- Artés, F.; Miguez, M.I.; Hornero, D. Analysing change in fruit pigments. In Colour in Food: Improving Quality; MacDougall, D.B., Ed.; CRC Press: Boca Raton, FL, USA; Woodhead Pub.: Cambridge, UK, 2002; pp. 248–282. [Google Scholar]
- Dashwood, R. Chlorophylls as anticarcinogens (review). Int J. Oncol. 1997, 10, 721–727. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L.; Groff, J.L. Advanced Nutrition and Human Metabolism; Thomson Wadsworth Publishing Co.: Belmont, CA, USA, 2005. [Google Scholar]
- Contreras, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; Garcia-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
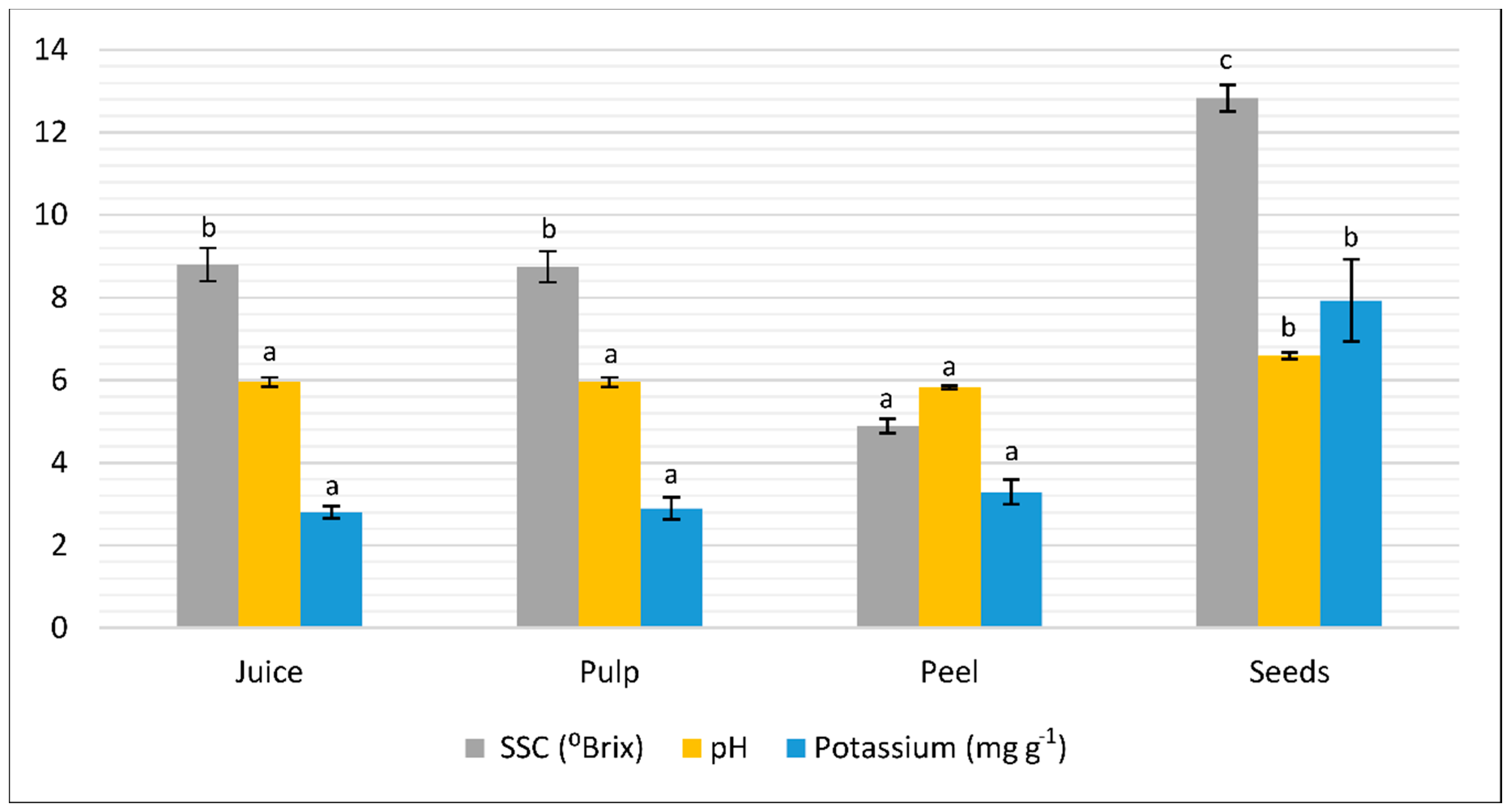
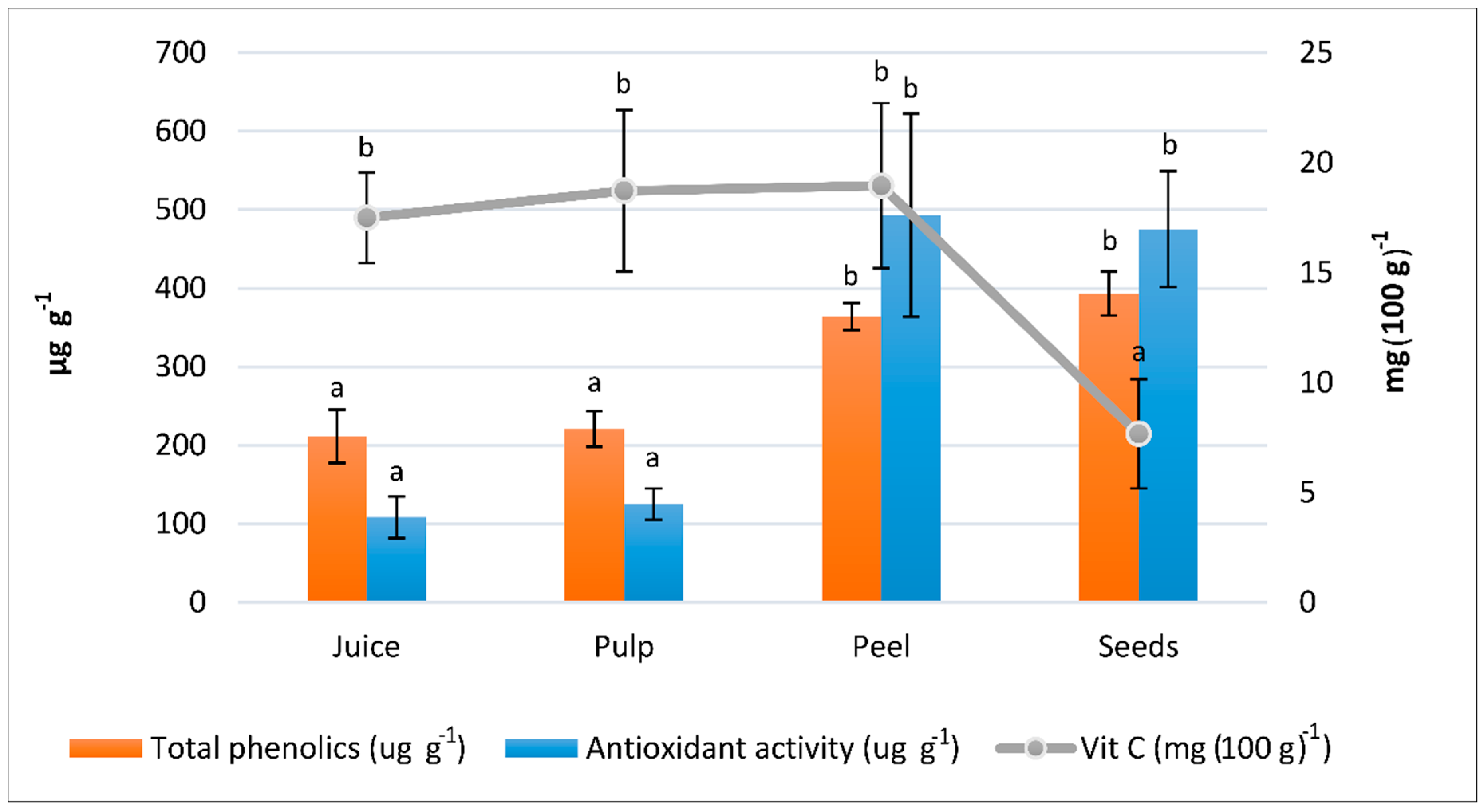
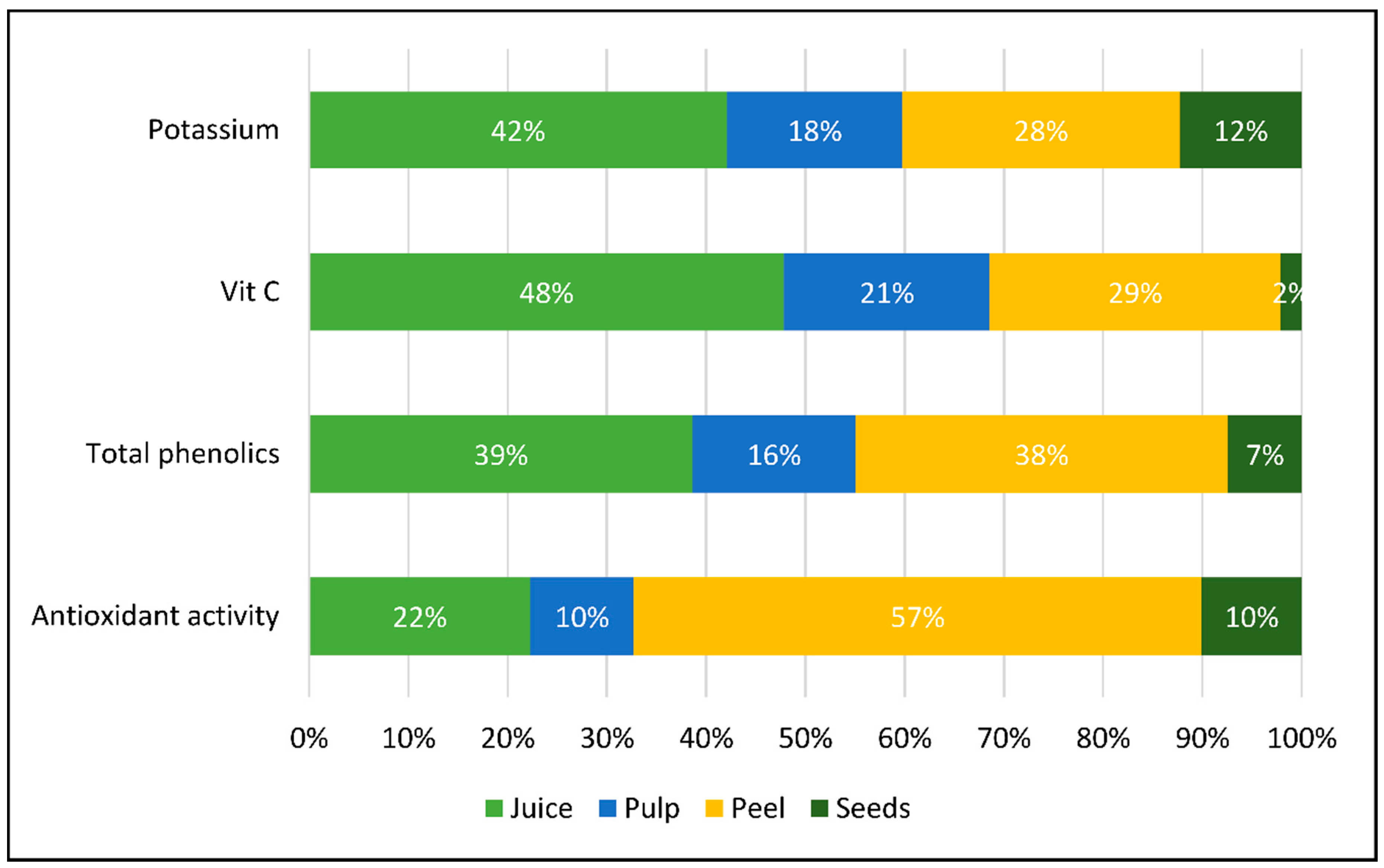
