Phenotypic and Phytochemical Variations in Wolfberry Varieties and Their Harvest Times
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Site
2.2. Plant Materials
2.3. Phenotypic Trait Measurement
2.4. Determination of Nutritional Characteristics
2.4.1. Polysaccharide Content
2.4.2. Total Flavonoid Content
2.4.3. Carotenoid Content
2.5. Multi-Criteria Assessment
2.6. Statistical Analyses
3. Results
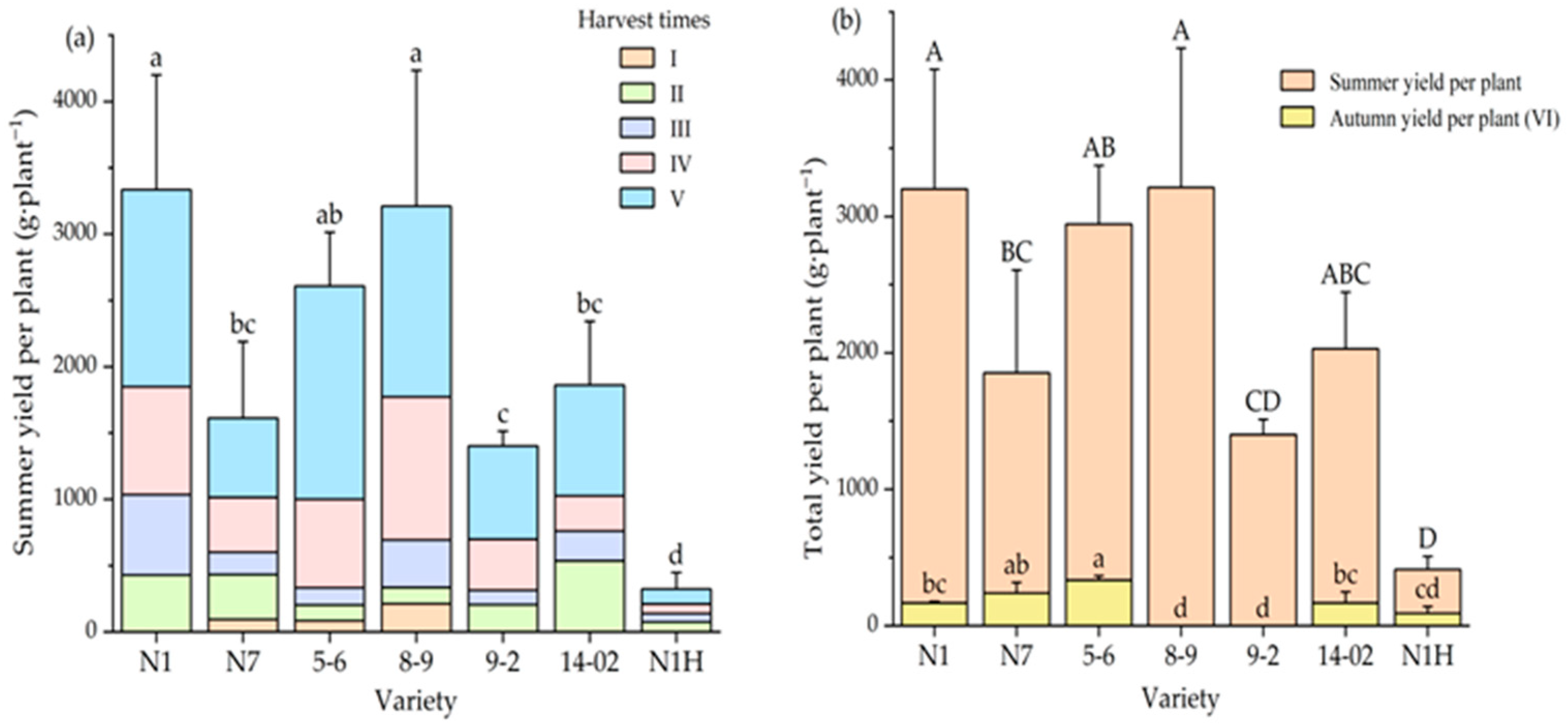
3.1. Variation in Fresh Fruit Yield per Plant
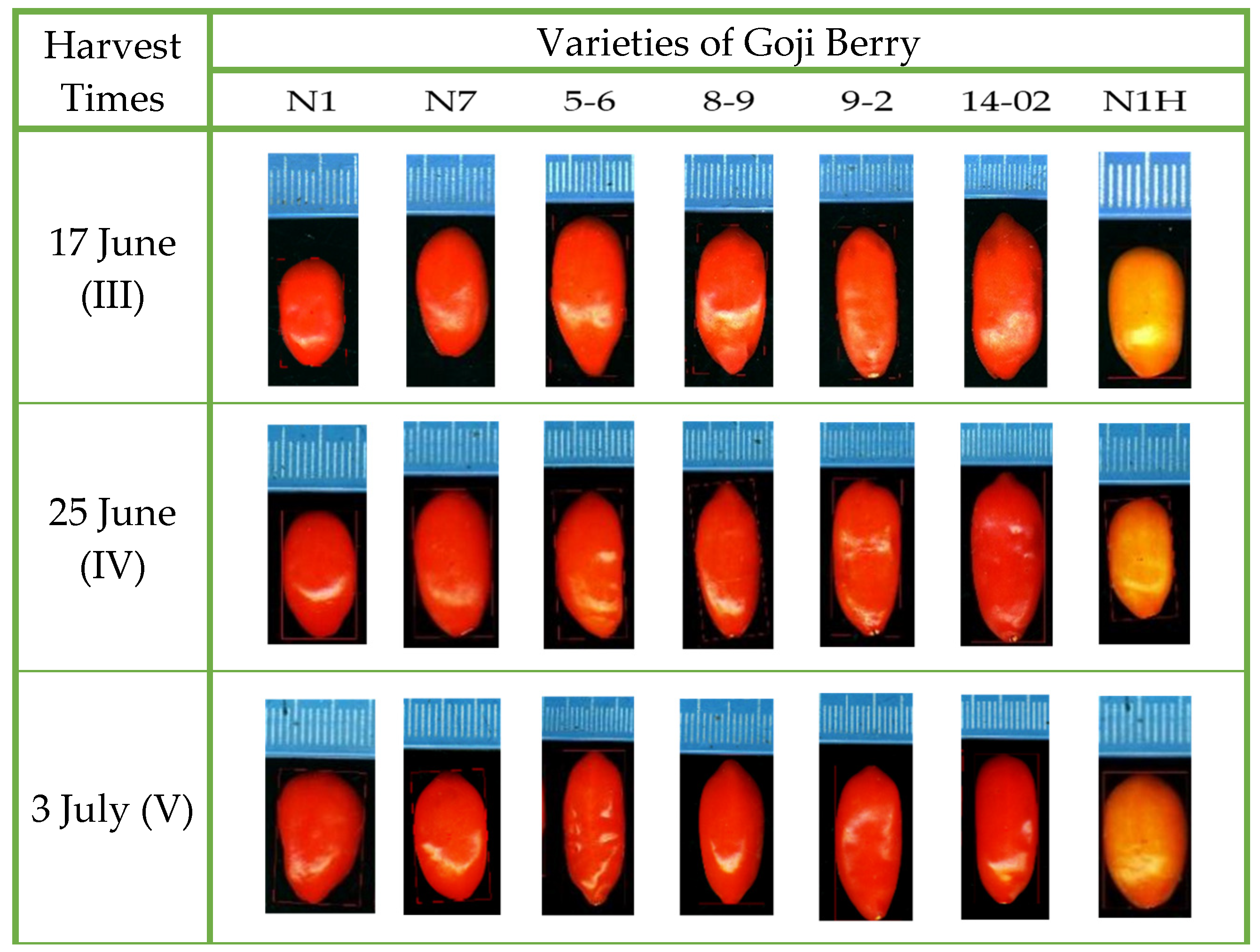
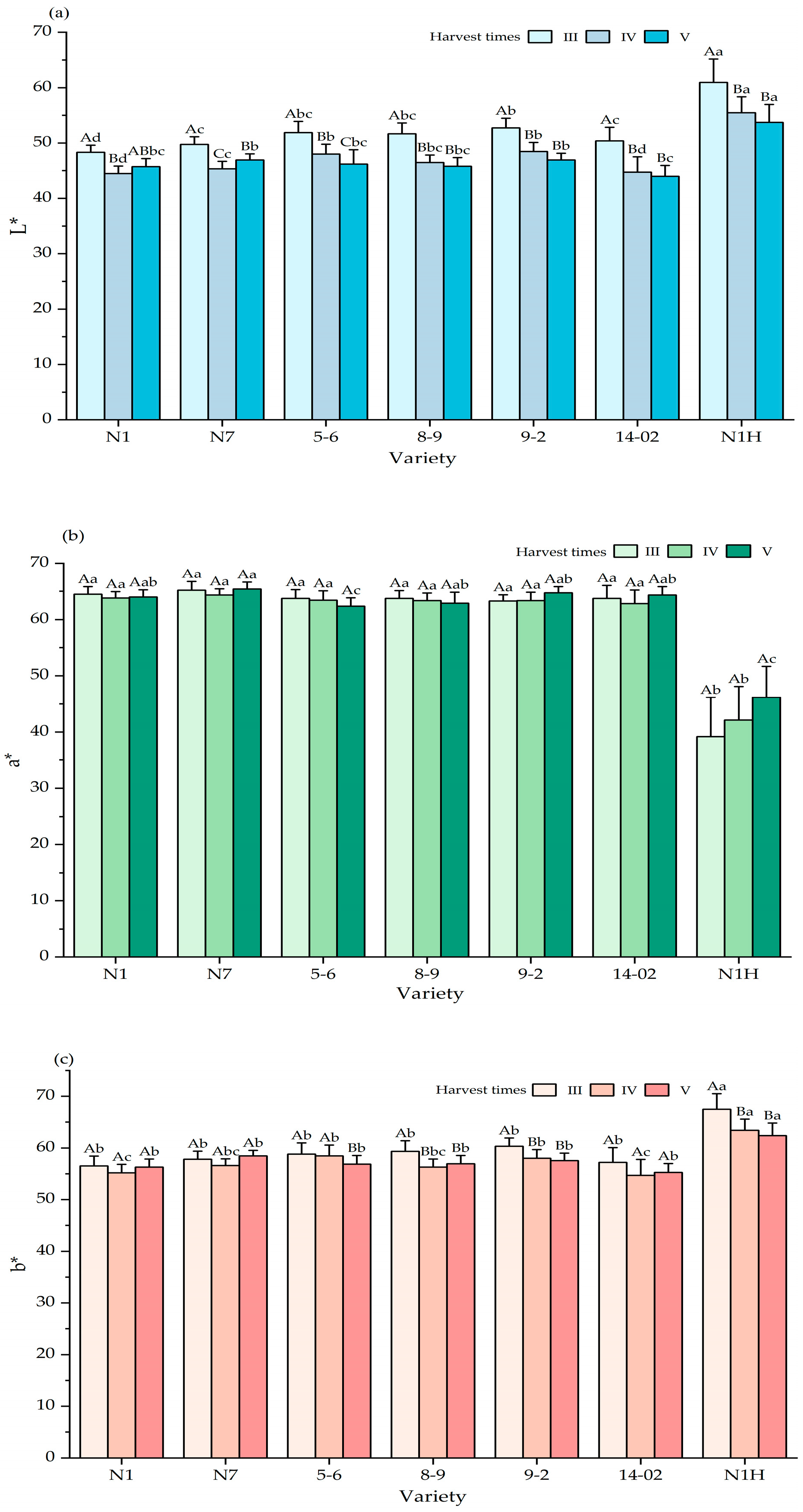
3.2. Variations in Phenotypic Characteristics
3.3. Variations in Key Metabolites
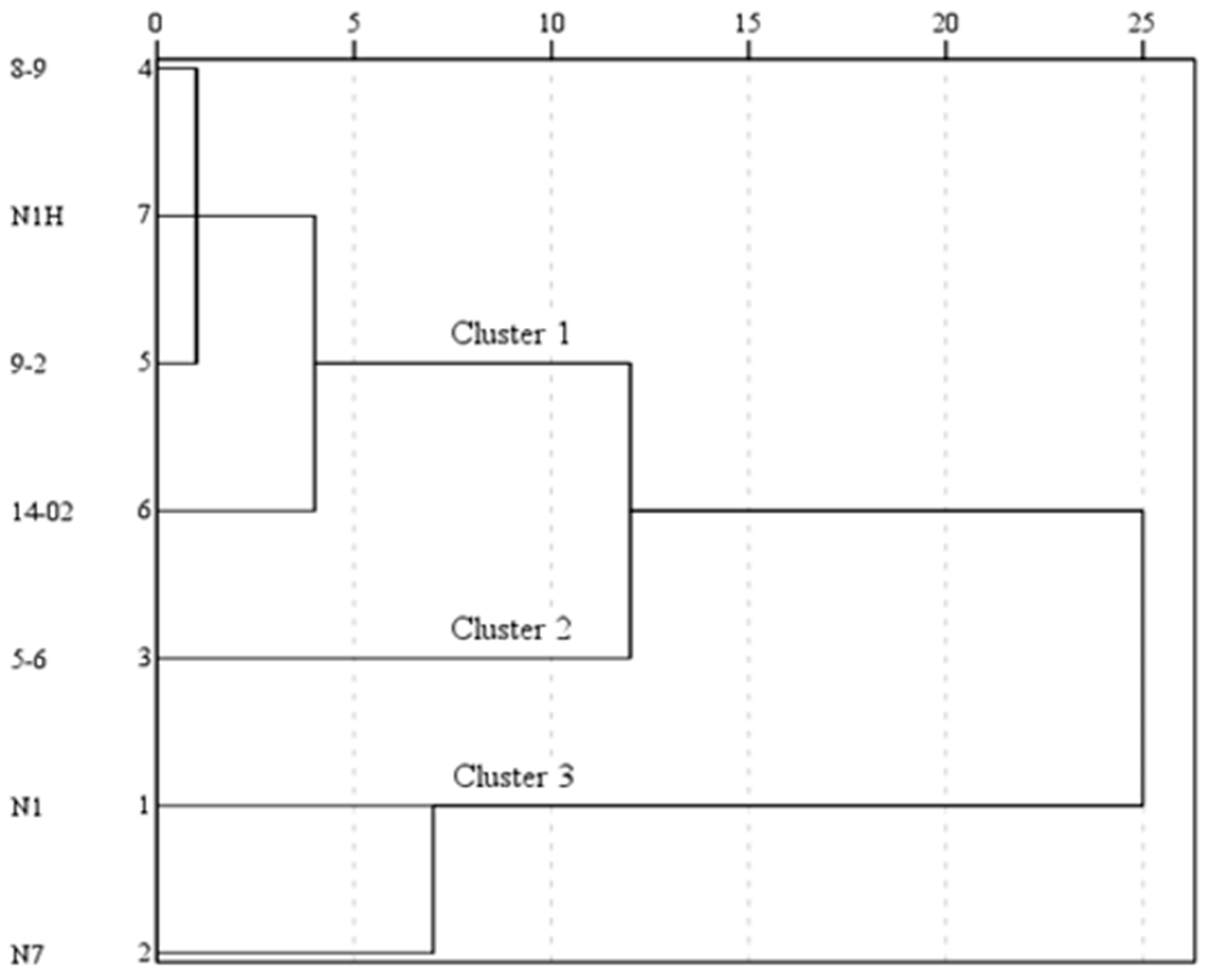
3.4. Comprehensive Evaluation on Fruit Quality Using TOPSIS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kulczyński, B.; Gramza-Michałowska, A. Goji Berry (Lycium barbarum): Composition and Health Effects—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Zhang, Z.; He, K.; Zhang, T.; Tang, D.; Li, R.; Jia, S. Physiological Responses of Goji Berry (Lycium barbarum L.) to Saline-alkaline Soil from Qinghai Region, China. Sci. Rep. 2019, 9, 12057. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, G.; Qin, K.; Wu, J.; Wang, Z.; Wang, C. Comprehensive Evaluation of Germplasm Resources in Various Goji Cultivars Based on Leaf Anatomical Traits. Forests 2025, 16, 187. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Zheng, Y.; Fu, L. Advances in the Study of Bioactive Compounds and Nutraceutical Properties of Goji Berry (Lycium barbarum L.). Appl. Sci. 2024, 15, 262. [Google Scholar] [CrossRef]
