Blue Light Does Not Affect Fruit Quality or Disease Development on Ripe Blueberry Fruit During Postharvest Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Collection and LED Light Source
2.2. Fruit Quality Attributes
2.3. Natural Postharvest Disease During Storage
2.4. Postharvest Disease Progression Following Artificial Inoculation
2.5. Progression of Color Development on Stem-End Green Spots
3. Results
3.1. Fruit Quality Attributes
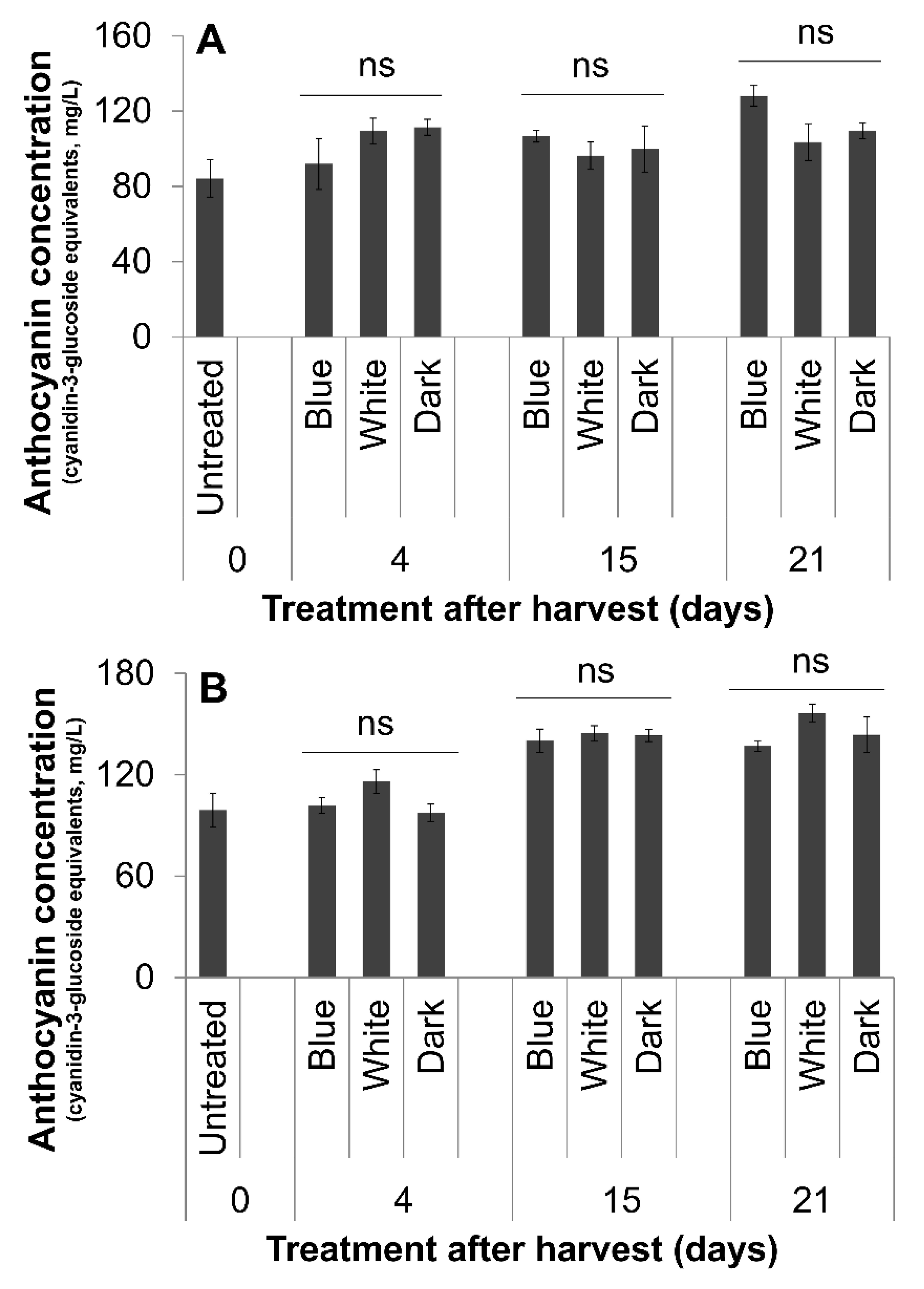
3.2. Fruit Color and Anthocyanin
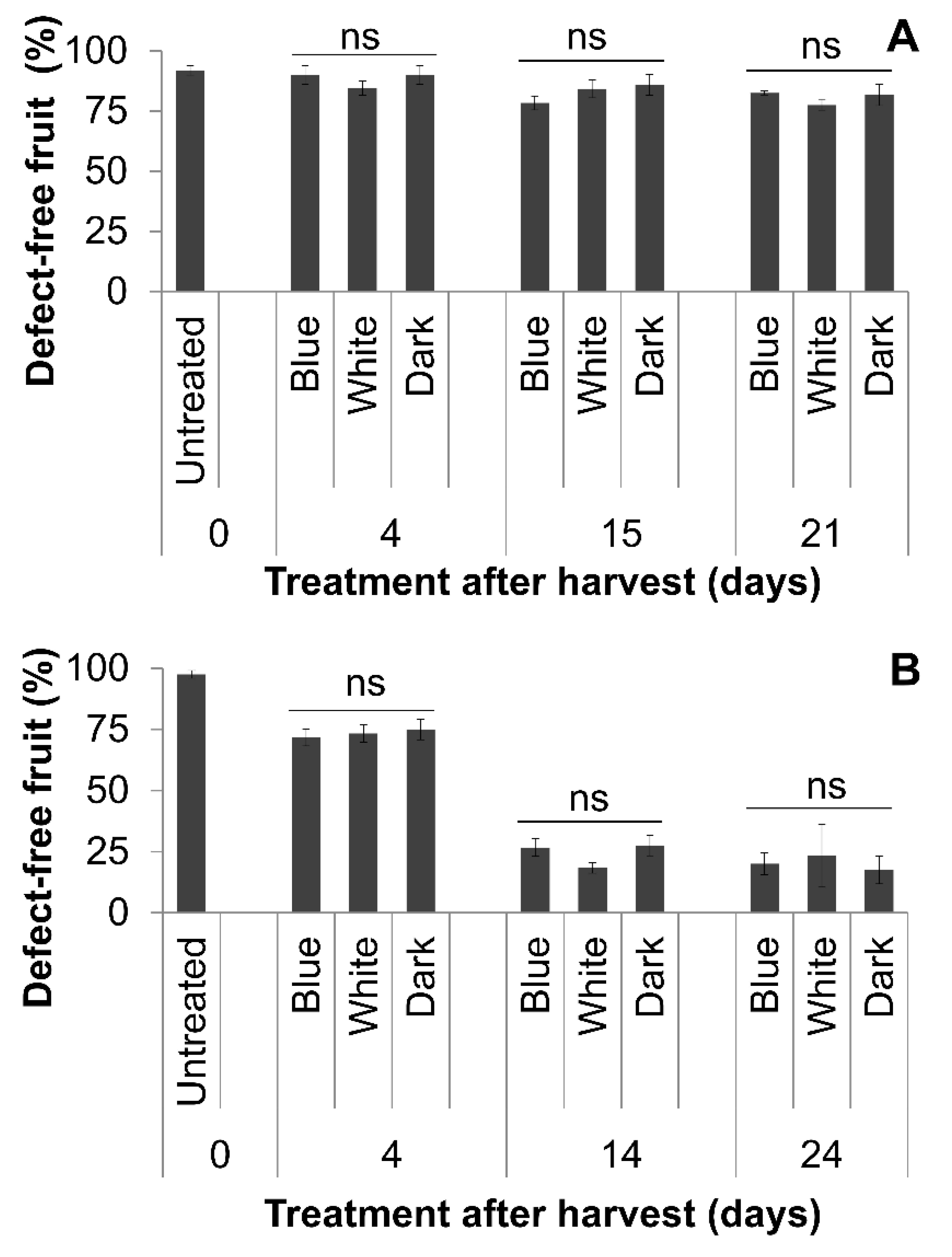
3.3. Natural Postharvest Disease During Storage
3.4. Postharvest Disease Progression Following Artificial Inoculation
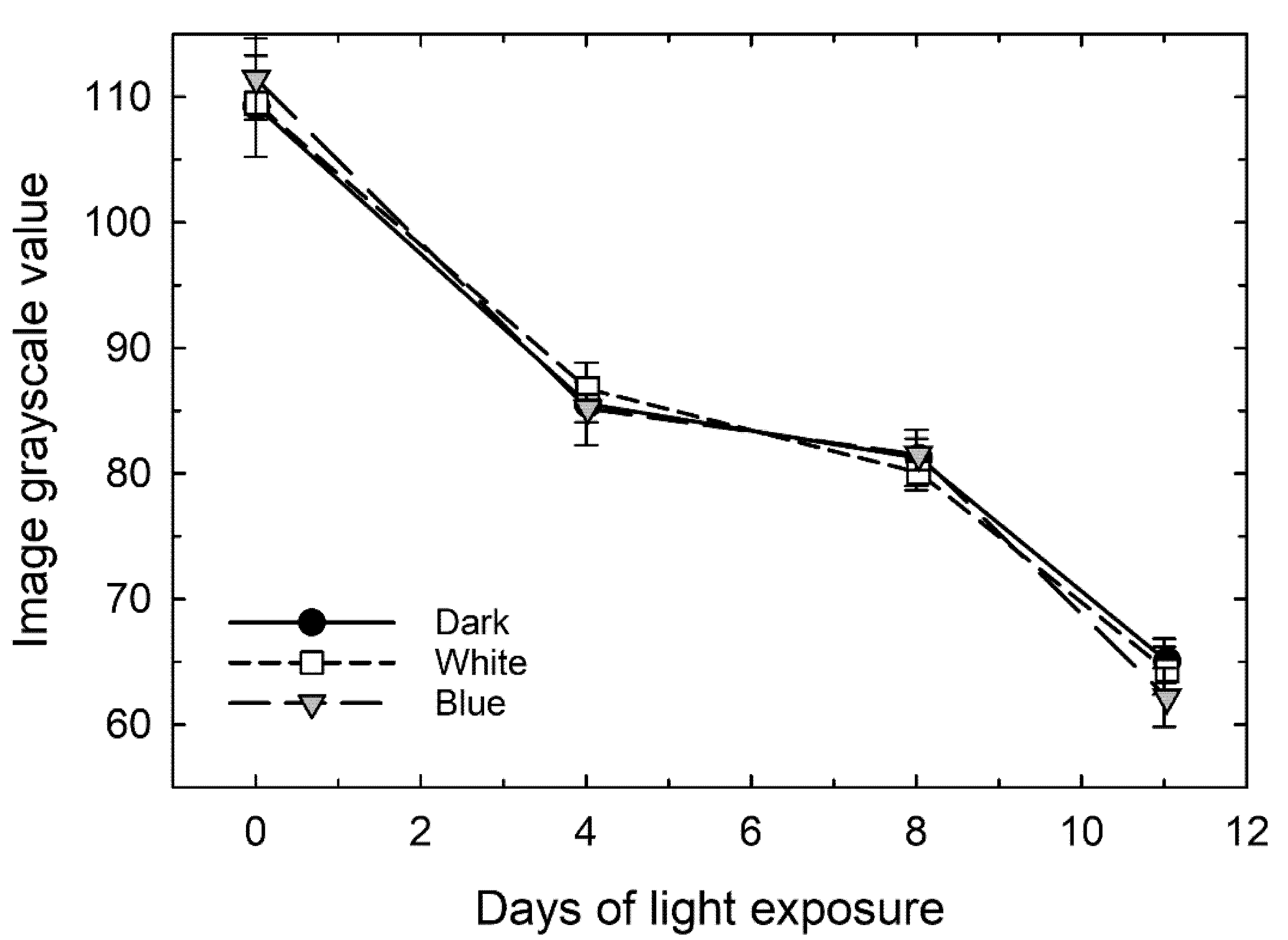
3.5. Progression of Color Development on Stem-End Green Spots
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, E.; Ballen, F. An Overview of US Blueberry Production, Trade and Consumption, with Special Reference to Florida; University of Florida, IFAS, Extension: Gainesville, FL, USA, 2017. [Google Scholar]
- Lang, G. A Southern highbush blueberries: Physiological and cultural factors important for optimal cropping of these complex hybrids. Acta Hortic. 1993, 346, 72–80. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Bassil, N.; Buck, E.; McCallum, S.; Graham, J.; Brown, A.; Wiedow, C.; Campbell, A.M.; Haynes, K.G.; et al. Construction of a genetic linkage map of an interspecific diploid blueberry population and identification of QTL for chilling requirement and cold hardiness. Mol. Breed. 2014, 34, 2033–2048. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Neto, C.C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; López, L.; Escalona, V.H.; Castro-Mandujano, O. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2015, 96, 619–626. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. Postharvest shelf life extension of blueberries using a biodegradable package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; MacLean, D. A Novel Instrument to Delineate Varietal and Harvest Effects on Blueberry Fruit Texture During Storage. J. Sci. Food Agric. 2011, 91, 1653–1658. [Google Scholar] [CrossRef]
