Response of Mediterranean Ornamental Plants to Drought Stress
Abstract
1. Introduction
2. Ornamental Plant Response to Drought Stress
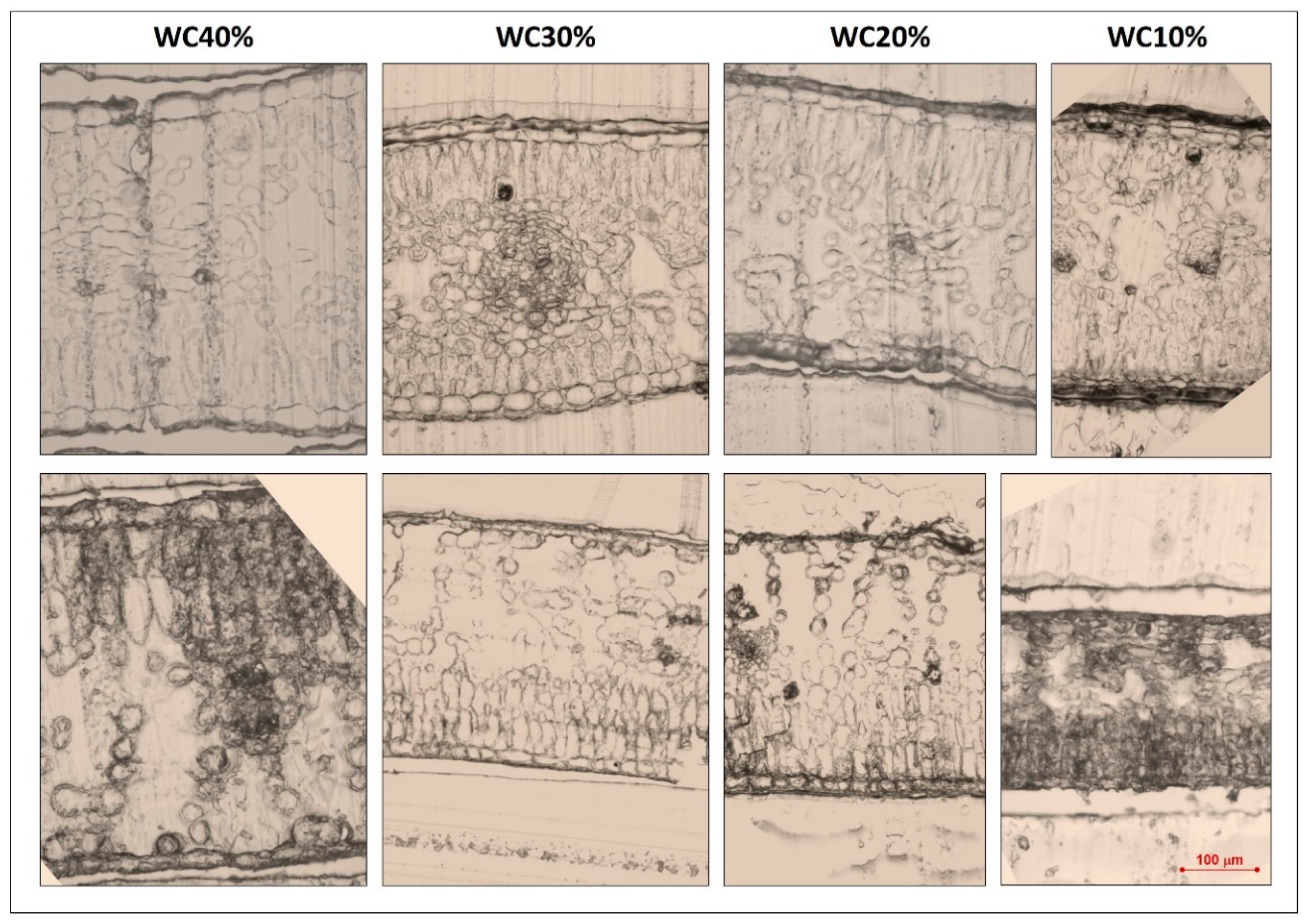
2.1. Growth and Morpho-Anatomical Modification
2.2. Physiological Parameters
2.2.1. Leaf Gas Exchange
2.2.2. Chlorophyll a Fluorescence
2.3. Oxidative Stress
3. Mechanism of Signal Transduction and Development of Drought Tolerance
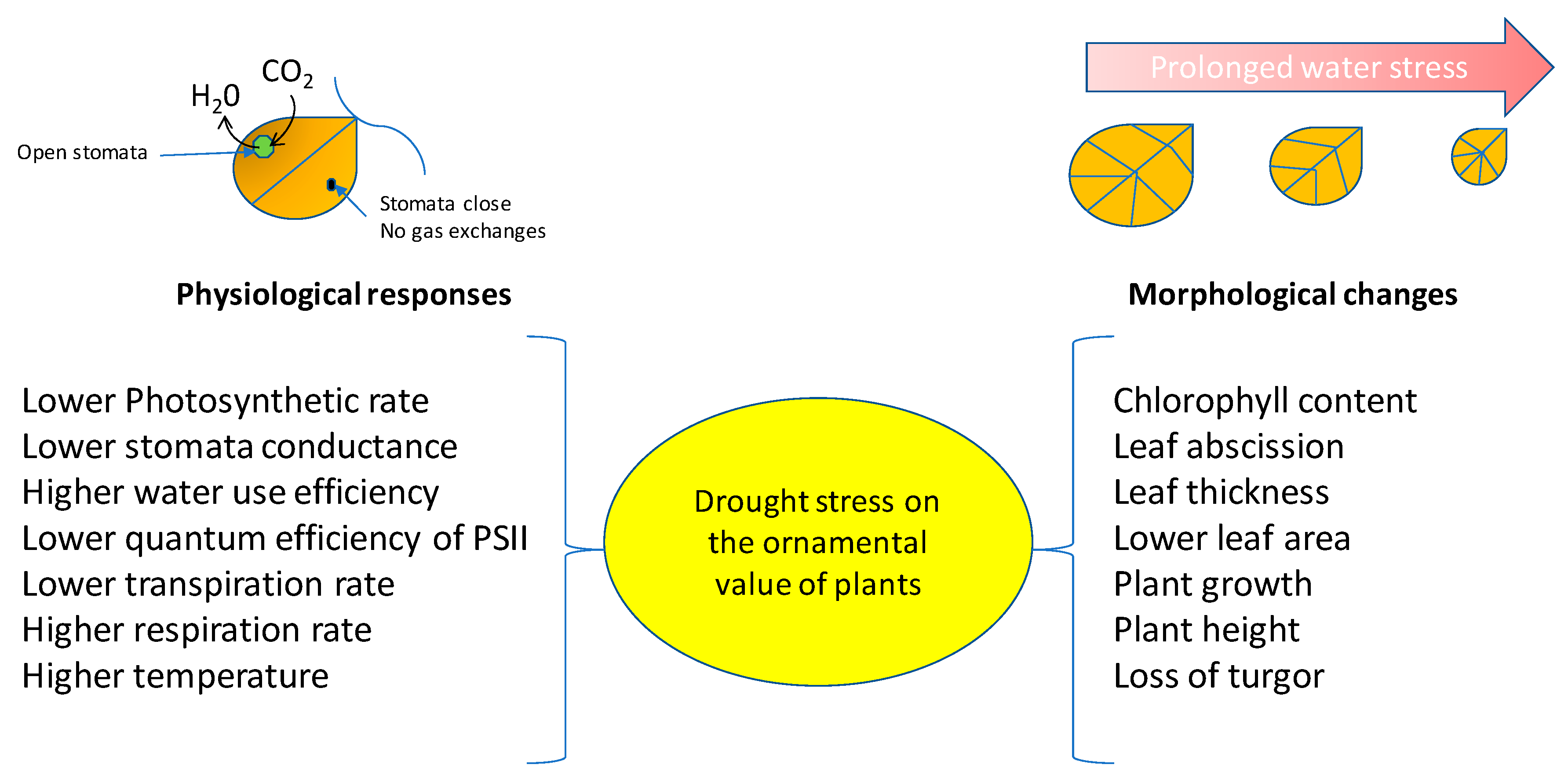
4. Effects of Drought Stress on the Ornamental Value of Plants
5. Use of Different Tools in Mitigating Drought-Induced Damages
6. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Pausas, J.G.; Vargas, P. Fire and plant diversification in mediterranean-climate regions. Front. Plant Sci. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Paz, S.; Negev, M.; Clermont, A.; Green, M.S. Health aspects of climate change in cities with mediterranean climate, and local adaptation plans. Int. J. Environ. Res. Public Health 2016, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- WWAP (World Water Assessment Programme). The United Nations World Water Development Report 2014: Water and Energy; UNESCO: Paris, France, 2014. [Google Scholar]
- Ouzounidou, G.; Vekiari, S.; Asfi, M.; Gork, M.G.; Sakcali, M.S.; Ozturk, M. Photosynthetic characteristics of carob tree (Ceratonia siliqua L.) and chemical composition of its fruit on diurnal and seasonal basis. Pak. J. Bot. 2012, 44, 1689–1695. [Google Scholar]
- Thayer, R.L. Visual ecology: Revitalizing the esthetics of landscape architecture. Landscape 1976, 20, 37–43. [Google Scholar]
- Savé, R. What is stress and how to deal with it in ornamental plants? Acta Hort. 2009, 813, 241–254. [Google Scholar] [CrossRef]
- Kjelgren, R.; Rupp, L.; Kilgren, D. Water conservation in urban landscapes. HortScience 2000, 35, 1037–1040. [Google Scholar]
- Cameron, R.W.F.; Wilkinson, S.; Davies, W.J.; Harrison Murray, R.S.; Dunstan, D.; Burgess, C. Regulation of plant growth in container-grown ornamentals through the use of controlled irrigation. Acta Hortic. 2004, 630, 305–312. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Aguiniga, L.; Mackay, W. Salinity tolerance of Lupinus havardii and Lupinus texenis. HortScience 2007, 42, 526–528. [Google Scholar]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of Pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Rodriguez, P.; Morales, M.A.; Torrecillas, A. Comparative growth and water relations of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci. 2002, 162, 107–113. [Google Scholar] [CrossRef]
- Torrecillas, A.; Rodriguez, P.; Sánchez-Blanco, M.J. Comparison of growth, leaf water relations and gas exchange of Cistus albidus and C. monspeliensis plants irrigated with water of different NaCl salinity levels. Sci. Hortic. (Amsterdam) 2003, 97, 353–368. [Google Scholar] [CrossRef]
- Farieri, E.; Toscano, S.; Ferrante, A.; Romano, D. Identification of ornamental shrubs tolerant to saline aerosol for coastal urban and peri-urban greening. Urban For. Urban Green. 2016, 18, 9–18. [Google Scholar] [CrossRef]
- Hansen, C.W.; Petersen, K.K. Reduced nutrient and water availability to Hibiscus rosa-sinensis ‘Cairo Red’ as a method to regulate growth and improve post-production quality. Eur. J. Hort. Sci. 2004, 69, 159–166. [Google Scholar] [CrossRef]
