How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Physical Design
2.2. Irrigation and Yield
2.3. Postharvest Fruit Quality Parameters
2.4. Statistical Analysis
3. Results
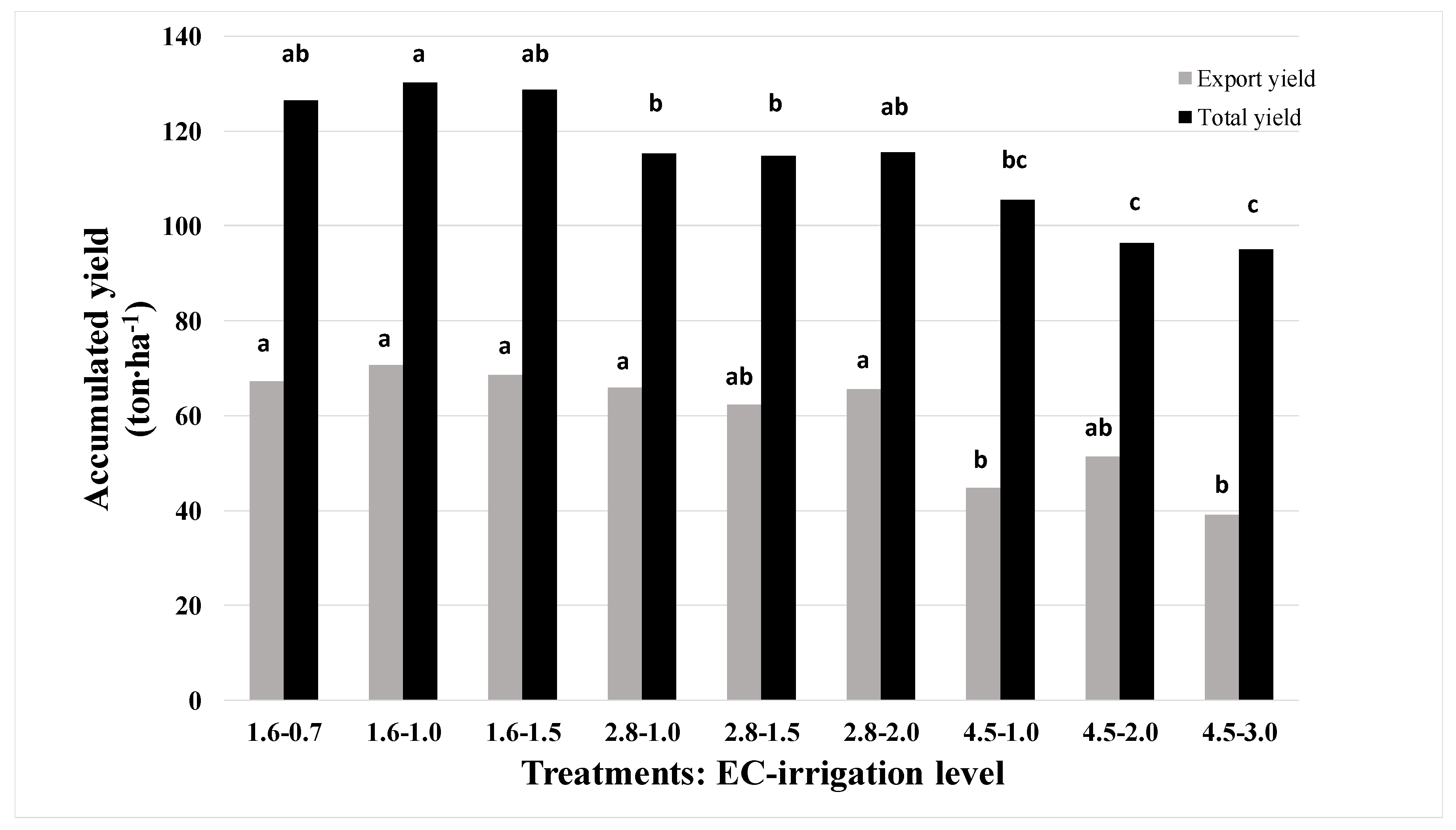
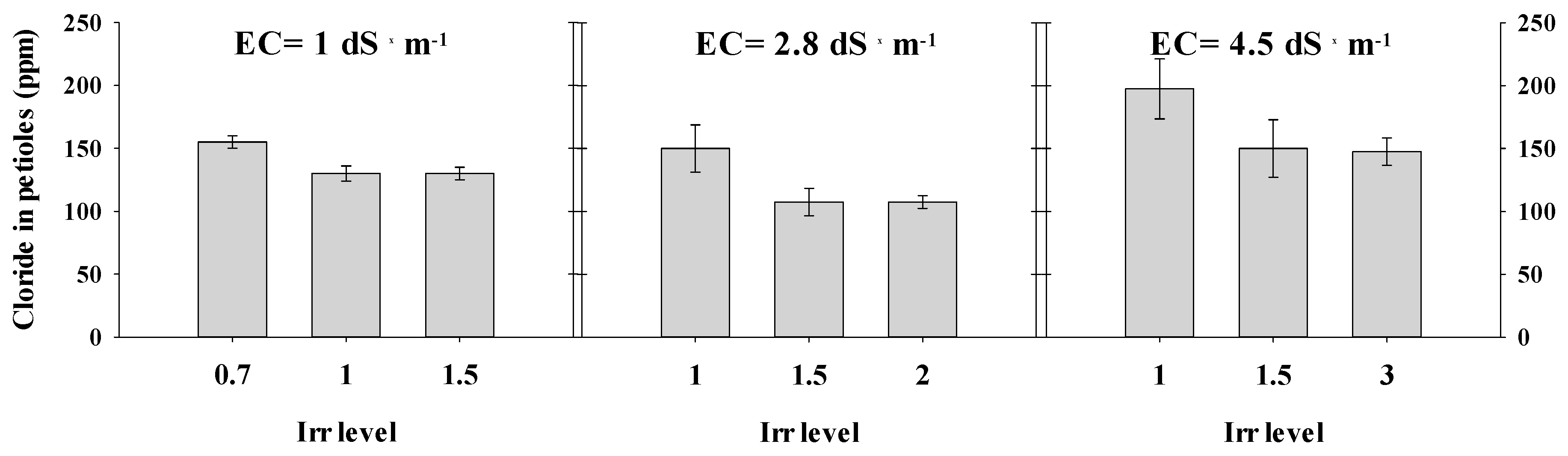
3.1. Yield and Chloride in Petiole
3.2. Fruit Quality
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandaopal, S. Water resources: Agricultural and environmental issues. BioScience 2005, 54, 909–918. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Singh, S.; Grover, K.; Begna, S.; Angadi, S.; Shukla, M.; Steiner, R.; Auld, D. Physiological response of diverse origin spring safflower genotypes to salinity. J. Arid Land Stud. 2014, 24, 169–174. [Google Scholar]
- Azuma, R.; Ito, N.; Nakayama, N.; Suwa, R.; Nguyen, N.T.; Larrinaga-Mayoral, J.A.; Esaka, M.; Fujiyama, H.; Saneoka, H. Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annuum L.). Sci. Hortic. 2010, 125, 171–178. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Ityel, E.; Dudley, L.; Cohen, S.; Yermiyahu, U.; Presnov, E.; Zigmond, L.; Shani, U. Effect of irrigation water salinity on transpiration and on leaching requirements: A case study for bell peppers. Agric. Water Manag. 2008, 95, 587–597. [Google Scholar] [CrossRef]
- Lee, S.K.D. Hot pepper response to interactive effects of salinity and boron. Plant Soil Environ. 2006, 52, 227–233. [Google Scholar]
- Tripler, E.; Haquin, G.; Koch, J.; Yehuda, Z.; Shani, U. Sustainable agricultural use of natural water sources containing elevated radium activity. Chemosphere 2014, 104, 205–211. [Google Scholar] [CrossRef]
- Suissa, A.; Silverman, D.; Friedman, O.; Tzieli, Y.; Cohen, S.; Ofenbach, R. Recommendation to Grow Spring Pepper in the Arava; Ministry of Agriculture and Rural Development, Extension Services, Vegetable Ward: Hanoi, Vietnam, 2017; p. 2. (In Hebrew)
- Fallik, E.; Grinberg, S.; Alkalai, S.; Yekutieli, O.; Wiseblum, A.; Regev, R.; Beres, H.; Bar-Lev, E. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biol. Technol. 1999, 15, 25–32. [Google Scholar] [CrossRef]
- Vinokur, Y.; Rodov, V. Method for determining total (hydrophilic and lipophilic) radical-scavenging activity in the same sample of fresh produce. Acta Hortic. 2006, 709, 53–60. [Google Scholar] [CrossRef]