- Mehra, L.K.; MacLean, D.D.; Savelle, A.T.; Scherm, H. Postharvest disease development on southern highbush blueberry fruit in relation to berry flesh type and harvest method. Plant Dis. 2013, 97, 213–221. [Google Scholar] [CrossRef]
- Paniagua, A.; East, A.; Hindmarsh, J.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Boil. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Scherm, H.; Nesmith, D.S.; Horton, D.L.; Krewer, G. A Survey of Horticultural and Pest Management Practices of the Georgia Blueberry Industry. Small Fruits Rev. 2001, 1, 17–28. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Quality evaluation of blueberries coated with chitosan and sodium alginate during postharvest storage. Intl. Food Res. J. 2017, 24, 1553–1561. [Google Scholar]
- Nambeesan, S.U.; Doyle, J.; Capps, H.D.; Starns, C.; Scherm, H. Effect of Electronic Cold-Pasteurizationtm (ECPTM) on Fruit Quality and Postharvest Diseases During Blueberry Storage. Horticulturae 2018, 4, 25. [Google Scholar] [CrossRef]
- Thang, K.; Au, K.; Rakovski, C.; Prakash, A. Effect of phytosanitary irradiation and methyl bromide fumigation on the physical, sensory, and microbiological quality of blueberries and sweet cherries. J. Sci. Food Agric. 2016, 96, 4382–4389. [Google Scholar] [CrossRef]
- Trigo, M.; Sousa, M.; Sapata, M.; Ferreira, A.; Curado, T.; Andrada, L.; Ferreira, E.; Antunes, C.; Horta, M.; Pereira, A.; et al. Quality of gamma irradiated blueberries. Acta Hortic. 2006, 715, 573–578. [Google Scholar] [CrossRef]
- Turtoi, M. Ultraviolet light treatment of fresh fruits and vegetables surface: A review. J. Agroaliment. Proc. Technol. 2013, 19, 325–337. [Google Scholar]
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Noji, S.; Takahashi, A. Phototropin 2 is involved in blue light-induced anthocyanin accumulation in Fragaria x ananassa fruits. J. Plant Res. 2013, 126, 847–857. [Google Scholar] [CrossRef]
