Pecan Propagation: Seed Mass as a Reliable Tool for Seed Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds Sampling and Classification
2.2. Seeds Sowing and Data Collection
2.3. Statistical Analysis
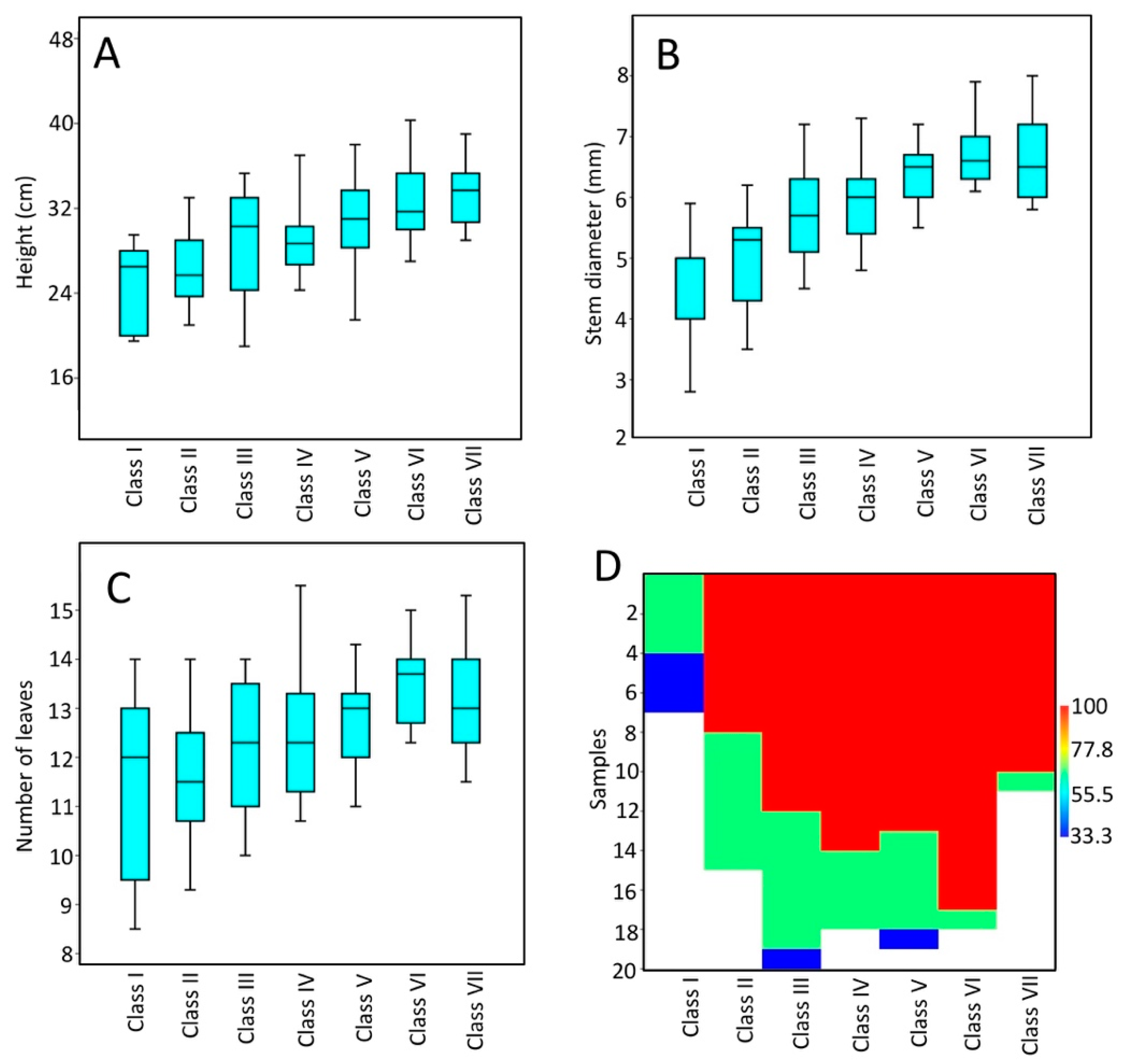
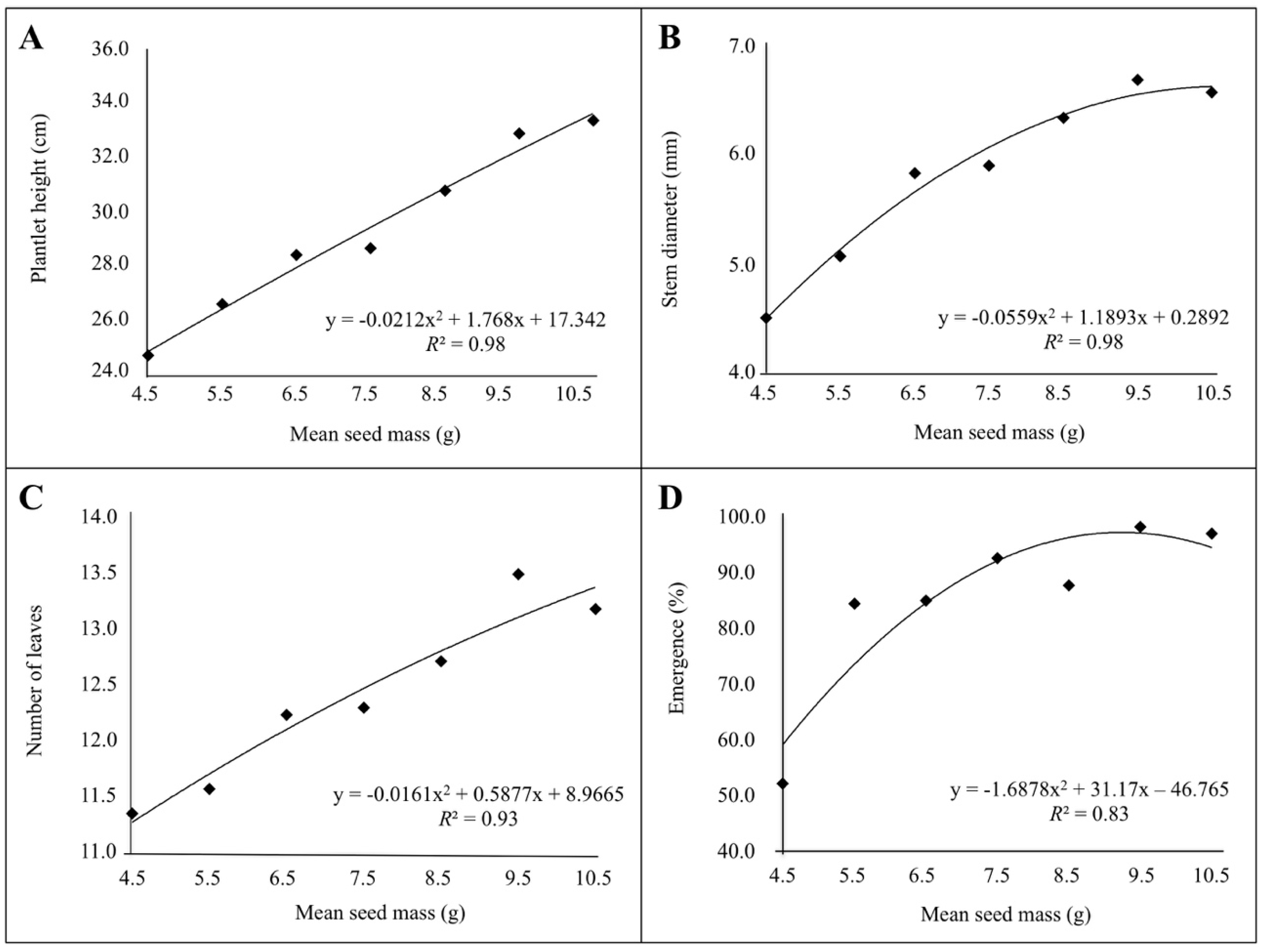
3. Results
3.1. Measures of Plantlet Vigor and Seedling Emergence
3.2. Correlation between Seed Mass, Seedling Emergence and Plantlet Vigor
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, T.E.; Conner, P.J. Pecan. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012; Volume 8, pp. 771–801. ISBN 978-1-4419-0762-2. [Google Scholar]
- Zhang, R.; Peng, F.; Li, Y. Pecan production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Cargnelutti Filho, A.; Poletto, T.; Muniz, M.F.B.; Baggiotto, C.; Poletto, I.; Fronza, D. Sampling design for height and diameter evaluation of pecan seedlings. Ciênc. Rural 2014, 44, 2151–2156. [Google Scholar] [CrossRef]
- Poletto, I.; Muniz, M.F.B.; Poletto, T.; Stefenon, V.M.; Baggiotto, C.; Ceconi, D.E. Germination and development of pecan cultivar seedlings by seed stratification. Pesqui. Agropecu. Bras. 2015, 50, 1232–1235. [Google Scholar] [CrossRef]
- Pereira, W.A.; Pereira, S.M.A.; Dias, D.C.F.S. Influence of seed size and water restriction on germination of soybean seeds and on early development of seedlings. J. Seed Sci. 2013, 35, 316–322. [Google Scholar] [CrossRef]