- Gündüz, E.; Dursun, R.; Zengin, Y.; İçer, M.; Durgun, H.M.; Kanıcı, A.; Kaplan, İ.; Alabalık, U.; Gürbüz, H.; Güloğlu, C. Lycium barbarum Extract Provides Effective Protection against Paracetamol-induced Acute Hepatotoxicity in Rats. Int. J. Clin. Exp. Med. 2015, 8, 7898–7905. [Google Scholar] [PubMed]
- Lu, Y.; Guo, S.; Zhang, F.; Yan, H.; Qian, D.W.; Shang, E.X.; Wang, H.Q.; Duan, J.A. Nutritional Components Characterization of Goji Berries from Different Regions in China. J. Pharm. Biomed. Anal. 2021, 195, 113859. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yan, Y.; Zhang, L.; Mi, J.; Yu, L.; Zhang, F.; Lu, L.; Luo, Q.; Li, X.; Zhou, X. A Comprehensive Review of Goji Berry Processing and Utilization. Food Sci. Nutr. 2023, 11, 7445–7457. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative Metabolic Profiling of Lycium Fruits (Lycium barbarum and Lycium chinense) from Different Areas in China and from Nepal. J. Food Qual. 2019, 2019, 4396027. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Ma, Q.; Li, Y.; Jing, H.; Sun, T.; Milne, E.; Easter, M.; Paustian, K.; Yong, H.W.A. Carbon Benefits of Wolfberry Plantation on Secondary Saline Land in Jingtai Oasis, Gansu—A Case Study on Application of the CBP Model. J. Environ. Manag. 2015, 157, 303–310. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Q.; Wei, N. Determining the Impact Bruising of Goji Berry Using a Pendulum Method. Horticulturae 2025, 11, 14. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Wang, Z.; Weckerle, C.S. Quality Control of Goji (Fruits of Lycium barbarum L. and L. chinense Mill.): A Value Chain Analysis Perspective. J. Ethnopharmacol. 2018, 224, 349–358. [Google Scholar] [CrossRef]
