Abstract
Climate change is increasing drought events and decreasing water availability. Tomato is commonly transplanted to an open field after seedling production in a nursery, requiring large volumes of water. Arbuscular mycorrhizal (AM) fungi help plants cope with drought stress; however, their effects depend on plant genotype and environmental conditions. In this study, we assessed the interactions among different tomato seedling genotypes and two AM fungi, Funneliformis mosseae and Rhizophagus intraradices, under two water regimes, full and reduced. Our results showed that F. mosseae was more effective than R. intraradices in the mitigation of drought stress both in old and modern genotypes. However, seedlings inoculated with R. intraradices recorded the highest values of leaf area. ‘Pearson’ and ‘Everton’ genotypes inoculated with F. mosseae recorded the highest values of root, leaf, and total dry weights under reduced and full irrigation regimes, respectively. In addition, ‘Pearson’ and ‘H3402’ genotypes inoculated with F. mosseae under a reduced irrigation regime displayed high values of water use efficiency. Our results highlight the importance of using AM fungi to mitigate drought stress in nursery production of tomato seedlings. However, the development of ad hoc AM fungal formulations, which consider genotype x AM fungi interactions, is fundamental for achieving the best agronomic performances.
1. Introduction
Crop growth, yield, and fruit quality are influenced by many abiotic factors, such as water, temperature, solar radiation, and salinity. When the potential transpiration rate exceeds water absorption by the roots from the soil, crops experience water stress. Water limitation causes the closure of plant stomata, leading to a decrease of carbon dioxide (CO2) uptake followed by a reduction in photosynthetic activity [1,2]. Additionally, drought stress reduces nutrient uptake, leading to a decrease in macro- and micro-element availability [3]. Finally, water deficits affect plant growth through the repression of gene expression related to cell division and proliferation [4,5].
Processing tomato is one of the most economically important and widespread horticultural crops in the world [6,7], and in 2018, its production was ~34 million tons [8]. In addition, processing tomato is extremely dependent on irrigation water, the average water requirement ranging from ~400 to 600 mm during the growing season based on the climatic conditions of the area [9]. Successful open field transplanting of nursery-grown seedlings is one of the key factors in producing high-yielding horticultural crops, such as processing tomato. Nonetheless, in many cases, the lack of closed and cooling systems for the production of tomato seedlings leads to an excessive use of irrigation water. In addition, climate change is increasing the frequency of heat waves, reducing water availability and increasing irrigation demands [10]. Hence, the excessive use of water for irrigation [11], combined with the effects of climate change [10,12], will limit the availability of fresh water for irrigation [10] even at early crop growth stages. As processing tomato requires huge volumes of irrigation water for their growth, a more scarce water availability during the growth cycle could lead to a decrease in fruit yield and quality [13].
Usually, when plants are subjected to water limitation, several strategies are used to overcome drought stress [14], and both morphological and physiological changes are observed as tolerance/resistance mechanisms used to cope with stress conditions [15]. In particular, plants accelerate phenological development, improve root growth and consequently water uptake, and control transpiration by stomatal regulation in order to avoid cellular damage caused by stress [15]. However, during severe and extended drought conditions, these mechanisms are not effective enough to preserve crop growth, fruit yield, and quality.
AM fungi are ubiquitous in soil and establish symbiotic relationships with the roots of many cultivated plants [6,16,17]. Plant–AM fungi interaction is mutualistic as the fungi can help plants overcome abiotic and biotic stresses and improve mineral nutrient and water uptake while the host plant provides photosynthates [16] and lipids [18]. During drought stress, AM fungi may influence physiological and cellular processes of the host plants [19]. Dell’Amico et al. [6] reported that Glomus clarum was able to stimulate ‘Amalia’ tomato growth under drought stress in a greenhouse, and the effects were more evident on leaves and shoots than on roots. Likewise, Duc et al. [20] reported that the use of AM fungus Septoglomus constrictum improved stomatal conductance, leaf water potential, leaf relative water content, and the activity of photosystem II of a greenhouse genotype ‘Moneymaker’ under combined heat and drought stress when grown in a greenhouse. However, studies have also revealed that microorganism effects depend on plant genotype [21,22].