- Silber, A.; Levi, M.; Cohen, M.; David, N.; Shtaynmetz, Y.; Assouline, S. Response of Leucadendron ‘Safari Sunset’ to regulated deficit irrigation: Effects of stress timing on growth and yield quality. Agric. Water Manag. 2007, 87, 162–170. [Google Scholar] [CrossRef]
- Bernal, M.; Estiarte, M.; Peñuelas, J. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biol. 2011, 13, 252–257. [Google Scholar] [CrossRef]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac-ethyl application. Sci. Hortic. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Cirillo, C.; De Micco, V.; Rouphael, Y.; Balzano, A.; Caputo, R.; De Pascale, S. Morpho-anatomical and physiological traits of two Bougainvillea genotypes trained to two shapes under deficit irrigation. Trees Struct. Funct. 2017, 31, 173–187. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Changes in growth rate, root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit. Sci. Hortic. 2013, 156, 54–62. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Gonçalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Chyliňsku, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Planta 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Rafi, Z.N.; Kazemi, F.; Tehranifar, A. Morpho-physiological and biochemical responses of four ornamental herbaceous species to water stress. Acta Physiol. Planta 2019, 41, 7. [Google Scholar] [CrossRef]
- Dghim, F.; Abdellaoui, R.; Boukhris, M.; Neffati, M.; Chaieb, M. Physiological and biochemical changes in Periploca angustifolia plants under withholding irrigation and rewatering conditions. S. Afr. J. Bot. 2018, 114, 241–249. [Google Scholar] [CrossRef]
- Kebbas, S.; Lutts, S.; Aid, F. Effect of drought stress on the photosynthesis of Acacia tortilis subsp. raddiana at the young seedling stage. Photosynthetica 2015, 53, 288–298. [Google Scholar] [CrossRef]
- Ugolini, F.; Bussotti, F.; Raschi, A.; Tognetti, R.; Ennos, A.R. Physiological performance and biomass production of two ornamental shrub species under deficit irrigation. Trees Struct. Funct. 2015, 29, 407–422. [Google Scholar] [CrossRef]
- Ugolini, F.; Tognetti, R.; Bussotti, F.; Raschi, A.; Ennos, A.R. Wood hydraulic and mechanical properties induced by low water availability on two ornamental species Photinia×fraseri var. Red Robin and Viburnum opulus L. Urban For. Urban Green. 2014, 13, 158–165. [Google Scholar] [CrossRef]
- Cirillo, C.; Rouphael, Y.; Caputo, R.; Raimondi, G.; De Pascale, S. The influence of deficit irrigation on growth, ornamental quality, and water use efficiency of three potted Bougainvillea genotypes grown in two shapes. HortScience 2014, 49, 1284–1291. [Google Scholar]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef]
- Álvarez, S.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in different phenological stages of potted geranium plants: Water consumption, water relations and ornamental quality. Acta Physiol. Plant. 2013, 35, 1257–1267. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Navarro, A.; Álvarez, S.; Castillo, M.; Bañón, S.; Sánchez-Blanco, M.J. Changes in tissue-water relations, photosynthetic activity, and growth of Myrtus communis plants in response to different conditions of water availability. J. Hortic. Sci. Biotechnol. 2009, 84, 541–547. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Álvarez, S.; Navarro, A.; Bañón, S. Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J. Plant Physiol. 2009, 166, 467–476. [Google Scholar] [CrossRef]