- Bie, Z.; Ito, T.; Shinohara, Y. Effects of sodium sulfate and sodium bicarbonate on the growth, gas exchange and mineral composition of lettuce. Sci. Hortic. 2004, 99, 215–224. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Khan, S.U.; Ali, A.; Rubab, T.; Schwinghamer, T.; Jilani, G.; Farid, A.; Zhang, J. Salicylic acid and kinetin mediated stimulation of salt tolerance in cucumber (Cucumis sativus L.) genotypes varying in salinity tolerance. Hortic. Environ. Biotechnol. 2018, 59, 461–471. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Baath, G.S.; Shukla, M.K.; Bosland, P.W.; Steiner, R.L. Irrigation water salinity influences growth stages of Capsicum annuum. Agric. Water Manag. 2017, 179, 246–253. [Google Scholar] [CrossRef]
- Rameshwaran, P.; Tepe, A.; Yazar, A.; Ragab, R. The effect of saline irrigation water on the yield of pepper: Experimental and modelling study. Irrig. Drain. 2015, 64, 41–49. [Google Scholar] [CrossRef]
- Yasour, H.; Tamir, G.; Stein, A.; Cohen, S.; Bar-Tal, A.; Ben-Gal, A.; Yermiyahu, U. Does water salinity affect pepper plant response to nitrogen fertigation? Agric. Water Manag. 2017, 191, 57–66. [Google Scholar] [CrossRef]
- Rubio, J.S.; Garcia-Sanchez, F.; Rubio, F.; Martinez, V. Yield, blossom-end rot incidence, and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca2+ fertilization. Sci. Hortic. 2009, 119, 79–87. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Chartzoulakis, K.S. Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric. Ecosyst. Environ. 2005, 106, 171–187. [Google Scholar] [CrossRef]
- Urrea-Lopez, R.; Diaz de la Garza, R.I.; Valiente-Banuet, J.I. Effects of substrate salinity and nutrient levels on physiological response, yield, and fruit quality of habanero pepper. HortScience 2014, 49, 812–818. [Google Scholar]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martınez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Aktas, H.; Abak, K.; Cakmak, I. Genotypic variation in the response of pepper to salinity. Sci. Hortic. 2006, 110, 260–266. [Google Scholar] [CrossRef]
- Botıa, P.; Navarro, J.M.; Cerda, A.; Martınez, V. Yield and fruit quality of two melon cultivars irrigated with saline water at different stages of development. Eur. J. Agron. 2005, 23, 243–253. [Google Scholar] [CrossRef]