Although there are many studies on the mitigation of crop drought stress by AM fungi conducted in open fields or on adult plants grown in greenhouses [2], research with processing tomato seedlings (at the four true expanded leaves stage, corresponding to 35 to 40 d after sowing) are scarce. Studies on tomato seedlings are necessary as seedlings at this stage are transplanted to the open field. Therefore, in the present study, we assessed the interactions among three genotypes of processing tomato seedlings and two AM fungi (Funneliformis mosseae and Rhizophagus intraradices) under two water regimes (full irrigation and reduced irrigation) in order to provide useful information to nursery growers.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The experiment was conducted in a growth chamber located at the University of Modena and Reggio Emilia (Reggio Emilia, Italy) following a fully randomized experimental design with five biological repetitions per treatment (genotype by AM species by irrigation regime). Three genotypes of processing tomato (‘Pearson’, ‘H3402’, and ‘Everton’) were used in the experiment. ‘Pearson’ is an old genotype released in the mid-1930s, with a semi-determinate growth habit, large fruits, and suitable for canning. ‘H3402’ is a modern genotype released in 2002, with a determinate growth habit, high vigor, and medium oval fruit, and it is suitable for medium and late transplanting and canning purposes. ‘Everton’ is a modern genotype released in 2008, with a determinate growth habit, medium vigor, and oval fruit suitable for medium transplanting and canning purposes [22,23].
Processing tomato seeds were provided by ISI Sementi S.p.A. (Fidenza, Italy) and Furia Seed (Monticelli Terme, Italy). Seeds were germinated on moistened filter paper in Petri dishes at 25 °C, and transferred after germination to pots (7 × 7 × 8 cm, 0.4 L) (one germinated seed per pot) containing the same quantity of neutralized peat (23% organic carbon, 0.5% nitrogen, pH 6, electrical conductivity 0.25 dS m−1, and dry apparent density 214 kg m−3; Dueemme S.r.l., Reggio Emilia). Pure inocula of AM fungi were obtained from MycAgro, LabTechnopôle Agro Environnement, Bretenière, France. F. mosseae and R. intraradices (10 propagules per 1 g) were separately mixed with peat 10:100 (w/w) as suggested by Rivero et al. [24]. Equal amounts of propagules/peat mix were added to pots before transferring germinated seeds to pots. After seedling emergence, each pot was covered with a transparent plastic sheet in order to reduce water evaporation.
Plants were cultivated at 25 °C day/19 °C night with a 16-h photoperiod under an irradiance of 180 µmol m−2 s−1 (white fluorescent tubes Fluora 18W/77, Osram, Munich, Germany). The seedlings were fully watered until 21 d after sowing. Subsequently, the seedlings were subjected to one of two different irrigation regimes for 15 d (fully-watered and reduced) based on relative soil water content (RSWC) that was controlled gravimetrically by weighing the pots every day [25]. In particular, every day the amount of water lost by transpiration was added to each pot in order to keep the soil water content at the desired levels of volumetric soil moisture (100% and 55%, respectively). Table 1 summarizes all the treatments of the experiment.

Table 1.
Genotypes, microorganisms, and water irrigation regimes of experiment.
2.2. Morphological and Physiological Parameters Assessed
Five seedlings per treatment were assessed at the end of drought stress. The impact of the different treatments on processing tomato seedling growth was assessed by recording the number of leaves, leaf area, seedling height, stem diameter, height/diameter ratio, and plant dry weight. Total and partitioned (leaves, stems, and roots) dry weights were obtained by oven-drying the fresh biomass at 65 °C until a constant weight. Leaf area was measured using an area meter LI-3000A (LI-COR, Lincoln, Nebraska, USA). In addition, leaf mass per area (LMA), a key parameter in plant growth and an important indicator of plant functioning, was calculated as the ratio between the leaf dry weight and leaf area.
Leaf chlorophyll, flavonoid, and anthocyanin content and nitrogen balance index were considered as physiological parameters. The leaf content of chlorophyll (Chl), flavonoids (Flav), and anthocyanins (Anth) was estimated on the youngest fully expanded leaf using Dualex 4 Scientific (Dx4) (FORCE-A, Orsay, France). The nitrogen balance index (NBI) was calculated as the ratio between Chl and Flav [26].
In order to understand the responses of seedlings to the different water regimes, the total water used by plants (TWU) was calculated as the sum of all the water applied during the experiment while the water use efficiency (WUE) was calculated as the ratio between the total dry weight of the seedlings the total water used.
2.3. SybrGreen PCR Analysis of AMF Presence
Root subsamples were randomly chosen from three plants per treatment. Roots were washed with tap water, frozen in liquid nitrogen, and stored at −80 °C for analysis. The AM fungal root presence was evaluated with a SybrGreen PCR approach. Frozen root samples were finely pulverized in a sterile mortar using liquid nitrogen. As reported in Caradonia et al. [22], the powder (300 mg) was mixed with 500 μL of extraction buffer (40 mM Tris-acetate, 20 mM sodium acetate, 1 mM EDTA, 1% w/v SDS (sodium dodecyl sulfate) pH 7.8), and 5 μL RNase (500 μg μL−1). After incubation at 37 °C for 600 s to digest the contaminating RNA, 150 μL of NaCl was added. The suspension was centrifuged at 12,000× g for 1200 s at 4 °C and the supernatant mixed with 400 μL of chloroform and 400 μL of phenol, then centrifuged at 12,000× g for 1200 s at 4 °C. The upper phase containing DNA was precipitated with 2 volumes of ethanol 95% (v/v). DNA was eluted with 50 μL of elution buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.8). The DNA concentrations were determined using a NanoDrop 1000 spectrophotometer at 260 and 280 nm (Thermo Fisher Scientific, Wilmington, DE, USA).