- Souza, P.U.; Lima, L.K.S.; Soares, T.L.; de Jesus, O.N.; Filho, M.A.C.; Girardi, E.A. Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci. Hortic. 2018, 229, 77–90. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Smirnoff, N. Plant resistance to environmental stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar] [CrossRef]
- Fraser, L.H.; Greenall, A.; Carlyle, C.; Turkington, R.; Ross Friedman, C. Adaptive phenotypic plasticity of Pseudoroegneria spicata: Response of stomatal density, leaf area and biomass to changes in water supply and increased temperature. Ann. Bot. 2009, 103, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Campbell, D.R.; Wu, C.A.; Travers, S.E. Photosynthetic and growth responses of reciprocal hybrids to variation in water and nitrogen availability. Am. J. Bot. 2010, 97, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, R.; Li, Y.; Wang, W.; Tai, F.; Xue, R.; Li, C. Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. J. Plant Growth Regul. 2010, 60, 225–235. [Google Scholar] [CrossRef]
- Ahmadi, U.; Baker, D.A. The effect of water stress on grain filling processes in wheat. J. Agric. Sci. 2001, 136, 257–269. [Google Scholar] [CrossRef]
- Del Blanco, I.A.; Rajaram, S.; Kronstad, W.E.; Reynolds, M.P. Physiological performance of synthetic hexaploid wheat–derived populations. Crop Sci. 2000, 40, 1257–1263. [Google Scholar] [CrossRef]
- Samarah, N.H.; Alqudah, A.M.; Amayreh, J.A.; McAndrews, G.M. The effect of late-terminal drought stress on yield components of four barley cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.M. Stress physiology of tropical pasture plants. Trop. Grassl. 1980, 14, 136–145. [Google Scholar]
- Nilsen, E.; Orcutt, D. The Physiology of Plants under Deficit. Abiotic Factors; Willey: New York, NY, USA, 1996; Volume 689, ISBN-13: 978-0471031529. [Google Scholar]
- Guerrier, G. Fluxes of Na+, K+ and Cl−, and osmotic adjustment in Lycopersicon pimpinellifolium and L. esculentum during short- and long-term exposures to NaCl. Physiol. Plant. 1996, 97, 583–591. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Inan, G.; Zhang, Q.; Li, P.H.; Wang, Z.L.; Cao, Z.Y.; Zhang, H.; Zhang, C.Q.; Quist, T.M.; Goodwin, S.M.; Zhu, J.; et al. Salt cress: A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 2004, 135, 1718–1737. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Younis, A.; Taj, A.R.; Riaz, S. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 2013, 45, 123–131. [Google Scholar]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor. Environ. Exp. Bot. 2007, 60, 105–111. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chiatanya, K.V.; Vivekanandan, M. Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Demmig, B.; Björkman, O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 1987, 171, 171–184. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Nicolás, E.; Sánchez-Blanco, M.J. Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Sci. Hortic. 2011, 129, 306–312. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Energy dissipation in C3 plants under drought. Funct. Plant Boil. 2002, 29, 1209–1215. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and dessication. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Schwanz, P.; Picon, C.; Vivin, P.; Dreyer, E.; Guehi, J.M.; Polle, A. Responses of antioxidative system to drought stress in pendunculata oak and maritime pine as modulated by elevated CO2. Plant Physiol. 1996, 110, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 8, 1056–1071. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and anti-oxidants in plants. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 131–147. [Google Scholar]