- Chung, R.; Chen, C.; Ng, L. Nitrogen Fertilization Affects the Growth Performance, Betaine and Polysaccharide Concentrations of Lycium barbarum. Ind. Crop. Prod. 2010, 32, 650–655. [Google Scholar] [CrossRef]
- Xue, J.; Lu, Y.; He, J.; Xie, M.; He, K.; Wang, H. Lycium barbarum Studies: A System Review on Molecular Biology, Cultivation, and Quality Characteristics of Goji Berries. Biochem. Syst. Ecol. 2025, 121, 105020. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A Review of Botanical Characteristics, Phytochemistry, Clinical Relevance in Efficacy and Safety of Lycium barbarum Fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quetglas-Llabrés, M.M.; Sureda, A.; Mardones, L.; Villagran, M.; Sönmez Gürer, E.; Živković, J.; Ezzat, S.M.; Zayed, A.; Gümüşok, S. Supercharging Metabolic Health with Lycium barbarum L.: A review of the Therapeutic Potential of This Functional Food for Managing Metabolic Syndrome. Food Front. 2024, 5, 420–434. [Google Scholar] [CrossRef]
- Gong, H.; Rehman, F.; Ma, Y.; A, B.; Zeng, S.; Yang, T.; Huang, J.; Li, Z.; Wu, D.; Wang, Y. Germplasm Resources and Strategy for Genetic Breeding of Lycium species: A review. Front. Plant Sci. 2022, 13, 802936. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qin, K.; Shang, X.; Gao, Y.; Wu, J.; Ma, H.; Wei, Z.; Dai, G. Mapping Quantitative Trait Loci Associated with Self-(in)compatibility in Goji Berries (Lycium barbarum). BMC Plant Biol. 2024, 24, 441. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Shi, Z.; Li, Y.; Huang, T.; Cao, Y.; An, W.; Zhang, X.; Zhao, J.; Qin, K.; Wang, X. Effect of Potassium on the Agronomic Traits and Fruit Quality of Goji (Lycium barbarum L.). Sci. Rep. 2024, 14, 21477. [Google Scholar] [CrossRef]