For SybrGreen PCR, the following primer pairs were used for F. mosseae [27]: MOSF 5′-GAAGTCAGTCATACCAACGGGAA-3′ and MOSR 5′-CTCGCGAATCCGAAGGC-3′ while GI-mtLSU-499F 5′-GAGGGAGTGGCAGTTTCTT-3′ and GI-mtLSU-632R 5′-GCATTCTTAGCCCAGCTATG-3′ were used for R. intraradices [28].
In order to check the amplification of DNA, all the samples were also amplified using primer pairs coding for Elongation factor 1-alpha X144449, a tomato housekeeping gene: EF1AFFxMF 5′ -CTCCGTCTTCCACTTCAGGAC-3′ and EF1AFFxMR 5′ -GTCACAACCATACCAGGCTTG [29].
The amplification was carried out in a 25-μL volume containing 12.5 μL of SYBR Green PCR, 2X GoTaq qPCR Master Mix (Promega), 0.25 μL of 100X Reference Dye (Promega), 0.3 μL of forward and reverse primers (10 μm), 5 μL of template DNA (10 ng μL−1), and water to 25 μL.
Reactions were repeated twice with a 7300 real-time PCR Systems (Applied Biosystems, Foster City, CA, USA) and with the following cycling protocol: 50 °C for 120 s, 95 °C for 600 s, 40 cycles of 95 °C for 15 s, and 60 °C for 60 s. A melting curve analysis (95 °C for 15 s, 60 °C for 30 s, 95 °C for 15 s) was always included in each run to control for false-positive results caused by primer-dimer hybridization and non-specific amplifications.
2.4. Statistical Analysis
The data were subjected to a three-way analysis of variance (ANOVA) to examine the effects of genotype, AM fungal inoculation, and irrigation regime by GenStat 17th edition. Means were compared using Duncan’s multiple range test (DMRT) at P < 0.05. In order to evaluate the relationships between the treatments and parameters analyzed, all data were analyzed by the principal component analysis (PCA) model [30,31] using PLS Toolbox software (Eigenvector Research Inc, Wenatchee, WA, USA). Difference between groups were assessed by multivariate analysis (MANOVA).
3. Results
3.1. Physiological and Morphological Results
The genotype and mycorrhizal treatment main effects on leaf chlorophyll content were not statistically significant (Table 2). However, the interaction between genotype and mycorrhizal inoculation was significant. In particular, the chlorophyll content was the highest in the genotype ‘Everton’ inoculated with R. intraradices under the reduced irrigation regime (Table S1).

Table 2.
Physiological parameters after treatment.
Leaf flavonoid content was affected by genotype, irrigation regime, and by the interactions genotype x AM fungal inoculation and genotype x irrigation (Table 2). The highest values were shown by ‘Everton’ inoculated with R. intraradices under the fully-watered irrigation regime, ‘H3402’ without mycorrhizal inoculation under the reduced irrigation regime, and ‘Pearson’ inoculated with F. mosseae under the fully-watered irrigation regime.
NBI was affected by the interaction among AM fungal inoculation, irrigation regime, and genotype. NBI values were the highest in ‘Everton’ inoculated with R. intraradices under the reduced irrigation regime. On the other hand, ‘H3402’ inoculated with F. mosseae showed the highest NBI values under the reduced irrigation regime (Table S1). Finally, ‘Pearson’ showed the highest values inoculated with AM fungus (independent of AM fungal species) under the reduced irrigation regime.
Interestingly, AMF inoculation and irrigation regimes influenced Anth values. The seedlings inoculated with F. mosseae showed the lowest Anth values in comparison with the seedlings without inoculation or inoculated with R. intraradices (Table 2).
Plant height is one of the most important parameters in processing tomato seedlings when grown in a nursery. In this study, plant height was influenced by the interaction among genotype x AM fungal inoculation x irrigation regime (Table 3). The highest value was displayed by ‘Pearson’ inoculated with R. intraradices under the fully-watered irrigation regime (Table S2). On the other hand, the lowest value was recorded by ‘Pearson’ inoculated with R. intraradices under the reduced irrigation regime.

Table 3.
Morphological parameters measured after treatment.