- Foyer, C.H.; Descourvieres, O.; Kunert, K.J. Protection against oxygen radicals: An important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Garratt, L.C.; Janagoudar, B.S.; Lowe, K.C.; Anthony, P.; Power, J.B.; Davey, M.R. Salinity tolerance and antioxidant status in cotton cultures. Free Radic. Biol. Med. 2002, 33, 502–511. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Catala, M.; Silva, J.M.D.; Branquinho, C.; Barreno, E. The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Ann. Bot. 2012, 110, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Matamorous, M.A.; Dalton, D.A.; Ramos, J.; Clemente, M.R.; Rubio, M.C.; Becana, M. Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol. 2003, 133, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plant. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Sankar, B.; Jaleel, C.A.; Manivannan, P.; Kishorekumar, A.; Somasundaram, R.; Panneerselvam, R. Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L. Colloids Surf B Biointerfaces 2007, 60, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Sankar, B.; Murali, P.V.; Gomathinayagam, M.; Lakshmanan, G.M.A.; Panneerselvam, R. Water deficit stress effects on reactive oxygen metabolism in Catharanthus roseus: Impacts on ajmalicine accumulation. Colloids Surf. B Biointerfaces 2008, 62, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, P.; Jaleel, C.A.; Somasundaram, R.; Panneerselvam, R. Osmoregulation and antioxidant metabolism in drought stressed Helianthus annuus under triadimefon drenching. C. R. Biol. 2008, 331, 418–425. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Gomathinayagam, M.; Murali, P.V.; Panneerselvam, R. Soil applied propiconazole alleviates the impact of salinity on Catharanthus roseus by improving antioxidant status. Pestic. Biochem. Phys. 2008, 90, 135–139. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Kishorekumar, A.; Sankar, B.; Somasundaram, R.; Panneerselvam, R. Protection of Vigna unguiculata (L.) Walp. plants from salt stress by paclobutrazol. Colloids Surf. B Biointerfaces 2008, 61, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Puckette, M.C.; Weng, H.; Mahalingam, R. Physiological and biochemical responses to acute ozone-induced oxidative stress in Medicago truncatula. Plant Physiol. Biochem. 2007, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dickman, M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Ines, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plant in pot under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Liao, W.; Lu, Q.; Wen, X.; Lu, C. Photosystem II photochemistry, photoinhibition, and the xanthophylls cycle in heat-stressed rice leaves. J. Plant Physiol. 2010, 167, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Lima, L.H.C.; Návia, D.; Inglis, P.W.; De Oliveira, M.R.V. Survey of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotypes in Brazil using RAPD markers. Genet. Mol. Res. 2000, 23, 781–785. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Biol. 2002, 53, 1331–1341. [Google Scholar]