- Liang, X.; An, W.; Li, Y.; Qin, X.; Zhao, J.; Su, S. Effects of Different Nitrogen Application Rates and Picking Batches on the Nutritional Components of Lycium barbarum L. Fruits. Front. Plant Sci. 2024, 15, 1355832. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Gong, H.; Li, Z.; Zeng, S.; Yang, T.; Ai, P.; Pan, L.; Huang, H.; Wang, Y. Identification of Fruit Size Associated Quantitative Trait Loci Featuring SLAF Based High-density Linkage Map of Goji Berry (Lycium spp.). BMC Plant Biol. 2020, 20, 474. [Google Scholar] [CrossRef]
- Huang, T.; Jia, N.; Zhu, L.; Jiang, W.; Tu, A.; Qin, K.; Yuan, X.; Li, J. Comparison of Phenotypic and Phytochemical Profiles of 20 Lycium barbarum L. Goji Berry Varieties During Hot Air-drying. Food Chem. 2025, 27, 102436. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum Polysaccharides: Extraction, Purification, Structural Characterization and Evidence about Hypoglycaemic and Hypolipidaemic Effects. A Review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Fakhfakh, J.; Athmouni, K.; Mallek-Fakhfakh, H.; Ayedi, H.; Allouche, N. Polysaccharide from Lycium arabicum: Structural Features, in vitro Antioxidant Activities and Protective Effect against Oxidative Damage in Human Erythrocytes. Chem. Biodivers. 2020, 17, e2000614. [Google Scholar] [CrossRef]
- Chang, R.; So, K.F. Use of Anti-aging Herbal Medicine, Lycium barbarum, against Aging-associated Diseases. What Do We Know So Far? Cell. Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential Therapeutic Agents for Cardiovascular Disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical Characterization, Antioxidant and Antimicrobial Properties of Goji Berries Cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef]