A significative interaction between AM fungal inoculation and irrigation regime was found for stem diameter. The highest values were achieved by ‘Pearson’, ‘H3402’, and ‘Everton’ inoculated with F. mosseae under the fully-watered regime. However, ‘H3402’ inoculated with F. mosseae under the reduced irrigation regime showed the lowest value in comparison with ‘Everton’ and ‘Pearson’.
For the ratio between the plant height and stem diameter, we noticed significant interactions among AM fungal inoculation, genotype, and the irrigation regime. The highest values of the plant height and stem diameter ratio were achieved by ‘H3402’ and ‘Pearson’ inoculated with R. intraradices and ‘Everton’ without AM fungal inoculations under the fully-watered irrigation regime.
Leaf traits (number of leaves, leaf area, leaf mass per area, and leaf dry weight) were influenced by interactions among all the treatments (Table 3; Table 4). ‘Everton’ and ‘H3402’ without mycorrhizal inoculation under the full irrigation regime displayed the highest number of leaves (Table S2). On the other hand, ‘Pearson’ showed the highest number of leaves when inoculated with F. mosseae under the reduced irrigation regime and inoculated with R. intraradices under the fully-watered irrigation regime (Table S2). ‘H3402’ and ‘Pearson’ inoculated with F. mosseae under the fully-watered irrigation regime achieved the highest leaf area values (Table S2). On the other hand, ‘Everton’ displayed the highest leaf area values when inoculated with F. mosseae under the fully-watered irrigation regime and inoculated with R. intraradices under the reduced irrigation regime. In the reduced irrigation regime, the decrease in the leaf area of the seedlings was accompanied by an increase in the leaf mass per area. These compensating changes led to little change in the leaf dry mass per seedling (decreased in ‘Everton’ inoculated with F. mosseae and increased in ‘H3402’ non-inoculated or inoculated with F. mosseae). ‘H3402’ showed the highest leaf dry weight values without inoculations under the reduced irrigation regime, ‘Pearson’ displayed the highest value inoculated with F. mosseae under the reduced irrigation regime, while ‘Everton’ was inoculated with F. mosseae under the full-water irrigation regime (Table S3).

Table 4.
Biomass parameters measured destructively after treatment.
Stem dry weight, total dry weight, and water use efficiency were influenced by interactions among genotype, AM fungal inoculation, and irrigation regime (Table 4 and Table 5). Stem dry weights and the total dry weights were highest in seedlings inoculated with F. mosseae. On the other hand, the highest water use efficiency values were reached by genotypes ‘H3402’ and ‘Pearson’. Finally, ‘Pearson’ and ‘H3402’ inoculated with F. mosseae under the reduced irrigation regime achieved the highest value of water use efficiency and a similar result was displayed by non-inoculated ‘Pearson’ plants (Table S4).

Table 5.
Water use parameters after treatment.
Root dry weight and root and shoot ratio were influenced by mycorrhizal treatments that interacted with genotype (Table 4). Root dry weight decreased in ‘Everton’ inoculated with F. mosseae under the reduced irrigation regime.
3.2. Relationships among Investigated Treatments and Parameters
The correlations among treatments and parameters were studied using PCA (Figure 1).
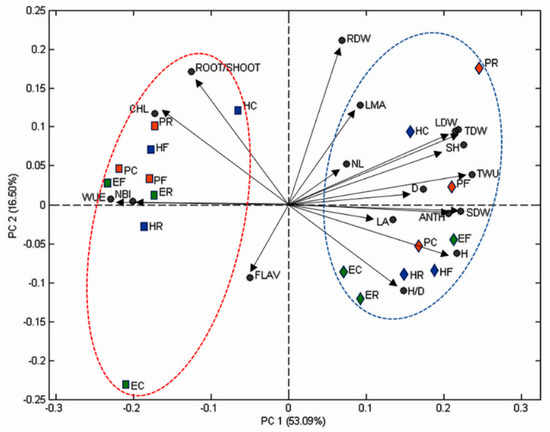
Figure 1.