- Kondo, S.; Tomiyama, H.; Rodyoung, A.; Okawa, K.; Ohara, H.; Sugaya, S.; Terahara, N.; Hirai, N. Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode (LED) irradiation at night. J. Plant Physiol. 2014, 171, 823–829. [Google Scholar] [CrossRef]
- Braidot, E.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Tubaro, F.; Wählby, U.; Coan, M.; Vianello, A.; Zancani, M. Low-intensity light cycles improve the quality of lamb’s lettuce (Valerianella olitoria [L.] Pollich) during storage at low temperature. Postharvest Biol. Technol. 2014, 90, 15–23. [Google Scholar] [CrossRef]
- Costa, L.; Montano, Y.M.; Carrión, C.; Rolny, N.; Guiamet, J.J. Application of low intensity light pulses to delay postharvest senescence of Ocimum basilicum leaves. Postharvest Boil. Technol. 2013, 86, 181–191. [Google Scholar] [CrossRef]
- Glowacz, M.; Mogren, L.M.; Reade, J.P.; Cobb, A.H.; Monaghan, J.M. High- but not low-intensity light leads to oxidative stress and quality loss of cold-stored baby leaf spinach. J. Sci. Food Agric. 2014, 95, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.; Yuk, H.-G.; Khoo, G.H.; Zhou, W. Application of Light-Emitting Diodes in Food Production, Postharvest Preservation, and Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Morrow, R.C. LED Lighting in Horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Both, A.-J.; Bourget, C.; Burr, J.; Kubota, C.; Lopez, R.; Morrow, R.; Runkle, E. LEDs: The future of greenhouse lighting! Chron. Hort. 2012, 52, 6–12. [Google Scholar]
- Kopsell, D.A.; Sams, C.E.; Morrow, R.C. Blue Wavelengths from LED Lighting Increase Nutritionally Important Metabolites in Specialty Crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue Light Irradiation Affects Anthocyanin Content and Enzyme Activities Involved in Postharvest Strawberry Fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Zoratti, L.; Sarala, M.; Carvalho, E.; Karppinen, K.; Martens, S.; Giongo, L.; Häggman, H.; Jaakola, L. Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Boil. 2014, 14, 377. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Choi, S.-J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Kook, K.K.H.-S.; Kim, H.-S.K.K. The Effect of Blue-Light-Emitting Diodes on Antioxidant Properties and Resistance to Botrytis Cinerea in Tomato. J. Plant Pathol. Microbiol. 2013, 4, 203. [Google Scholar] [CrossRef]
- Alferez, F.; Liao, H.-L.; Burns, J.K. Blue light alters infection by Penicillium digitatum in tangerines. Postharvest Boil. Technol. 2012, 63, 11–15. [Google Scholar] [CrossRef]
- Barash, I.; Angel, E. Isolation and properties of an exopoly-galacturonase produced by Penicillium dsgitatum during infection of Lemon fruits. Israel J. Bot. 1970, 19, 599–608. [Google Scholar]
- Barmore, C.R. Role of Pectolytic Enzymes and Galacturonic Acid in Citrus Fruit Decay Caused by Penicillium Digitatum. Phytopathol. 1979, 69, 675. [Google Scholar] [CrossRef]
- Liao, H.-L.; Alferez, F.; Burns, J.K. Assessment of blue light treatments on citrus postharvest diseases. Postharvest Boil. Technol. 2013, 81, 81–88. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; Martin, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants and Wines by the Ph Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons.: New York, NY, USA, 1990. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; American Phytopathological Society: St. Paul, MN, USA, 1998. [Google Scholar]
- Wharton, P.; Schilder, A. Blueberry Fruit Rot Identification Guide; Michigan State University Extension: East Lansing, MI, USA, 2015. [Google Scholar]
- Barnett, H.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; Macmillan Publishing Coy: New York, NY, USA; London, UK, 1987. [Google Scholar]
