Abstract
Vigor-reducing rootstocks are now commonly used in sweet cherry cultivation. However, their application in Hungary presents challenges due to the drier summer climate and limited availability of irrigation water. The aim of this study was to determine the water transport characteristics and potential drought tolerance of three vigor-reducing rootstocks that may be suitable for cherry production in Hungary. The stomatal conductance (gs), midday stem water potential (MSWP), and sap flow velocity were measured in four-year-old Carmen and Regina cherry trees grafted onto MaxMa 14, WeiGi 2, and GiSelA 6 rootstocks. Measurements were taken after harvest during a period of severe drought. Among the rootstocks studied, MaxMa 14 trees exhibited the lowest MSWP values, even after irrigation and during periods with a relatively adequate water supply. No significant or consistent differences in the gs values were observed between the rootstocks. However, the variation in the gs and MSWP values before and after irrigation was the greatest in MaxMa 14 trees and the smallest in GiSelA 6 trees. Furthermore, the sap flow velocity in MaxMa 14 trees showed no significant difference between the pre- and post-irrigation measurements, indicating stable water transport. In contrast, trees on GiSelA 6 and WeiGi 2 rootstocks exhibited significant differences between dry and irrigated conditions. Although MaxMa 14 showed lower MSWP values, its gs responded more dynamically to changes in the water availability, and it maintained consistent water transport parameters across both dry and wet conditions. Based on the evaluated parameters, GiSelA 6 and WeiGi 2 showed similar behavior. However, in regard to some traits—such as the dynamic change in stomatal conductance—WeiGi 2 appeared to be more similar to MaxMa 14. Our results suggest that MaxMa 14 may be the most adaptable to drought among the tested rootstocks.
1. Introduction
Vigor-reducing rootstocks are now widely used in sweet cherry production. As fruit production trends move toward faster and more economical solutions, these rootstocks facilitate the adoption of mechanical pruning and thinning techniques, and they simplify both pruning and harvesting operations [1]. Trees grafted onto vigor-reducing rootstocks can be planted at higher densities and tend to be more generative than those on more vigorous rootstocks, allowing for increased yields even in smaller orchard areas [2].
Vigor-reducing rootstocks are generally characterized by reduced vegetative growth, as well as lower hydraulic conductivity [3,4] and smaller root systems. These characteristics make them more sensitive to moisture availability in the soil, particularly in the upper soil layers [5]. Additionally, their water uptake capacity may be limited due to a smaller average vessel diameter in the roots and a more complex graft union between the rootstock and scion [6]. Several studies indicate that drought tolerance is not invariably correlated with the growth vigor of rootstocks. Stott et al. (2019) [7] investigated three members of the Gisela® rootstock series differing in growth vigor and found that Gisela 3, the least vigorous among them, exhibited greater drought tolerance than Gisela 5, which possessed a slightly more robust growth habit. Among two semi-dwarfing Prunus rootstocks, ROOTPAC® 40 was identified as drought-tolerant, whereas ROOTPAC® 20 was classified as drought-sensitive [8]. Similarly, Toro et al. (2023) [9], after evaluating ‘Bing’ and ‘Lapins’ sweet cherry cultivars grafted onto vigorous Mx60 and Colt rootstocks, reported that the most drought-sensitive combination was ‘Bing’/Colt, despite the fact that the ungrafted Colt rootstock itself demonstrated drought tolerance. These findings suggest that the drought tolerance of a rootstock is not necessarily expressed in the performance of the grafted scion, underscoring the necessity of evaluating each rootstock–scion combination individually in this regard. Among growth-reducing rootstocks, MaxMa 14, GiSelA 6, and WeiGi 2 are becoming increasingly popular in Hungarian sweet cherry production, alongside more vigorous types. MaxMa 14 has a relative vigor of 80–90%, and GiSelA 6 has a vigor about 70–80% compared to a mahaleb seedling rootstock [10], while WeiGi 2 has a vigor comparable to that of GiSelA 5, i.e., approximately 50–60% [11]. GiSelA 6 and WeiGi 2 were developed in Germany, while MaxMa 14 originates from the United States, where it was selected under more humid climatic conditions than those found in Hungary. Although limited data are available on the performance of WeiGi 2 under Hungarian conditions, international experiences suggest that it may exhibit better drought tolerance than other rootstocks of a similar vigor [12].