- Matsui, A.; Ishida, J.; Morosawa, T.; Mochizuki, Y.; Kaminuma, E.; Endo, T.A.; Satou, M. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol. 2008, 49, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.; Bräutigam, K.; Campbell, M.M. Time of day shapes Arabidopsis drought transcriptomes. Plant J. 2010, 63, 715–727. [Google Scholar] [CrossRef]
- Tommasini, L.; Svensson, J.T.; Rodriguez, E.M.; Wahid, A.; Malatrasi, M.; Kato, K.; Wanamaker, S.; Resnik, J.; Close, T.J. Dehydrin gene expression provides an indicator of low temperature and drought stress: Transcriptome-based analysis of barley (Hordeum vulgare L.). Funct. Integr. Genom. 2008, 8, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Klay, I.; Gouia, S.; Liu, M.; Mila, I.; Khoudi, H.; Bernadac, A.; Bouzayen, M.; Pirrello, J. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018, 274, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Leon-Kloosterziel, K.M.; Schwartz, S.H.; Zeevaart, J.A. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem. 1998, 36, 83–89. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Bray, E.A. Plant responses to water deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Yoshiba, Y.; Kiyosue, T.; Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997, 38, 1095–1102. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 2018, 156, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M.; Nadal, M. Gas exchange and hydraulics during drought in crops: Who drives whom? J. Exp. Bot. 2018, 69, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses-drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Wang, Y.T. Impact of drought and temperature on growth and leaf gas exchange of six bedding plant species under greenhouse conditions. HortScience 2006, 41, 1408–1411. [Google Scholar]
- García-Castro, A.; Volder, A.; Restrepo-Diaz, H.; Starman, T.W.; Lombardini, L. Evaluation of different drought stress regimens on growth, leaf gas exchange properties, and carboxylation activity in purple Passionflower plants. J. Am. Soc. Hortic. Sci. 2017, 142, 57–64. [Google Scholar] [CrossRef]
- Zollinger, N.; Kjelgren, R.; Cerny-Koenig, T.; Kopp, K.; Koenig, R. Drought responses of six ornamental herbaceous perennials. Sci. Hortic. 2006, 109, 267–274. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S. Ethylene and abscission. HortScience 1985, 20, 45–50. [Google Scholar]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Fiorani, F.; Brzobohatý, B.; Cerný, M.; Spichal, L.; et al. Cytokinins: Their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.W.; Letham, D.S.; Wong, S.C.; Farquhar, G.D. Effects of root restriction on growth and associated cytokinin levels in cotton (Gossypium hirsutum). Funct. Plant Biol. 2010, 37, 974–984. [Google Scholar] [CrossRef]
- Franco, J.A.; Martinéz-Sanchéz, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid and environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Lenzi, A.; Pittas, L.; Martinelli, T.; Lombardi, P.; Tesi, R. Response to water stress of some oleander cultivars suitable for pot plant production. Sci. Hortic. 2009, 122, 426–431. [Google Scholar] [CrossRef]