- Cohen, Y.; Ben-Naim, Y.; Falach, L.; Rubin, A.E.; Rubin, A.E. Epidemiology of Basil Downy Mildew. Phytopathology 2017, 107, 1149–1160. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ha, J.Y.; Oh, J.E.; Cho, M.S. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Sci. Biotechnol. 2014, 23, 1087–1093. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Lafuente, M.T. LED Blue Light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017, 218, 575–583. [Google Scholar] [CrossRef]
- Yamaga, I.; Nakamura, S. Blue LED Irradiation Induces Scoparone Production in Wounded Satsuma Mandarin ‘Aoshima Unshu’ and Reduces Fruit Decay During Long-Term Storage. Hortic. J. 2018, 87, 474–480. [Google Scholar] [CrossRef]
- Yamaga, I.; Takahashi, T.; Ishii, K.; Kato, M.; Kobayashi, Y. Suppression of Blue Mold Symptom Development in Satsuma Mandarin Fruits Treated by Low-Intensity Blue LED Irradiation. Food Sci. Technol. Res. 2015, 21, 347–351. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.-H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Boil. Technol. 2014, 90, 73–77. [Google Scholar] [CrossRef]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Shi, L.; Cao, S.; Chen, W.; Yang, Z. Blue light induced anthocyanin accumulation and expression of associated genes in Chinese bayberry fruit. Sci. Hortic. 2014, 179, 98–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H.-R. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]

| Days after Treatment | Treatment | Compression (kg) | Puncture (kg) | Weight (g) | TSS a (oBrix) | TA b (%) | pH |
|---|---|---|---|---|---|---|---|
| 0 | - | 0.23 | 0.13 | 2.07 | 13.4 | 0.58 | 3.43 |
| 4 | Blue | 0.24 | 0.14 | 2.11 | 13.1 b | 0.55 | 3.35 |
| White | 0.23 | 0.14 | 2.14 | 14.1 a | 0.41 | 3.53 | |
| Dark | 0.24 | 0.14 | 2.28 | 13.2 b | 0.52 | 3.40 | |
| Prob > F | ns | ns | ns | 0.0134 | ns | ns | |
| 15 | Blue | 0.27 | 0.13 | 2.17 ab | 13.4 | 0.40 | 3.50 b |
| White | 0.25 | 0.12 | 2.02 b | 13.5 | 0.42 | 3.50 b | |
| Dark | 0.27 | 0.12 | 2.20 a | 13.3 | 0.40 | 3.68 a | |
| Prob > F | ns | ns | 0.0263 | ns | ns | 0.0282 | |
| 21 | Blue | 0.27 | 0.11 | 2.08 | 13.0 | 0.39 | 3.63 |