The Hungarian climate has changed significantly in recent decades and is now characterized by hot, dry summers and mild, dry winters [13]. The average annual precipitation is approximately 550 mm, and the average summer temperature in 2023 was 21.6 °C, higher than the 20-year average of 20.8 °C [14]. In most years, the summer precipitation is both insufficient and poorly distributed, typically occurring as infrequent but intense rainfall events. Under these conditions, irrigation has become essential for fruit production. However, many regions lack sufficient surface water sources, and groundwater use is strictly regulated. These constraints make it crucial for growers to conserve water and adopt efficient irrigation practices.
In sweet cherry production, the fruit-bearing period is relatively short and concludes early in the growing season, followed by a prolonged post-harvest period. During summer, it is important to support tree health, encourage biomass production, and facilitate preparation for winter. Although the tree water demand during this period is lower, it presents an opportunity for water conservation through regulated deficit irrigation (RDI). Studies have shown that post-harvest RDI in sweet cherry can reduce vegetative growth without negatively impacting the following season’s flowering, fruit set, or fruit development [15,16,17,18,19].
Water potential measurement is considered the most sensitive and cost-effective method for assessing the plant water status and detecting water stress, even among different Prunus species, including sweet cherry [20,21,22]. It is also a relatively simple and rapid process, making it suitable for field use. The water potential is typically measured at two times of day: before sunrise, when the relative humidity is the highest, and around midday, when it is the lowest. The latter, known as the midday stem water potential (MSWP), has proven to be the most useful indicator for irrigation management, with a threshold of −1.3 MPa being recommended for sweet cherry during post-harvest RDI [23].
The main objective of this study was to investigate the water relations of MaxMa 14, GiSelA 6, and WeiGi 2 rootstocks during the post-harvest summer period using several different widely adopted methods.
2. Materials and Methods
2.1. Experimental Site
The experiment was conducted in a 0.93-hectare intensive sweet cherry orchard located in Érd, in the central region of Hungary. The soil type was chernozem-like, with a humus content of 2.5–3.0% and a pH ranging from 7.0 to 7.3. The climate was continental, characterized by an average annual precipitation of 550 mm and a mean annual temperature of 11.0 °C [14]. In 2024, the first half of the growing season was average in terms of the weather conditions; however, from late June through early September, a particularly hot and dry period occurred (Table 1).

Table 1.
Main meteorological data for vegetation period in 2024. Érd, Elvira Major.
The orchard was planted in autumn 2020 with a planting density of 4.5 × 1.75 m. Trees were trained on a wire trellis support system and irrigated using a drip irrigation system. Vegetation in the alleyways was regularly mowed, while the tree rows were mechanically cultivated. A total of 19 cultivars grafted onto four rootstocks were planted in a randomized block design. For this study, combinations of two cultivars and three rootstocks were selected to assess their physiological characteristics.
2.2. Plant Materials
The cultivars evaluated in this study were Regina and Carmen, each grafted onto MaxMa 14, GiSelA 6, and WeiGi 2 rootstocks (Supplementary Materials). A total of 5 trees were selected for each of the six cultivar–rootstock combinations, resulting in a total of 30 trees (2 cultivars × 3 rootstocks × 5 trees). The Carmen and Regina varieties were planted in two separate blocks, and the trees grafted onto different rootstocks were located randomly within the two scion variety blocks. In terms of their ripening times, ‘Carmen’ is categorized as a mid-season cultivar, while ‘Regina’ belongs to the late ripening group.
In the study year, flowering began on 31 March for Carmen and 2 April for Regina. Full bloom occurred on 7 April and 10 April, respectively. A difference of approximately one week was observed in the harvest dates. ‘Carmen’ was harvested on 2 June, while ‘Regina’ was harvested on 10 June.