- Rafi, Z.N.; Kazemi, F.; Tehranifar, A. Effects of various irrigation regimes on water use efficiency and visual quality of some ornamental herbaceous plants in the field. Agric. Water Manag. 2019, 212, 78–87. [Google Scholar] [CrossRef]
- Iles, J.K. The science and practice of stress reduction in managed landscapes. Acta Hortic. 2003, 618, 117–124. [Google Scholar] [CrossRef]
- Cho, K.; Toler, H.; Lee, J.; Ownley, B.; Stutz, J.C.; Moore, J.L.; Augé, R.M. Mycorrhizal symbiosis and response of sorghum plants to combined drought and salinity stresses. J. Plant Physiol. 2006, 163, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Barea, J.M.; Azcón-Aguilar, C. Production of plant growth-regulating substances by the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Appl. Environ. Microbiol. 1982, 43, 810–813. [Google Scholar]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Yong, J.W.H. The importance of phytohormones and microbes in biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem; Springer: Cham, Switzerland, 2015; pp. 105–158. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2018, 41, 396–409. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Vernieri, P.; Ferrante, A.; Borghesi, E.; Mugnai, S. I biostimolanti: Uno strumento per migliorare la qualità delle produzioni. Fertil. Agrorum 2006, 1, 17–22. [Google Scholar]
- Davies, M.J.; Harrison-Murray, R.; Atkinson, C.J.; Grant, O.M. Application of deficit irrigation to container-grown hardy ornamental nursery stock via overhead irrigation, compared to drip irrigation. Agric. Water Manag. 2016, 163, 244–254. [Google Scholar] [CrossRef]
| Species | Plant Habit | Treatments | Growth stage | Modified Parameter by Drought Stress | Ref. |
|---|---|---|---|---|---|
| Rudbeckia hirta, Callistephus chinensis, Althaea rosea, Malva sylvestris | forbs | 4 levels of irrigation treatments: 25%, 50%, 75% and 100% of the reference evapotranspiration (ET0) | Seedling one month after transplanting | Plant fresh weight (−); SLA (−); Stomatal Conductance (−); Δ Canopy Temperature (+); water use efficiency index (WUEi) (+); water use efficiency biomass (WUEb) (+) | [28] |
| Periploca angustifolia | bushy-branched shrub | Full irrigation (FI), Water Deficit (WD), and Rehydrated (R) | 11-month-old seedlings | Relative water content (RWC) (−); osmotic potential (ψπ) (−); water potential (ψw) (−); transpiration rate (−); net CO2 assimilation rate (ACO2) (−); stomatal conductance (gs) (−); water use efficiency (WUE) (+); Proline (+); MDA (+); chlorophyll (a, b, total and a/b) and carotenoid content (−); | [29] |
| Pistacia lentiscus | bushy shrub | C = 100% water holding capacity; Moderate Water irrigation (MW, 60% of the control) and Severe Water deficit (SW, 40% of the C) | 1-year-old seedlings | Dry weight (−), plant height (−), pre-dawn leaf water potential (Ψl) (−); RWC (−) in SW | [12] |
| Lantana camara, Ligustrum lucidum | bushy shrubs | C = container capacity, or irrigated at 100% of water container capacity (WCC); light deficit irrigation (LDI), irrigated at 75% of WCC; moderate deficit irrigation (MDI), irrigated at 50% of WCC; and severe deficit irrigation (SDI), irrigated at 25% of WCC. | Two-month-old rooted cuttings | Dry weight (−); leaf number (−); total leaf area (−); leaf thickness (−); photosynthesis (−); stomatal conductance (−); variable to maximal fluorescence (Fv/Fm) (−); water potential (−). | [23] |