- Bispo, J.S.; Costa, D.C.C.; Gomes, S.E.V.; Oliveira, G.M.; Matias, J.R.; Ribeiro, R.C.; Dantas, B.F. Size and vigor of Anadenanthera colubrina (Vell.) Brenan seeds harvested in Caatinga areas. J. Seed Sci. 2017, 39, 363–373. [Google Scholar] [CrossRef]
- Adams, J.C.; Thielges, B.A. Seed sizes effects on first-and second-year pecan and hybrid Pecan growth. Tree Planters’ Notes 1979, 30, 31–33. [Google Scholar]
- Hammer, Ø.; Harper, D.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–4. [Google Scholar]
- Wessa, P. Pearson Correlation (v1.0.13) in Free Statistics Software (v1.2.1), Office for Research Development and Education. 2017. Available online: https://www.wessa.net/rwasp_correlation.wasp/ (accessed on 5 March 2018).
- Tao, Y.; Mace, E.S.; Tai, S.; Cruickshank, A.; Campbell, B.C.; Zhao, X.; Van Oosterom, E.J.; Godwin, I.D.; Botella, J.R.; Jordan, D.R. Whole-Genome Analysis of Candidate genes Associated with Seed Size and Weight in Sorghum bicolor Reveals Signatures of Artificial Selection and Insights into Parallel Domestication in Cereal Crops. Front. Plant Sci. 2017, 8, 1237. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ruan, C.; Guan, Y.; Krishna, P. Identification of microRNAs involved in lipid biosynthesis and seed size in developing sea buckthorn seeds using high throughput sequencing. Sci. Rep. 2018, 8, 4022. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Wang, N.; Jiang, X.; Mao, H.; Zhu, C.; Wen, F.; Wang, F.; Lu, Z.; Yue, G.; et al. Manipulation of Auxin Response Factor 19 affects seed size in the woody perennial Jatropha curcas. Sci. Rep. 2017, 7, 40844. [Google Scholar] [CrossRef] [PubMed]