Biplot of principal component analysis. Abbreviations: CHL = index of leaf chlorophyll content; FLAV = index of leaf flavonoid content; NBI = nitrogen balance index; ANTH = index of leaf anthocyanin content; H = height of seedling; D = diameter of seedling; H/D = height to diameter ratio of seedling; NL = number of fully expanded leaves per seedling; LA = leaf area of the seedling; LMA = leaf mass per area of the seedling; LDW = leaf dry weight; SDW = stem dry weight; RDW = root dry weight; SH = shoot weight (LDW + SDW); TDW = total dry weight of the seedling; ROOT/SHOOT = root and shoot ratio; TWU = total water used by seedling; WUE = water use efficiency; PC = ‘Pearson’ without mycorrhizal inoculation; PF = ‘Pearson’ inoculated with F. mosseae; PR = ‘Pearson’ inoculated with R. intraradices; HC = ‘H3402’ without mycorrhizal inoculation; HF = ‘H3402’ inoculated with F. mosseae; HR = ‘H3402’ inoculated with R. intraradices; EC = ‘Everton’ without mycorrhizal inoculation; EF = ‘Everton’ inoculated with F. mosseae; ER = ‘Everton’ inoculated with R. intraradices. Symbols: squares = reduced irrigation regime; diamonds = fully-watered irrigation regime; green = ‘Everton’; blue = ‘H3402’; red = ‘Pearson’: red ellipse groups all the genotypes under reduced irrigation regime; blue ellipse groups all the genotypes under fully-watered irrigation regime.
The contributions of the two first principal components were 53.1% (PC1) and 16.5% (PC2) and their sum explained 69.6% of the total variation. The irrigation regime clustered in two groups. In fact, all the combinations of genotype x mycorrhizae treatment under the reduced irrigation regime were on the negative side of the PC1 while all the combinations of genotype x mycorrhizae treatment under the full irrigation regime were on the positive side of PC1. Therefore, PC1 was clearly related with the irrigation regime. In addition, MANOVA analysis confirmed that the two clusters were statistically different at P < 0.05. In addition, ‘H3402’ without mycorrhizal inoculation (HC) and ‘Pearson’ inoculated with R. intraradices (PR) were in the positive square while ‘H3402’ inoculated with R. intraradices (HR) and ‘Everton’ without mycorrhizal inoculation (EC) were in the negative one. In addition, WUE was positive correlated with CHL and the root/shoot ratio (Figure S1). Finally, focusing the attention on the genotype, the old genotype ‘Pearson’ that was not inoculated (PC) performed as well as PF under drought stress in terms of WUE while the same trend was not observed in the modern genotypes.
3.3. AM Fungal Root Presence
A melting curve profile was used to track the presence of inoculated mycorrhizal species. As reported in Figure 2, all the samples of three processing tomato genotypes (1A-C) were amplified by primer pairs of the tomato housekeeping gene, confirming the amplifiability of root DNA. Based on SybrGreen PCR analysis, the presence of F. mosseae and R. intraradices DNA was confirmed in the roots of all three processing tomato genotypes inoculated with F. mosseae and R. intraradices, respectively (Figure 2). In contrast, all non-inoculated roots (controls) were negative for F. mosseae and R. intraradices DNA presence (Figure 2).
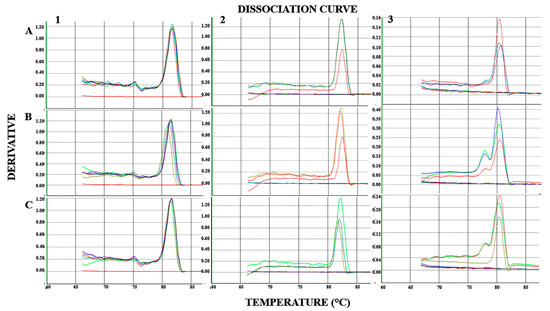
Figure 2.
Dissociation curves to confirm presence of arbuscular mycorrhizal fungi in roots. Columns: non-inoculated (1), F. mosseae presence (2) and R. intraradices presence (3). Rows: ‘Pearson’ (A), ‘H3402’ (B), and ‘Everton’ (C). Single peaks were obtained from different samples (each peak of different color represents a samples). Flat lines are no template control (NTC) technical replicates.
4. Discussion
Climate change can affect water availability in many agricultural areas [11,13]. Water management is a crucial factor for processing tomato, and limited availability of water resources in terms of both irrigation volumes and quality could reduce fruit yield and quality [13,32]. Hence, farmers and researchers are facing this problem in every operation of the production system. Possible solutions include reduction of irrigation volumes required for seedling production in the nursery as well as the volume of water used in the field by the processing tomato, and the inoculation of the crop with AM fungi to help the crop cope with this stress. While several attempts have been undertaken in an open field [33] or with adult plants in a greenhouse [2,33], to our knowledge, little information has been reported on the reduction of irrigation water and inoculation with AM fungi for seedlings at the stage of four true, expanded leaves.
In the present study we showed how different genotypes of processing tomato seedlings at the stage of 35 to 40 d after germination responded to different mycorrhizal symbioses under different irrigation regimes. Physiological parameters were assessed at the end of a drought stress period including leaf chlorophyll, flavonoid, and anthocyanin content. Chlorophyll is a key pigment involved in photosynthesis while the accumulation of anthocyanins and flavonoids in plants is often linked to the plant response to stress conditions [22,28]. In our experiment, beneficial AM fungal effects were observed on leaf chlorophyll content for all genotypes. However, the beneficial effects varied with the genotype, AM fungal species, and water availability (Table S1). These results agree with the findings by Londoño et al. [34], who noticed that inoculation with Rhizophagus clarus led to an increase in total chlorophyll concentration which varied between maize genotypes.