2.3. Measurement Methods
Measurements were performed after harvest during two extended dry periods. Between 10 June and 16 July (36 days), the total precipitation was 6.4 mm. From 19 July to 15 August (26 days), only 2.8 mm of precipitation was recorded, and the daily rainfall never exceeded 5 mm. During these dry periods, irrigation was applied when soil moisture fell below 15% v/v (approximately 20% of the available water content), with 50 L of water supplied per tree.
The MSWP measurements [24] were conducted weekly between 11:00 and 12:30 on one leaf located close to the trunk on each tree. Leaves were covered with aluminum foil and a plastic bag at least 90 min before measurement to prevent transpiration and equilibrate the water potential between the leaf and stem. MSWP was measured using a Scholander pressure chamber (Model 1000, PMS Instrument Company, Albany, OR, USA).
Stomatal conductance (gs) was assessed as an indicator of leaf gas exchange. Measurements were taken from a single, well-exposed leaf in the middle section of a current-year extension shoot. An infrared gas analyzer (TARGAS-1 Portable Photosynthesis System, PP Systems, Camas, Oregon, USA) with a chamber area of 4.5 cm2 was used. The measurements were conducted on clear days between 10:00 and 12:00.
Sap flow was measured using the thermal dissipation method [25,26] with SFS2 sensors (33 mm length, 2.0 mm diameter; UP GmbH, Ibbenbüren, Germany). The sensors were installed at a height of 50 cm, below the first branching point of the trunk. Data were logged every 5 min using a LoLoP data logger (UP GmbH, Ibbenbüren, Germany), and hourly means were used for statistical analysis. Six sensor pairs were deployed, enabling simultaneous measurements on six trees. Measurements were conducted on six Carmen trees from 12 to 23 July and on six Regina trees from 25 July to 13 August. Statistical evaluation was based on data collected during 3-day periods before and after each irrigation event.
Volumetric soil water content (% v/v) was measured near the selected trees using SMT-150 sensors and a GP2 data logger (Delta-T Devices, Burwell, Cambridge, UK). The sensors were installed at 30 cm and 60 cm depths in the center of the cultivated soil strip under the drip line.
Meteorological parameters were recorded by an automated weather station (Boreas Ltd., Érd, Hungary) located approximately 100 m from the experimental site. Reference evapotranspiration (ET0) was calculated using the Penman–Monteith FAO method [27].
2.4. Statistical Analysis
Statistical analyses were performed using paired-sample t-tests or one-way ANOVA on natural log-transformed data. Homogeneity of variance was tested using Levene’s test. Tukey’s HSD test was used for post hoc comparisons when the condition of homogeneity was met. In cases of unequal variance, the Games–Howell test was applied. All statistical analyses were conducted using IBM SPSS Statistics, version 29.
3. Results
The first part of the summer was characterized by adequate rainfall, with more than 50 mm falling during the first 10 days of June. However, it was followed by an extended period of hot and dry weather, leading to a marked reduction in soil moisture within the root zone by early July. Following each irrigation event, soil moisture levels temporarily increased but declined rapidly thereafter (Figure 1).
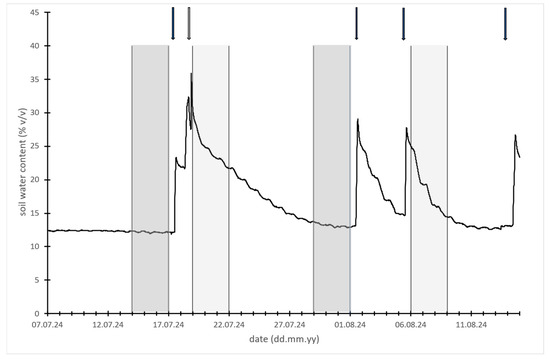
Figure 1.
Soil water content (% v/v) measured 30 cm deep in tree rows. Arrows with continuous line indicate irrigation events (50 L/tree), while arrow with dashed line marks natural rainfall (27.2 mm in 6 h). Moderately and light gray zones indicate 3-day periods of sap flow measurements.