| Colour Parameters | Titratable Acidity (meq L−1) | MI (%) | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | Chroma | Hue Angle (°) | |||
| Juice | 38.79 ± 2.10 a | −1.18 ± 0.34 c | 7.57 ± 3.74 a | 7.69 ± 3.74 a | 104.18 ± 3.43 b | 13.1 ± 1.8 a | 67.2 |
| Pulp | 60.20 ± 2.36 c | −3.20 ± 0.18 b | 10.08 ± 0.66 a | 10.60 ± 0.67 a | 107.98 ± 0.60 c | 13.2 ± 2.3 a | 66.3 |
| Peel | 49.68 ± 2.19 b | −10.78 ± 0.74 a | 29.54 ± 1.87 b | 31.46 ± 1.97 c | 110.07 ± 0.68 c | 19.4 ± 7.0 b | - |
| Seeds | 75.88 ± 1.69 d | 4.51 ± 0.66 d | 25.85 ± 1.69 b | 26.25 ± 1.77 b | 80.28 ± 0.83 a | 36.9 ± 3.5 c | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, F.A.; Fundo, J.F.; Garcia, E.; Santos, J.R.; Silva, C.L.M.; Brandão, T.R.S. Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon. Horticulturae 2020, 6, 60. https://doi.org/10.3390/horticulturae6040060
Miller FA, Fundo JF, Garcia E, Santos JR, Silva CLM, Brandão TRS. Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon. Horticulturae. 2020; 6(4):60. https://doi.org/10.3390/horticulturae6040060
Chicago/Turabian StyleMiller, Fátima Alves, Joana Freitas Fundo, Ester Garcia, João Rodrigo Santos, Cristina Luisa Miranda Silva, and Teresa Ribeiro Silva Brandão. 2020. "Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon" Horticulturae 6, no. 4: 60. https://doi.org/10.3390/horticulturae6040060
APA StyleMiller, F. A., Fundo, J. F., Garcia, E., Santos, J. R., Silva, C. L. M., & Brandão, T. R. S. (2020). Physicochemical and Bioactive Characterisation of Edible and Waste Parts of “Piel de Sapo” Melon. Horticulturae, 6(4), 60. https://doi.org/10.3390/horticulturae6040060