The content of leaf secondary metabolites, expressed as leaf anthocyanins and flavonoids, was influenced by the genotype and AM fungal inoculation (Table 2 and Table S1). In fact, AM fungi are known to affect not only plant development and nutrient uptake but also plant secondary metabolism, especially in root and shoot tissues [35].
Observing morphological parameters (Table 3 and Table S2), an increase in chlorophyll content in seedlings under moderate drought stress was not reflected in an increase of the net assimilation rate corresponding to a higher plant growth. In fact, the seedlings under the reduced irrigation regime were smaller in plant height, stem diameter, and number of leaves. These data highlight the importance of irrigation water in processing tomato development during seedling production. Nonetheless, AM fungi positively influenced leaf area, and the seedlings inoculated with R. intraradices achieved the highest values. This observation, together with the increased primary metabolism, might be an interesting aspect to take into account for seedling production in nursery conditions.
One of the most important parameters, both in agronomic and physiological studies, is the plant total dry weight. We observed a different partitioning of dry weight biomass to roots and stem; seedlings under drought stress had the highest allocation to the roots while the stem showed the highest value under normal irrigation. As previously reported, this pattern of biomass accumulation confirmed the ability of tomato plants to cope with drought stress, inducing morphological changes. However, when genotype and the AM fungal inoculation effects were considered, there was a different behavior between old and modern genotypes. ‘Pearson’ recorded the highest values of the root, stem, leaf, and total dry weights when inoculated with F. mosseae under partial irrigation, while ‘H3402’ showed the highest dry weight values for the different tissues when not inoculated and grown under partial irrigation. The ability of tomato to produce high biomass under reduced irrigation was previously reported by Patanè et al. [36] in an experiment performed with the processing tomato genotype ‘Brigade’ in the open field. Finally, ‘Everton’ performed better under full irrigation and when inoculated with F. mosseae. Our results also confirmed that AM fungal effects on plant biomass depend on interactions with genotypes and cropping systems as previously shown by Londoño et al. and Steinkellner et al. [34,37]. In contrast, a negative effect of R. intraradices on plant biomass was observed. These results could be explained by the parasitic behavior of AM fungi due to environmental conditions that limit plant growth and are not symbiotic [38,39,40].
Water use efficiency is the most important parameter which assesses the ability of plants to maximize the use of the available water in the production of biomass. In our results, plants inoculated with F. mosseae showed higher values of WUE compared to those inoculated with R. intraradices. In fact, ‘H3402’ inoculated with F. mosseae displayed the highest values of WUE during drought stress. In contrast to these findings, Chitarra et al. [2] and Volpe et al. [41] observed that tomato plants inoculated with R. intraradices achieved the highest WUE. These differences could be due to the different growth conditions as Chitarra et al. [2] worked under severe drought stress conditions and with adult plants and a different genotype (‘San Marzano nano’), confirming the importance of assessing the interactions among the available AM fungi and genotypes in different plant phenological stages and cropping systems.
The AM fungal root presence was displayed for all the genotype x irrigation regimes, confirming the data reported by Steinkellner et al. [38], who investigated old and modern genotypes of tomatoes bred for greenhouse and fresh market purposes.
Our findings highlighted that the breeding program for processing tomato (the introgression of favorable traits to the harvesting machinery associated with reduced plant height, average fruit weight, and increased marketable yields) [42] did not select against AM fungal symbiosis in processing tomato. These results corroborated those previously reported by De Vita et al. [43] for durum wheat.