- Norden, N.; Daws, M.I.; Antoine, C.; Gonzales, M.A.; Garwood, N.C.; Chave, J. The relationship between seed mass and mean time to germination for 1037 tree species across five tropical forests. Funct. Ecol. 2009, 23, 203–210. [Google Scholar] [CrossRef]
- Prataviera, J.S.; Lamarca, E.V.; Teixeira, C.C.; Barbedo, C.J. The germination success of the cut seeds of Eugenia pyriformis depends on their size and origin. J. Seed Sci. 2017, 37, 47–54. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Jijeesh, C.M.; Sudhakara, K. Larger drupe size and earlier geminants for better seedling attributes of teak (Tectona grandis Linn. f.). Ann. For. Res. 2013, 56, 307–316. [Google Scholar]
- Ramírez-Valiente, J.A.; Valladares, F.; Gil, L.; Aranda, G. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Chaisurisri, K.; Edwards, D.G.W.; El-Kassaby, Y.A. Effects of seed size on seedling attributes in Sitka spruce. New For. 1994, 8, 81–87. [Google Scholar] [CrossRef]
- Dalkiliç, Z. Effects of drying on germination rate of pecan seeds. J. Food Agric. Environ. 2013, 11, 879–882. [Google Scholar] [CrossRef]
- Henery, M.L.; Westoby, M. Seed mass and seed nutrient content as predictors of seed output variation between species. Oikos 2001, 92, 479–490. [Google Scholar] [CrossRef]
| Class | I | II | III | IV | V | VI | Class | I | II | III | IV | V | VI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | II | 1.00 | Stem diameter | 1.00 | ||||||||||
| III | 1.00 | 1.00 | 0.23 | 0.45 | ||||||||||
| IV | 0.71 | 1.00 | 1.00 | 0.06 | 0.04 | 1.00 | ||||||||