The midday stem water potential (MSWP) values were the most negative in trees grafted onto the MaxMa 14 rootstock, with significantly lower values compared to trees on WeiGi 2 and GiSelA 6 rootstocks. No significant difference was observed between the latter two (Figure 2). This pattern remained consistent across both dry (pre-irrigation) and moist (post-irrigation) periods, indicating that trees on MaxMa 14 experienced greater water stress irrespective of the soil moisture availability.
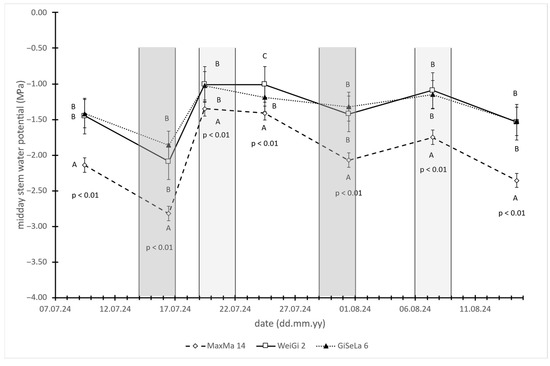
Figure 2.
Midday stem water potential (MSWP) values of cherry trees on MaxMa 14, WeiGi 2, or GiSelA 6 rootstocks. Every data point represents average of 10 individual measurements (n = 10), while error bars indicate standard errors of mean (SEs). Different letters on the same day denote significant differences among rootstocks, according to Tukey HSD range test. Moderately and light gray zones indicate 3-day-long periods of sap flow measurements.
In contrast, the differences in stomatal conductance (gs) between the rootstocks were less pronounced and only partially statistically significant (Figure 3). During water deficit periods, trees on MaxMa 14 exhibited the lowest gs values, though this was statistically significant only on two dates: 16 July and 14 August. Under improved moisture conditions, GiSelA 6 showed the lowest gs values; however, these differences were not statistically significant on any sampling date.
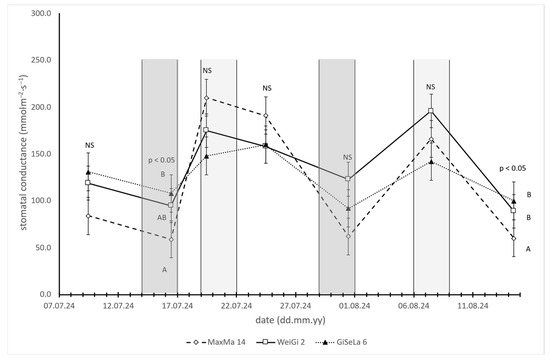
Figure 3.
Stomatal conductance (gs) values of cherry trees on MaxMa 14, WeiGi 2, or GiSelA 6 rootstocks. Every data point represents average of 10 individual measurements (n = 10), while error bars indicate standard errors of mean (SEs). Different letters on the same day denote significant differences among rootstocks, according to Tukey HSD range test. Moderately and light gray zones indicate 3-day periods of sap flow measurements.
Following irrigation or rainfall events, both gs and MSWP values increased significantly across all the rootstocks (Table 2). However, the magnitude of change varied among them. For gs, the increases were 51.0 and 50.5 mmol·m−2·s−1 in GiSelA 6, 80.0 and 72.5 mmol·m−2·s−1 in WeiGi 2, and 150.5 and 110.0 mmol·m−2·s−1 in MaxMa 14 trees at the two observed time points, respectively (Table 3).


The MSWP values followed a similar trend: increases of 0.83 and 0.17 MPa were recorded for GiSelA 6, 1.09 and 0.34 MPa for WeiGi 2, and 1.47 and 0.32 MPa for MaxMa 14. Thus, MaxMa 14 showed the greatest response to irrigation, while GiSelA 6 showed the smallest response, with WeiGi 2 consistently showing an intermediate response.