- Qian, J. The Efficiency of Flavonoids in Polar Extracts of Lycium chinense Mill Fruits as Free Radical Scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar] [CrossRef]
- Solomando González, J.C.; Rodríguez Gómez, M.J.; Ramos García, M.; Nicolás Barroso, N.; Calvo Magro, P. Characterization and Selection of Lycium barbarum Cultivars Based on Physicochemical, Bioactive, and Aromatic Properties. Horticulturae 2025, 11, 924. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, Y.; Liu, J.; Fan, Y.; Zheng, A.; Gao, P.; Wang, L.; Liu, D. Assessment of the Bioaccessibility of Carotenoids in Goji Berry (Lycium barbarum L.) in Three Forms: In vitro Digestion Model and Metabolomics Approach. Foods 2022, 11, 3731. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, X.; Ni, Z.; Thakur, K.; Wang, W.; Yan, Y.; Cao, Y.; Zhang, J.; Rengasamy, K.R.; Wei, Z. Lycium barbarum (Goji) as Functional Food: A Review of Its Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10621–10635. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C.S. Quality Variation of Goji (Fruits of Lycium spp.) in China: A Comparative Morphological and Metabolomic Analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Ramón-Canul, L.G.; Margarito-Carrizal, D.L.; Limón-Rivera, R.; Morales-Carrera, U.A.; Rodríguez-Buenfil, I.M.; Ramírez-Sucre, M.O.; Cabal-Prieto, A.; Herrera-Corredor, J.A.; de Jesús Ramírez-Rivera, E. Technique for Order of Preference by Similarity to Ideal Solution (TOPSIS) Method for the Generation of External Preference Mapping Using Rapid Sensometric Techniques. J. Sci. Food Agric. 2021, 101, 3298–3330. [Google Scholar] [CrossRef]