- Grieve, A.M.; Prior, L.D.; Bevington, K.B. Long-term effects of saline irrigation water on growth, yield, and fruit quality of ‘Valencia’ orange trees. Aust. J. Agric. Res. 2007, 58, 342–348. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, C.R.; Li, X.Y.; Yao, Y.X.; Hao, Y.J. Modifications of Kyoho grape berry quality under long-term NaCl treatment. Food Chem. 2013, 139, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sakaguchi, S.; Furukawa, H.; Ikeda, H. Effects of NaCl application to hydroponic nutrient solution on fruit characteristics of tomato (Lycopersicon esculentum Mill). Sci. Hortic. 2006, 109, 248–253. [Google Scholar] [CrossRef]
- Petreikov, M.; Yeselson, L.; Shen, S.; Levin, I.; Schaffer, A.A.; Efrati, A.; Bar, M. Carbohydrate balance and accumulation during development of near-isogenic tomato lines differing in the AGPase-L1 allele. J. Am. Soc. Hortic. Sci. 2009, 134, 134–140. [Google Scholar]
- Laribi, A.I.; Palou, L.; Intrigliolo, D.S.; Nortes, P.A.; Rojas-Argudo, C.; Taberner, V.; Bartual, J.; Pérez-Gago, M.B. Effect of sustained and regulated deficit irrigation on fruit quality of pomegranate cv. “Mollar de Elche” at harvest and during cold storage. Agric. Water Manag. 2013, 125, 61–70. [Google Scholar] [CrossRef]
- Elmann, A.; Garra, A.; Alkalai-Tuvia, S.; Fallik, E. Influence of organic and mineral-based conventional fertilization practices on nutrient levels, anti-proliferative activities and quality of sweet red peppers following cold storage. Isr. J. Plant Sci. 2016, 63, 51–57. [Google Scholar] [CrossRef]
- Ehret, D.L.; Usher, K.; Helmer, T.; Block, G.; Steinke, D.; Fret, B.; Kuang, T.; Diarra, M. Tomato fruit antioxidants in relation to salinity and greenhouse climate. J. Agric. Food Chem. 2013, 61, 1138–1145. [Google Scholar] [CrossRef]
| Electrical Conductivity of the Irrigation Water (dS·m−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.8 | 4 | ||||||||||
| Water Application Levels (% from ETp) | Fertilizer Application | |||||||||||
| DAP | ETp | 70 | 100 | 150 | 100 | 150 | 200 | 100 | 200 | 300 | N-P-K | N |
| (mm·d−1) | Daily Irrigation Water Depths (mm·d−1) | (%) | (mg·L−1) | |||||||||
| 0–35 | 1.3 | 0.91 | 1.3 | 1.95 | 1.3 | 1.95 | 2.6 | 1.3 | 2.6 | 3.9 | 6-6-6 | 50 |
| 36–51 | 3.3 | 2.31 | 3.3 | 4.95 | 3.3 | 4.95 | 6.6 | 3.3 | 6.6 | 9.9 | 6-6-6 | 50 |