The trunk sap flow velocity, which reflects the whole-tree transpiration, was also used to assess the differences in water transport capacity among rootstocks. The relationship between sap flow velocity and reference evapotranspiration (ET0), an indicator of the evaporative demand, followed a saturation-type response and was modeled using the following exponential function (Figure 4):
where
- u is the sap flow velocity in the trunk (cm·h−1);
- A represents the asymptotic maximum sap flow velocity (i.e., maximum transpiration rate);
- c is a rate constant related to the curve’s steepness;
- ET0 is the reference evapotranspiration (mm·h−1).
Parameter A indicates the tree’s maximum water transport capacity, which is influenced by its hydraulic conductivity. The parameter c reflects how quickly the sap flow increases with a rising evaporative demand.
Equation (1) was fitted to datasets of measurements taken for 3 days before and after irrigation events for each rootstock. The A parameter increased moderately after irrigation in all the rootstocks, but the change was not statistically significant. In contrast, c increased significantly in WeiGi 2 and GiSelA 6 trees, while it remained unchanged in MaxMa 14 (Table 4). A higher c value suggests that the maximum sap flow is achieved at lower levels of evaporative demand, indicating an improved water transport response under less stressful conditions.

Table 4.
Parameters A and c of the curve describing the relationship between the trunk sap flow velocity and the reference evapotranspiration (see Equation (1)) in trees on different rootstocks before and after irrigation events.
These findings suggest that after irrigation, the water transport capacity of trees on WeiGi 2 and GiSelA 6 rootstocks improved primarily during times of low evaporative demand. Conversely, the water transport dynamics of MaxMa 14 trees remained largely unchanged, suggesting that this rootstock maintains similar hydraulic behavior during both drought stress and post-irrigation periods.
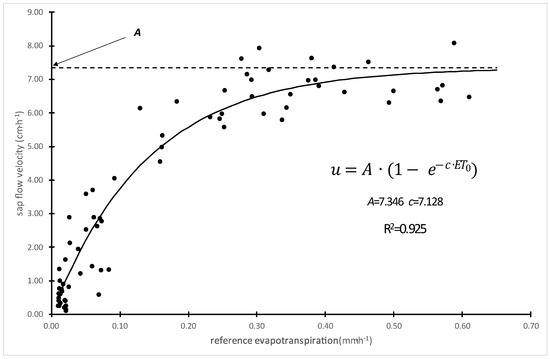
Figure 4.
Trunk sap flow velocity as a function of the reference evapotranspiration. Data points represent hourly averages over a 3-day period (n = 72). Carmen/MaxMa 14 tree. R2 is defined as 1-(residual sum of squares/corrected sum of squares).
4. Discussion
This study primarily focused on identifying physiological differences among sweet cherry rootstocks under conditions of severe drought stress. Accordingly, the data analysis was restricted to rootstock effects, and the potential influences of the scion cultivars were not examined, despite evidence that the scion genotype can also affect the canopy’s physiological traits [28]. Despite this limitation, significant differences between the rootstocks were observed, particularly in the midday stem water potential (MSWP).
Contrary to our initial expectations and previous findings [29], trees grafted onto the most vigorous rootstock, MaxMa 14, exhibited the lowest MSWP values, indicating the highest levels of water stress. These values were significantly lower than those of the less vigorous rootstocks, WeiGi 2 and GiSelA 6, both during pre-irrigation drought periods and under post-irrigation conditions with improved soil moisture. Notably, even under better moisture conditions, the MSWP values for MaxMa 14 remained below −1.3 MPa—the recommended irrigation threshold for sweet cherry under regulated deficit irrigation (RDI) strategies [23]. In contrast, the MSWP values for WeiGi 2 and GiSelA 6 fluctuated around this threshold.
In a study by Morandi et al. [29], the GiSelA 6 rootstock generally exhibited lower MSWP values compared to the semi-vigorous CAB6 P rootstock. However, their observations were conducted before and after the fruit vernalization phase, rather than during the post-harvest period. Their results demonstrated significant differences in fruit water potential values among rootstocks with varying growth habits, which, according to their hypothesis, affect the overall water relations of the trees. In contrast, our own investigations were performed in the post-harvest period, under markedly different environmental and physiological conditions, which may account for the differences in the MSWP outcomes between the two studies.