| White | 0.27 | 0.11 | 2.00 | 13.1 | 0.37 | 3.68 | |
| Dark | 0.28 | 0.12 | 2.11 | 12.7 | 0.41 | 3.53 | |
| Prob > F | ns | ns | ns | ns | ns | ns |
| Days after Treatment | Treatment | Compression (kg) | Puncture (kg) | Weight (g) | TSS a (oBrix) | TA b (%) | pH |
|---|---|---|---|---|---|---|---|
| 0 | - | 0.15 | 0.11 | 1.13 | 14.0 | 0.41 | 3.30 |
| 4 | Blue | 0.15 | 0.10 | 1.08 | 14.0 | 0.35 | 3.33 |
| White | 0.15 | 0.10 | 1.16 | 14.2 | 0.36 | 3.35 | |
| Dark | 0.16 | 0.10 | 1.08 | 13.7 | 0.37 | 3.38 | |
| Prob > F | ns | ns | ns | ns | ns | ns | |
| 14 | Blue | 0.13 | 0.10 | 1.14 | 14.1 | 0.292 b | 3.58 |
| White | 0.15 | 0.11 | 1.06 | 14.2 | 0.295 ab | 3.63 | |
| Dark | 0.15 | 0.11 | 1.10 | 14.1 | 0.298 a | 3.75 | |
| Prob > F | ns | ns | ns | ns | 0.0415 | ns | |
| 24 | Blue | 0.16 | 0.11 | 1.10 | 13.8 | 0.28 | 3.53 |
| White | 0.17 | 0.12 | 1.13 | 13.9 | 0.27 | 3.48 | |
| Dark | 0.17 | 0.11 | 1.05 | 13.7 | 0.29 | 3.48 | |
| Prob > F | ns | ns | ns | ns | ns | ns |
| Days after Treatment | Treatment | L* | a* | b* | c* | h* |
|---|---|---|---|---|---|---|
| 0 | - | 29.37 | −0.72 | −3.60 | 3.71 | 258.71 |
| 4 | Blue | 30.23 a | −0.87 | −4.01 | 4.12 | 258.52 |
| White | 29.15 b | −0.64 | −3.44 | 3.55 | 261.12 | |
| Dark | 30.19 ab | −0.90 | −4.05 | 4.16 | 257.92 | |
| Prob > F | 0.0269 | ns | ns | ns | ns | |
| 15 | Blue | 30.66 | −0.89 | −4.24 | 4.40 | 257.35 |
| White | 29.79 | −0.65 | −3.86 | 4.07 | 258.62 | |
| Dark | 30.27 | −0.81 | −3.92 | 4.04 | 260.90 | |
| Prob > F | ns | ns | ns | ns | ns | |
| 21 | Blue | 30.41 | −1.00 | −4.37 | 4.50 | 257.78 |
| White | 30.07 | −1.03 | −4.22 | 4.36 | 256.47 | |
| Dark | 30.69 | −1.04 | −4.32 | 4.47 | 255.43 | |
| Prob > F | ns | ns | ns | ns | ns |
| Days after Treatment | Treatment | L* | a* | b* | c* | h* |
|---|---|---|---|---|---|---|
| 0 | - | 29.35 | −0.92 | −3.7 | 3.83 | 256.57 |
| 4 | Blue | 29.56 | −0.97 | −3.41 | 3.57 | 255.08 |
| White | 29.59 | −0.90 | −3.28 | 3.41 | 255.13 | |