5. Conclusions
The present study provides useful information to nursery growers on the application of AM fungi for the production of processing tomato seedlings during drought stress. Our results showed how AM fungi could improve drought tolerance and enhance tomato seedling growth. The present study suggests that the two AMF studied activated different physiological strategies in processing tomato seedlings to cope with drought stress. However, F. mosseae seemed more effective than R. intraradices on influencing several morphological and physiological traits. In fact, ‘Pearson’ and ‘Everton’ inoculated with F. mosseae recorded the highest values of root, leaf, and total dry weights under reduced and fully-watered irrigation regimes, respectively. In addition, seedlings of ‘Pearson’ and ‘H3402’ inoculated with F. mosseae under reduced irrigation displayed the highest values of leaf chlorophyll content, nitrogen balance index, and water use efficiency. On the other hand, seedlings inoculated with R. intraradices recorded the highest values of leaf area. Our results confirmed the importance of developing ad hoc formulates based on AM fungal consortia that take into account the environmental conditions, plant genotype, and AM fungi interactions in order to achieve the best outcomes in terms of plant resilience/tolerance to adverse conditions. These aspects are particularly important in the current scenario of climate change characterized by a reduction of water availability for agricultural purposes. Hence, a multidisciplinary approach to investigate the interactions between the most cultivated genotypes and AM fungi is urgently needed and is fundamental to obtaining useful agronomic strategies that can promptly improve the sustainability of processing tomato production.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-7524/5/4/79/s1, Table S1; Physiological parameters measured at the end of the experiment; Table S2: Morphological non-destructive parameters measured at the end of the experiment; Table S3: Morphological destructive parameters measured at the end of the experiment; Table S4: Parameters measured at the end of the experiment in order to understand the responses of seedlings to the different water regime; Figure S1: Correlation plot.
Author Contributions
Conceptualization, V.T. and D.R.; methodology, F.-W.B. and F.R.; investigation, F.C., D.R., C.M. and R.G.; data curation, D.R. and F.C.; Writing—Original draft preparation, F.C. and D.R.; Review and editing, E.F., C.M., R.G., F.-W.B., F.R., V.T.; supervision, F.-W.B. and V.T.; funding acquisition, E.F. and V.T.
Funding
This work was partially supported by the GENBACCA project, Regione Emilia Romagna, POR-FESR 2014/2020 and by BIOPRIME MiPAAF project (DIQPAI - N.0003400 del 20/12/2018).
Acknowledgments
We thank Justyna Anna Milc for the critical comments and suggestions on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Increasing drought decreases phosphorus availability in an evergreen Mediterranean forest. Plant Soil 2004, 267, 367–377. [Google Scholar] [CrossRef]
- Claeys, H.; Inze, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.-S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amico, J.; Torrecilla, A.; Rodríguez, P.; Morte, A.; Sánchez-Blanco, M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002, 138, 387–393. [Google Scholar] [CrossRef]
- Ronga, D.; Gallingani, T.; Zaccardelli, M.; Perrone, D.; Francia, E.; Milc, J.; Pecchioni, N. Carbon footprint and energetic analysis of tomato production in the organic vs. the conventional cropping systems in Southern Italy. J. Clean. Prod. 2019, 220, 836–845. [Google Scholar] [CrossRef]
- World Processing Tomato Council (WPTC). Available online: www.wptc.to/pdf/releases/WPTC%20crop%20update%20as%20of%2025%20October%202018.pdf (accessed on 24 July 2019).
- Rana, G.; Rinaldi, M.; Introna, M.; Cicirietti, L. Determinazione Sperimentale Dei Consumi Idrici Del Pomodoro Da Industria in Capitanata. In Atti del Convegno “Modelli di Agricoltura Sostenibile per la Pianura Meridionale: Gestione Delle Risorse Idriche Nelle Pianure Irrigue”; Gutenberg: Salerno, Italy, 6 November 2000; pp. 99–106. [Google Scholar]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Cammarano, D.; Ceccarelli, S.; Grando, S.; Romagosa, I.; Benbelkacem, A.; Akar, T.; Al-Yassin, A.; Pecchioni, N.; Francia, E.; Ronga, D. The impact of climate change on barley yield in the Mediterranean basin. Eur. J. Agron. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Cammarano, D.; Hawes, C.; Squire, G.; Holland, J.; Rivington, M.; Murgia, T.; Roggero, P.P.; Fontana, F.; Casa, R.; Ronga, D. Rainfall and temperature impacts on barley (Hordeum vulgare L.) yield and malting quality in Scotland. Field Crop Res. 2019, 241, 107559. [Google Scholar] [CrossRef]
- Bisbis, M.; Gruda, S.N.; Blanke, M.M. Securing Horticulture in a Changing Climate—A Mini Review. Horticulturae 2019, 5, 56. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Bakr, J.; Pék, Z.; Helyes, L.; Posta, K. Mycorrhizal Inoculation Alleviates Water Deficit Impact on Field-Grown Processing Tomato. Pol. J. Environ. Stud. 2018, 27, 1949–1958. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Arbuscular mycorrhizal dialogues: Do you speak “plantish” or “fungish”? Trends Plant Sci. 2015, 20, 150–154. [Google Scholar] [CrossRef]