Unlike MSWP, the stomatal conductance (gs) did not show consistent or statistically significant differences among the rootstocks. While gs was generally the lowest in MaxMa 14 during dry periods, this trend was not consistently significant, and no clear differences among the rootstocks were observed under better moisture conditions.
Irrigation significantly increased both MSWP and gs across all the rootstocks. However, the magnitude of these responses varied substantially: MaxMa 14 exhibited the largest increases, while GiSelA 6 showed the smallest. Specifically, the increase in the gs following irrigation was up to three times greater in MaxMa 14 than in GiSelA 6, and the MSWP values changed by nearly twice as much. These results suggest that trees on MaxMa 14 respond more dynamically to changes in soil moisture.
While gs provides insight into gas exchange at the leaf level, its representativeness of the whole-canopy transpiration is limited due to the small measurement area relative to the total foliage [30]. The MSWP is more representative of the overall tree water status but offers only a midday snapshot and indirectly indicates transpiration. In contrast, the sap flow rate, measured at the trunk level, provides direct and continuous information on the whole-tree water use and is suitable for 24 h monitoring.
In this study, we analyzed the sap flow as a function of reference evapotranspiration (ET0). This relationship was well described by a saturation-type curve, whose parameters offered a clear physiological interpretation and enabled a comparison across the rootstocks. For MaxMa 14, the sap flow–ET0 curves did not differ significantly between the drought and post-irrigation conditions, suggesting a stable water transport capacity. However, in GiSelA 6 and WeiGi 2, the slope parameter of the curve was significantly lower during drought, indicating that the maximum sap flow was reached only at higher evaporative demands. This implies that the rootstock differences are the most evident during moderate ET0 periods, rather than at times of peak transpiration demand.
Based on their physiological responses to a water deficit, plants are traditionally classified as either isohydric or anisohydric [31]. Isohydric species strive to maintain a relatively constant tissue water status by rapidly closing their stomata as the water availability declines—a conservative strategy that limits water loss but also reduces carbon assimilation. In contrast, anisohydric species delay stomatal closure under an increasing water deficit, thereby sustaining gas exchange and photosynthetic activity for a longer period—a productive strategy. Among drought-tolerant Prunus rootstocks, a delayed reduction in transpiration during water shortages has been reported, suggesting that anisohydric behavior is characteristic of these genotypes [32].
In the present study, of the three evaluated rootstocks, MaxMa 14 displayed an isohydric response pattern, as inferred from the dynamics of stomatal conductance under both drought and well-watered conditions. In contrast, GiSelA 6 and WeiGi 2 exhibited a more anisohydric response. Typically, the leaf water potential in isohydric plants remains more stable across different water availability regimes compared to that in anisohydric plants. However, in our experiments, the leaf water potential was not measured; only the midday stem water potential (MSWP) was assessed, which was insufficient for a definitive classification of the three rootstocks into isohydric or anisohydric categories.
According to Stott et al. (2019) [7], the whole-plant transpiration recovery following drought is a key indicator of drought tolerance.
Furthermore, Opazo et al. (2020) [8] reported that the organic matter production of the drought-sensitive ROOTPAC® 20 rootstock (isohydric behavior) was significantly higher during the post-drought recovery phase under a normal water supply (recovery period) than that of the drought-tolerant ROOTPAC® 40 rootstock. This finding implies that, under typical agricultural conditions—where irrigation results in alternating periods of water deficits and adequate water availability—rootstocks with anisohydric behavior and greater drought tolerance are not necessarily more productive when the overall yield is assessed across the entire growing season.
Assuming a proportional relationship between the stomatal conductance and leaf transpiration, our results suggest that, among the rootstocks studied, MaxMa 14 is the best able to adapt to the alternating periods of drier and better-water-supply conditions that occur post-harvest, while GiSelA 6 appears to be the least tolerant, with WeiGi 2 showing intermediate characteristics.