| 52–62 | 2.7 | 1.89 | 2.7 | 4.05 | 2.7 | 4.05 | 5.4 | 2.7 | 5.4 | 8.1 | 7-3-7 | 120 |
| 63–94 | 2.5 | 1.75 | 2.5 | 3.75 | 2.5 | 3.75 | 5 | 2.5 | 5 | 7.5 | 7-3-7 | 150 |
| 95–104 | 1.7 | 1.19 | 1.7 | 2.55 | 1.7 | 2.55 | 3.4 | 1.7 | 3.4 | 5.1 | 7-3-7 | 100 |
| 105–114 | 1.2 | 0.84 | 1.2 | 1.8 | 1.2 | 1.8 | 2.4 | 1.2 | 2.4 | 3.6 | 7-3-7 | 100 |
| 115–124 | 1.2 | 0.84 | 1.2 | 1.8 | 1.2 | 1.8 | 2.4 | 1.2 | 2.4 | 3.6 | 7-3-7 | 100 |
| 125–134 | 0.8 | 0.56 | 0.8 | 1.2 | 0.8 | 1.2 | 1.6 | 0.8 | 1.6 | 2.4 | 4-2-6 | 100 |
| 135–144 | 0.8 | 0.56 | 0.8 | 1.2 | 0.8 | 1.2 | 1.6 | 0.8 | 1.6 | 2.4 | 4-2-6 | 100 |
| 145–154 | 1.1 | 0.77 | 1.1 | 1.65 | 1.1 | 1.65 | 2.2 | 1.1 | 2.2 | 3.3 | 4-2-6 | 100 |
| 155–164 | 1.3 | 0.91 | 1.3 | 1.95 | 1.3 | 1.95 | 2.6 | 1.3 | 2.6 | 3.9 | 4-2-6 | 100 |
| 165–194 | 2 | 1.4 | 2 | 3 | 2 | 3 | 4 | 2 | 4 | 6 | 4-2-6 | 100 |
| 195–224 | 3 | 2.1 | 3 | 4.5 | 3 | 4.5 | 6 | 3 | 6 | 9 | 4-2-6 | 100 |
| 225–243 | 4 | 2.8 | 4 | 6 | 4 | 6 | 8 | 4 | 8 | 12 | 4-2-6 | 100 |
| 244–272 | 5 | 3.5 | 5 | 7.5 | 5 | 7.5 | 10 | 5 | 10 | 15 | 4-2-6 | 100 |
| Treatment | Water Quality | Amount of Water z | Weight Loss (%) y | Flexibility (mm) x | TSS (%) w | Decay (%) |
|---|---|---|---|---|---|---|
| 1 | 1.6 | 0.7 | 4.13 a v | 2.70 a | 7.58 b | 14.5 a |
| 2 | 1.6 | 1.0 | 4.05 a | 2.58 a | 7.53 b | 9.3 a |
| 3 | 1.6 | 1.5 | 3.90 a | 2.52 a | 7.55 b | 7.2 a |
| 4 | 2.8 | 1.0 | 3.53 a | 2.32 a | 7.83 ab | 7.5 a |
| 5 | 2.8 | 1.5 | 3.53 a | 2.17 a | 8.13 ab | 6.0 a |
| 6 | 2.8 | 2.0 | 3.58 a | 2.43 a | 8.12 ab | 8.5 a |
| 7 | 4.5 | 1.0 | 3.87 a | 3.12 a | 8.05 ab | 7.0 a |
| 8 | 4.5 | 2.0 | 3.77 a | 3.02 a | 8.37 ab | 7.2 a |
| 9 | 4.5 | 3.0 | 3.62 a | 3.13 a | 8.72 a | 7.2 a |
| LSD | 0.31 | 0.40 | 0.32 | 5.61 | ||
| Mean of water quality | ||||||
| 1.6 | 4.03 a | 2.60 ab | 7.56 b | 10.33 a | ||
| 2.8 | 3.55 b | 2.31 b | 8.03 a | 7.33 a | ||
| 4.5 | 3.75 ab | 3.09 a | 8.38 a | 7.11 a | ||
| LSD | 0.18 | 0.23 | 0.19 | 3.24 | ||
| Mean of amount of water | ||||||
| Low | 3.84 a | 2.71 a | 7.82 a | 9.67 a | ||
| Moderate | 3.78 a | 2.59 a | 8.01 a | 7.50 a | ||
| High | 3.70 a | 2.69 a | 8.12 a | 7.61 a | ||
| LSD | 0.18 | 0.23 | 0.19 | 3.24 | ||
| Analysis of Variance (F-Value) | ||||||
| WQ u | 0.04 * | 0.0058 *** | 0.0003 *** | 0.54 NS | ||