- Krak, K.; Janoušková, M.; Caklová, P.; Vosátka, M.; Štorchová, H. Intraradical dynamics of two coexisting isolates of the arbuscular mycorrhizal fungus Glomus intraradices sensu lato as estimated by real-time PCR of mitochondrial DNA. Appl. Environ. Microbiol. 2012, 78, 3630–3637. [Google Scholar] [CrossRef]
- Bravo, A.; Brands, M.; Wewer, V.; Dörmann, P.; Harrison, M.J. Arbuscular mycorrhiza-specific enzymes fatm and ram2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017, 214, 1631–1645. [Google Scholar] [CrossRef]
- Sanchez-Romera, B.; Calvo-Polanco, M.; Ruiz-Lozano, J.M.; Zamarreno, A.M.; Arbona, V.; Garcia-Mina, J.M.; Gomez-Cadenas, A.; Aroca, R. Involvement of the def-1 Mutation in the Response of Tomato Plants to Arbuscular Mycorrhizal Symbiosis Under Well-Watered and Drought Conditions. Plant Cell Physiol. 2018, 59, 248–261. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Garcia de Salamone, I.E.; Funes, J.M.; Di Salvo, L.P.; Escobar Ortega, J.S.; D’Auria, F.; Ferrando, L.; Fernandez Scavino, A. Inoculation of paddy rice with Azospirillum brasilense and Pseudomonas fluorescens: Impact of plant genotypes on the rhizosphere microbial communities and field crop production. Appl. Soil Ecol. 2012, 61, 196–204. [Google Scholar] [CrossRef]
- Caradonia, F.; Francia, E.; Morcia, C.; Ghizzoni, R.; Moulin, L.; Terzi, V.; Ronga, D. Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria Avoid Processing Tomato Leaf Damage during Chilling Stress. Agronomy 2019, 9, 299. [Google Scholar] [CrossRef]
- Ronga, D.; Rizza, F.; Badeck, F.W.; Milc, J.; Laviano, L.; Montevecchi, G.; Nicola, P.; Francia, E. Physiological responses to chilling in cultivars of processing tomato released and cultivated over the past decades in Southern Europe. Sci. Hortic. 2018, 231, 118–125. [Google Scholar] [CrossRef]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 2015, 6, 598. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Morcia, C.; Carletti, P.; Ghizzoni, R.; Badeck, F.W.; Rizza, F.; Lucini, L.; Terzi, V. Proteomic insight into the mitigation of wheat root drought stress by arbuscular mycorrhizae. J. Proteom. 2017, 169, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Cartelat, A.; Goulas, Y.; Meyer, S. In-the-field assessment of wheat leaf polyphenolics using the new optical leaf-clip DUALEX. Precis. Agric. 2005, 5, 243–249. [Google Scholar]
- Alkan, N.; Gadkar, V.; Yarden, O.; Kapulnik, Y. Analysis of quantitative interactions between two species of arbuscular mycorrhizal fungi, Funneliformis mosseae and G. intraradices, by real-time PCR. Appl. Environ. Microbiol. 2006, 72, 4192–4199. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemometr. Intel. Lab. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Jackson, J.E. A Users Guide to Principal Components; Nr. 1; Wiley & Sons Ltd.: New York, NY, USA, 1991. [Google Scholar]
- Francaviglia, R.; Di Bene, C. Deficit Drip Irrigation in Processing Tomato Production in the Mediterranean Basin. A Data Analysis for Italy. Agriculture 2019, 9, 79. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hort. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Londoño, D.M.M.; Meyer, E.; González, D.; Hernández, A.G.; Soares, C.R.S.F.; Lovato, P.E. Landrace maize varieties differ from conventional and genetically modified hybrid maize in response to inoculation with arbuscular mycorrhizal fungi. Mycorrhiza 2019, 29, 237–249. [Google Scholar]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Pseudomonads Increases Anthocyanin Concentration in Strawberry Fruits (Fragaria x ananassa var. Selva) in Conditions of Reduced Fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Horti. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Steinkellner, S.; Hage-Ahmed, K.; Garcia-Garrido, J.M.; Illna, A.; Ocampo, J.A.; Vierheilig, H. A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f. sp. lycopersici. Mycorrhiza 2011, 22, 189–194. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi-’Pearson’, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Jones, M.D.; Smith, S.E. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Can. J. Bot. 2004, 82, 1089–1109. [Google Scholar] [CrossRef]
- Veiga, R.S.L.; Jansa, J.; Frossard, E.; Van Der Heijden, M.G.A. Can arbuscular mycorrhizal fungi reduce the growth of agricultural weeds? PLoS ONE 2011, 6, e27825. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
- Ronga, D.; Francia, E.; Rizza, F.; Badeck, F.W.; Caradonia, F.; Montevecchi, G.; Pecchioni, N. Changes in yield components, morphological, physiological and fruit quality traits in processing tomato cultivated in Italy since the 1930’s. Sci. Hortic. 2019, 257, 108726. [Google Scholar] [CrossRef]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Sci. Rep. 2018, 8, 10612. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).