| V | 0.09 | 0.09 | 1.00 | 1.00 | 0.01 | 0.00 | 0.63 | 1.00 | ||||||
| VI | 0.01 | 0.00 | 0.55 | 0.02 | 1.00 | 0.00 | 0.00 | 0.02 | 0.02 | 1.00 | ||||
| VII | 0.02 | 0.00 | 0.19 | 0.03 | 1.00 | 1.00 | 0.02 | 0.00 | 0.27 | 0.72 | 1.00 | 1.00 | ||
| Number of leaves | II | 1.00 | % of germination | 0.13 | ||||||||||
| III | 1.00 | 1.00 | 0.05 | 1.00 | ||||||||||
| IV | 1.00 | 1.00 | 1.00 | 0.00 | 1.00 | 1.00 | ||||||||
| V | 1.00 | 0.23 | 1.00 | 1.00 | 0.05 | 1.00 | 1.00 | 1.00 | ||||||
| VI | 0.15 | 0.00 | 0.03 | 0.06 | 0.19 | 0.00 | 0.15 | 0.29 | 1.00 | 0.94 | ||||
| VII | 1.00 | 0.11 | 1.00 | 1.00 | 1.00 | 1.00 | 0.01 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poletto, T.; Stefenon, V.M.; Poletto, I.; Muniz, M.F.B. Pecan Propagation: Seed Mass as a Reliable Tool for Seed Selection. Horticulturae 2018, 4, 26. https://doi.org/10.3390/horticulturae4030026
Poletto T, Stefenon VM, Poletto I, Muniz MFB. Pecan Propagation: Seed Mass as a Reliable Tool for Seed Selection. Horticulturae. 2018; 4(3):26. https://doi.org/10.3390/horticulturae4030026
Chicago/Turabian StylePoletto, Tales, Valdir Marcos Stefenon, Igor Poletto, and Marlove Fátima Brião Muniz. 2018. "Pecan Propagation: Seed Mass as a Reliable Tool for Seed Selection" Horticulturae 4, no. 3: 26. https://doi.org/10.3390/horticulturae4030026
APA StylePoletto, T., Stefenon, V. M., Poletto, I., & Muniz, M. F. B. (2018). Pecan Propagation: Seed Mass as a Reliable Tool for Seed Selection. Horticulturae, 4(3), 26. https://doi.org/10.3390/horticulturae4030026