In summary, MaxMa 14 trees maintained water transport rates similar to those under well-watered conditions even during drought, while their stomatal conductance exhibited the most dynamic response to the soil moisture availability. This suggests that the low MSWP observed in MaxMa 14 may not solely reflect higher water stress but rather an adaptive mechanism to maintain transpiration under dry conditions. We hypothesize that the more negative stem water potential in MaxMa 14 trees facilitates a greater water potential gradient, supporting continued water uptake and transport. These trees also exhibited more stable sap flow rates across irrigation regimes and appeared visually more hydrated (i.e., with turgid leaves) compared to GiSelA 6 and WeiGi 2. However, this visual assessment requires confirmation through quantitative measurements, which also include the examination of leaf water potential and measurements taken during the phenological phases prior to harvest.
5. Conclusions
In conclusion, the water-related physiological parameters assessed in this study indicate that the MaxMa 14 rootstock exhibits the greatest adaptive capacity to continental climates characterized by prolonged drought periods. It demonstrated the most dynamic stomatal conductance response to fluctuations in the water availability and was able to maintain consistent water transport performance under both dry and well-watered conditions. In contrast, GiSelA 6 and WeiGi 2 rootstocks showed different behavior across most parameters, although WeiGi 2 exhibited slightly greater responsiveness—particularly in the dynamic changes in its stomatal conductance—suggesting a physiological profile more closely aligned with that of MaxMa 14. Based on our findings, MaxMa 14 is suitable for use in dry continental regions and adapts well to regulated deficit irrigation applied in the post-harvest period.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11090997/s1.
Author Contributions
Conceptualization and methodology, T.L.; investigation, P.M.; writing and editing, P.M. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
The funding for this research was provided by the Doctoral School of Horticultural Science, Hungarian University of Agriculture and Life Sciences (in connection with the PhD study of P. Mohay, unique identifier: I9AFMY).
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ET0 | Reference evapotranspiration |
| gs | Stomatal conductance |
| MSWP | Midday stem water potential |
| RDI | Regulated deficit irrigation |
References
- Iglesias, I.; Dallabetta, N.; Whiting, M.; Long, L.E.; Iezzoni, A. Development of innovative high-density orchards aiming for an efficient and sustainable sweet cherry production. Act. Hort. 2024, 1408, 41. [Google Scholar] [CrossRef]
- Stone, C.H.; Close, D.C.; Bound, S.A.; Hunt, I. Training Systems for Sweet Cherry: Light Relations, Fruit Yield and Quality. Agronomy 2022, 12, 643. [Google Scholar] [CrossRef]
- Olmstead, A.M.; Lang, N.S.; Lang, A.G.; Ewers, F.W.; Owens, S.A. Examining the vascular pathway of sweet cherry grafted onto dwarfing rootstocks. HortScience 2006, 41, 677–678. [Google Scholar] [CrossRef]
- Ljubojević, M.; Ognjanov, V.; Zorić, L.; Maksimović, I.; Merkulov, L.; Bošnjaković, D.; Barać, G. Modeling of water movement through cherry plant as preselecting tool for prediction of tree vigor. Sci. Hortic. 2013, 160, 189–197. [Google Scholar] [CrossRef]
- Hrotkó, K.; Rozpara, E. Rootstocks and improvement. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Wallingford, UK, 2017; pp. 117–139. [Google Scholar] [CrossRef]
- Gonçalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Ferreira, H.; Moutinho-Pereira, J.M. Variation in xylem structure and function in roots and stems of scion-rootstock combinations of sweet cherry tree. Trees 2006, 21, 127–129. [Google Scholar] [CrossRef]
- Stott, L.V.; Black, B.; Bugbee, B. Differences in drought tolerance of Gisela cherry rootstocks determined using automated weighing lysimeter. HortScience 2019, 54, 1847–1852. [Google Scholar] [CrossRef]