| Dark | 29.42 | −0.93 | −3.36 | 3.50 | 255.28 | |
| Prob > F | ns | ns | ns | ns | ns | |
| 14 | Blue | 27.97 | −0.79 b | −3.08 | 3.27 | 253.69 |
| White | 28.35 | −0.58 ab | −2.83 | 2.96 | 259.74 | |
| Dark | 27.85 | −0.42 a | −2.74 | 2.87 | 261.24 | |
| Prob > F | ns | 0.0398 | ns | ns | ns | |
| 24 | Blue | 29.02 | −0.72 | −2.95 | 3.11 | 255.15 |
| White | 29.43 | −0.89 | −3.25 | 3.39 | 254.59 | |
| Dark | 28.84 | −0.58 | −2.55 | 2.83 | 256.67 | |
| Prob > F | ns | ns | ns | ns | ns |
| ‘Star’ | ‘Alapaha’ | |||
|---|---|---|---|---|
| Treatment | Final Disease Incidence (%) | AUDPC (%-Days) | Final Disease Incidence (%) | AUDPC (%-Days) |
| Blue | 27.3 | 343.5 a | 51.9 | 591.5 |
| White | 19.0 | 201.5 b | 44.9 | 656.5 |
| Dark | 21.0 | 222.1 b | 56.2 | 558.3 |
| Prob > F | ns | 0.0204 | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-W.; Ludwig, H.D.; Scherm, H.; van Iersel, M.W.; Nambeesan, S.U. Blue Light Does Not Affect Fruit Quality or Disease Development on Ripe Blueberry Fruit During Postharvest Cold Storage. Horticulturae 2020, 6, 59. https://doi.org/10.3390/horticulturae6040059
Wang Y-W, Ludwig HD, Scherm H, van Iersel MW, Nambeesan SU. Blue Light Does Not Affect Fruit Quality or Disease Development on Ripe Blueberry Fruit During Postharvest Cold Storage. Horticulturae. 2020; 6(4):59. https://doi.org/10.3390/horticulturae6040059
Chicago/Turabian StyleWang, Yi-Wen, Helaina D. Ludwig, Harald Scherm, Marc W. van Iersel, and Savithri U. Nambeesan. 2020. "Blue Light Does Not Affect Fruit Quality or Disease Development on Ripe Blueberry Fruit During Postharvest Cold Storage" Horticulturae 6, no. 4: 59. https://doi.org/10.3390/horticulturae6040059
APA StyleWang, Y.-W., Ludwig, H. D., Scherm, H., van Iersel, M. W., & Nambeesan, S. U. (2020). Blue Light Does Not Affect Fruit Quality or Disease Development on Ripe Blueberry Fruit During Postharvest Cold Storage. Horticulturae, 6(4), 59. https://doi.org/10.3390/horticulturae6040059