| Bougainvillea buttiana ‘Rosenka’ and B. ‘Lindleyana’ | shrubby vines | C = substrate moisture close to container capacity and irrigation applied when 20% of the water was leached; deficit irrigation (DI), 25% of the amount of water supplied in C. | Two-year-old plants | Leaf, flower, total biomass dry weight, total leaf area (−); stomatal resistance (+); Ψl and Ψp (+); Stomatal length and width (−) | [20] |
| Spirea nipponica (S), Pittosporum eugenioides (P), Viburnum nudum (V) | bushy shrubs | 4 irrigation levels (100, 70, 50, and 25% of container capacity) and Trinexapac-ethyl (TE) treatments (0.1, 0.2, and 0.3 L ha−1) | Plant heights 10 (S and V) and 40 cm (P) | Leaf number and area (−), plant dry weight and height (−), root dry weight (+). A, E, and gs (−). The application of 0.2 and 0.3 L ha−1 TE enhanced S, P and V tolerance to drought stress | [19] |
| Acacia tortilis subsp. raddiana | medium-sized tree | C = 80% of field capacity; Stress = withholding irrigation for 25 d. | 6-week-old seedlings | Leaf number (−), dry mass (−), shoot length and total leaf area (−), water potential (−), stomatal conductance (−); transpiration rates (−); chlorophyll fluorescence (−) only when soil WC was < 40%, soluble sugars (+). | [30] |
| Viburnum opulus and Photinia X fraseri ‘Red Robin’ | bushy shrubs | C = 100% ET; Moderate Water Deficit plants (MWD) received 60% ET and Severe Water Deficit (SWD) received 30% ET. | Plants grown in pots (24 cm in diameter) | Water potentials (−); Pn and gs (−) in SWD in P. x fraseri; gs and leaf transpiration (Tr) (−) in V. opolus | [31] |
| Callistemon laevis | bushy shrub | Control (0.8 dS m−1, 100% water holding capacity), WD (0.8 dS m−1, 50% of the amount of water supplied in control), saline (4.0 dS m−1, same amount of water supplied as control) and saline water deficit (4.0 dS m−1, 50% of the water supplied in the control). | 2-year-old rooted cuttings | Total biomass (−); plant height (−); osmotic adjustment (−), leaf tissue elasticity (−) | [21] |
| Viburnum opulus L. and Photinia x fraseri ‘Red Robin’ | bushy shrubs | Control with 600 mLday−1 (C), moderate WD (MWD) 66% of C and severe water deficit (SWD) received 33% of C. | One-year-old plants | Stem diameter (−). Modulus of elasticity (−) only in Photinia | [32] |
| B. glabra ‘Sanderiana’, B. xbuttiana ‘Rosenka’, B. ‘Lindleyana’ | shrubby vine | Three irrigation levels based on the daily water use 100% (C), 50% (MDI) or 25% (SDI) | Rooted cuttings | SDW (−), total DW (−), leaf number (−), leaf area (−), macronutrient concentration (−) in SDI. Stomatal resistance (+), leaf water potential (−), leaf osmotic potential. (−) | [33] |
| Nerium oleander | bushy shrub | C (field capacity); WD (withholding irrigation) | One-year-old plants | Stem elongation (−); Leaf FW (−); Leaf WC (−); Chl (a, b and total) (−); Proline (+); Glycine betaine (+); Total soluble sugar (+); Total phenolic compounds (+); Total flavonoids (+); ascorbate peroxidase (+); glutathione reductase (+). | [34] |
| Callistemon citrinus ‘Firebrand’ | bushy shrub | C (substrate moisture close to container capacity); moderate deficit irrigation (MDI) by applying 50% of the amount of C and severe deficit irrigation (SDI) by applying 25% of the C irrigation | 2-year-old rooted cuttings | RGR (−) in MDI; R/S ratio (+); WUE (+); gs in MDI and SDI (−); Pn/gs ratios (+); Stem water potential (−); Pn (−) in SDI | [24] |