| AOW t | 0.8 NS | 0.86 NS | 0.31 NS | 0.75 NS | ||
| WA × AOW | 0.97 NS | 0.87 NS | 0.61 NS | 0.83 NS | ||
| Treatment | Water Quality | Amount of Water | Vitamin C (mg/100 g FW) | AOX TEAC (µM TE/g FW) |
|---|---|---|---|---|
| 1 | 1.6 | 0.7 | 121 a z | 4.1 cd |
| 2 | 1.6 | 1.0 | 124 a | 4.1 cd |
| 3 | 1.6 | 1.5 | 123 a | 4.3 bcd |
| 4 | 2.8 | 1.0 | 124 a | 4.4 abc |
| 5 | 2.8 | 1.5 | 133 a | 4.8 a |
| 6 | 2.8 | 2.0 | 133 a | 4.6 ab |
| 7 | 4.5 | 1.0 | 126 a | 4.2 bcd |
| 8 | 4.5 | 2.0 | 119 a | 4.0 cd |
| 9 | 4.5 | 3.0 | 118 a | 3.9 d |
| LSD | 10.6 | 0.13 | ||
| Mean of water quality | ||||
| 1.6 | 123 a | 4.1 b | ||
| 2.8 | 130 a | 4.6 a | ||
| 4.5 | 121 a | 4.0 b | ||
| LSD | 6.13 | 0.08 | ||
| Mean of water amount | ||||
| Low | 123 a | 4.2 a | ||
| Middle | 125 a | 4.3 a | ||
| High | 125 a | 4.2 a | ||
| LSD | 6.13 | 0.08 | ||
| Analysis of Variance (F-Value) | ||||
| WQ y | 0.33 NS | <0.0001 **** | ||
| AOW x | 0.96 NS | 0.76 NS | ||
| WA × AOW | 0.81 NS | 0.02 * | ||
| WL | Firm | TSS | Decay | VC | |
|---|---|---|---|---|---|
| Firm | −0.065 | ||||
| TSS | −0.223 | 0.259 | |||
| Decay | 0.342 ** | −0.310 ** | 0.193 | ||
| VC | −0.471 **** | 0.099 | −0.014 | −0.202 | |
| AOX | −0.101 | −0.195 | 0.076 | −0.155 | 0.059 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D.; Zaaroor-Presman, M.; Offenbach, R.; Cohen, S.; Tripler, E. How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality. Horticulturae 2019, 5, 4. https://doi.org/10.3390/horticulturae5010004
Fallik E, Alkalai-Tuvia S, Chalupowicz D, Zaaroor-Presman M, Offenbach R, Cohen S, Tripler E. How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality. Horticulturae. 2019; 5(1):4. https://doi.org/10.3390/horticulturae5010004
Chicago/Turabian StyleFallik, Elazar, Sharon Alkalai-Tuvia, Daniel Chalupowicz, Merav Zaaroor-Presman, Rivka Offenbach, Shabtai Cohen, and Effi Tripler. 2019. "How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality" Horticulturae 5, no. 1: 4. https://doi.org/10.3390/horticulturae5010004
APA StyleFallik, E., Alkalai-Tuvia, S., Chalupowicz, D., Zaaroor-Presman, M., Offenbach, R., Cohen, S., & Tripler, E. (2019). How Water Quality and Quantity Affect Pepper Yield and Postharvest Quality. Horticulturae, 5(1), 4. https://doi.org/10.3390/horticulturae5010004