- Opazo, I.; Toro, G.; Salvatierra, A.; Pastenes, C.; Pimentel, P. Rootstocks modulate the physiology and growth responses to water deficit and long-term recovery in grafted stone fruit trees. Agric. Water Manag. 2020, 228, 105897. [Google Scholar] [CrossRef]
- Toro, G.; Pastenes, C.; Salvatierra, A.; Pimientel, P. Trade-off between hydraulic sensitivity, root hydraulic conductivity and water use efficiency in grafted Prunus under water deficit. Agric. Water Manag. 2023, 282, 108284. [Google Scholar] [CrossRef]
- Long, L.E.; Kaiser, C. Sweet cherry rootstocks. In A Pacific Northwest Extension Publication 2010; Oregon State University: Corvallis, OR, USA, 2010. [Google Scholar]
- Balmer, M. Improving profitability: Assessment of new rootstocks and planting density. Italus Hortus 2019, 26, 35–40. [Google Scholar] [CrossRef]
- Stoppel, P. WeiGi® 2. The New Generation of Rootstocks for Cherry Trees. 2015. Available online: https://weigi.com/en/pdf/Weigi2_E_A4_web.pdf (accessed on 20 June 2025).
- Izsák, B.; Szentimrey, T. To what extent does the detection of climate change in Hungary depend on the choice of statistical methods? Int. J. Geomath. 2020, 11, 17. [Google Scholar] [CrossRef]
- Hungaro-Met. Weather Archive. Available online: https://www.met.hu (accessed on 23 June 2025).
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef]
- Carrasco-Benavides, M.; Espinoza Meza, S.; Olguín-Cáceres, J.; Muñoz-Concha, D.; von Bennewitz, E.; Ávila-Sánchez, C.; Ortega-Farías, S. Effects of regulated post-harvest irrigation strategies on yield, fruit quality and water productivity in a drip-irrigated cherry orchard. N. Z. J. Crop Hortic. Sci. 2020, 48, 97–116. [Google Scholar] [CrossRef]
- Houghton, E.; Bevandicka, K.; Neilsen, D.; Hannam, K.; Nelson, L.M. Effects of postharvest deficit irrigation on sweet cherry (Prunus avium) in five Okanagan Valley, Canada, orchards: II. Phenology, cold hardiness, fruit yield, and quality. Can. J. Plant Sci. 2023, 103, 184–200. [Google Scholar] [CrossRef]
- Houghton, E.; Bevandicka, K.; Neilsen, D.; Hannam, K.; Nelson, L.M. Effects of postharvest deficit irrigation on sweet cherry (Prunus avium) in five Okanagan Valley, Canada, orchards: I. Tree water status, photosynthesis, and growth. Can. J. Plant Sci. 2023, 103, 73–92. [Google Scholar] [CrossRef]
- McCutchan, H.J.; Shackel, K.H. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Shackel, K. A plant-based approach to deficit irrigation in trees and vines. HortScience 2011, 46, 173–177. [Google Scholar] [CrossRef]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water relations and quality changes throughout fruit development and shelf life of sweet cherry grown under regulated deficit irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Blanco, V.; Domingo, R.; Pérez-Pastor, R.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and plant water indicators for deficit irrigation management of field-grown sweet cherry trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. Ann. Sci. For. 1985, 42, 193–200. [Google Scholar] [CrossRef]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements. In FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Peschiutta, M.L.; Bucci, S.J.; Scholz, F.G.; Koval, R.F.; Goldstein, G. Leaf and stem hydraulic traits in relation to growth, water use and fruit yield in Prunus avium L. cultivars. Trees 2013, 27, 1559–1569. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Corelli Grappadelli, L. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, F. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Opazo, I.; Toro, G.; Solis, S.; Salvatierra, A.; Franck, N.; Albornoz, F.; Pimentel, P. Late reduction on transpiration is an important trait for water deficit tolerance in interspecific Prunus rootstock hybrids. Theor. Exp. Plant Physiol. 2019, 31, 493–506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).