| Pelargonium x hortorum | forb | C (100% of water field capacity = WFC); sustainable deficit irrigation (SDI), irrigated at 75% WFC throughout the experiment; regulated deficit irrigation I (RDI I), irrigated at 75% throughout the experiment, except during the flowering phase when plants were irrigated at 100%; regulated deficit irrigation II (RDI II), irrigated at 100% throughout the experiment, except during the flowering phase when plants were irrigated at 75%. | Rooted cuttings (4- to 5-cm tall and with 6–7 leaves) | Height (−), Flowering (−) RDI II; SDW (−), Number of leaves (−); Total leaf area (−). | [35] |
| Eugenia uniflora ‘Etna Fire’, P. x fraseri ‘Red Robin’ | bushy shrubs | Well-watered (WW), moderate drought stress (MD, 75%), severe drought stress (35%, SD). | Three months old rooted cuttings | A, gs and E (−); RWC (−); Fv/Fm (−); Proline and MDA (+) in Eugenia; MDA (+) in SD. | [36] |
| Myrtus communis | bushy shrub | Control (C), 100% water holding capacity [leaching 15% (v/v) of the applied water;]; moderate water deficit; MWD, 60% of the C; severe water deficit; SWD,40% of the C. | Seedlings of 2-year-old | SDW (−); root dry weights (−), leaf numbers (−), Total leaf area (−), plant height (−) in SWD; plant height (−) in MDW. Root hydraulic resistance (+); leaf water potential pre-dawn (−); Pn (−). | [37] |
| Pelargonium x hortorum | forb | Control, C, container capacity; Moderate deficit irrigation, MDI, 60% of the C; Severe deficit irrigation, SDI 40% of C. After 2 months, all the plants were exposed to a recovery period of 15 days with the same irrigation regime applied to control plants, until the end of the experiment. | Rooted cuttings | SDW (−); leaf area (−); R/S ratio (+); Height (−); Width (−); gs (−); Pn (−). | [38] |
| Callistemon citrinus, Laurus nobilis, Pittosporum tobira, Thunbergia erecta | bushy shrubs | Two consecutive cycles of suspension/rewatering (S-R) compared with plants that were watered daily (C). | Six-month-old plants | SDW (−); R/S ratio (+); RWC (−); Leaf water potential (−), gs (−); Pn (−). | [22] |
| Passiflora alata, P edulis, P. gibertii, P. setacea, P. cincinnata | climbing vines | Two soil water regimes: soil field capacity and interruption of irrigation until the stomatal closure and apparent wilting of the whole plant. | Six month after sowing | Height (−); Dry weight of leaves, branches, roots (−); gs (−); palisade parenchyma thickness (+); leaf limb and spongy parenchyma thicknesses (+); stomatal diameter (+). | [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. https://doi.org/10.3390/horticulturae5010006
Toscano S, Ferrante A, Romano D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae. 2019; 5(1):6. https://doi.org/10.3390/horticulturae5010006
Chicago/Turabian StyleToscano, Stefania, Antonio Ferrante, and Daniela Romano. 2019. "Response of Mediterranean Ornamental Plants to Drought Stress" Horticulturae 5, no. 1: 6. https://doi.org/10.3390/horticulturae5010006
APA StyleToscano, S., Ferrante, A., & Romano, D. (2019). Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae, 5(1), 6. https://doi.org/10.3390/horticulturae5010006