- Sater, H.; Ferrão, L.F.V.; Olmstead, J.; Munoz, P.R.; Bai, J.; Hopf, A.; Plotto, A. Exploring Environmental and Storage Factors Affecting Sensory, Physical and Chemical Attributes of Six Southern Highbush Blueberry Cultivars. Sci. Hortic. 2021, 289, 110468. [Google Scholar] [CrossRef]
- Jurikova, T.; Tinakova, S.M.; Ziarovska, J.; Szekeres, L.; Mlcek, J.; Fatrcova-Sramkova, K.; Knazicka, Z.; Skrovankova, S. Polyphenolic Spectrum of Goji Berries and Their Health-promoting Activity. Foods 2025, 14, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, Y. Comprehensive Analysis of Phenolic Compounds in Four Varieties of Goji Berries at Different Ripening Stages by UPLC–MS/MS. J. Food Compos. Anal. 2022, 106, 104279. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Guo, S.; Li, Y.; Zhang, B.; Yin, Y.; An, W.; Cao, Y.; Zhao, J. Evaluation of Nutrients and Related Environmental Factors for Wolfberry (Lycium barbarum) Fruits Grown in the Different Areas of China. Biochem. Syst. Ecol. 2019, 86, 103916. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, M.; Zhang, D.; Xie, Z. Characterization of Goji Quality at Different Harvest Stages in Qaidam Basin Based on Transcriptome and Widely Targeted Metabolome. J. Food Biochem. 2024, 2024, 1139944. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, M.; Wang, Y.; Zhang, L.; Yao, W.; Feng, M. Integrated Metabolome and Transcriptome Analysis of Differences in Quality of Ripe Lycium barbarum L. Fruits Harvested at Different Periods. BMC Plant Biol. 2024, 24, 82. [Google Scholar] [CrossRef]
- Polat, M.; Mertoglu, K.; Eskimez, I.; Okatan, V. Effects of the Fruiting Period and Growing Seasons on Market Quality in Goji Berry (Lycium Barbarum L.). Folia Hortic. 2020, 32, 229–239. [Google Scholar] [CrossRef]
| Soil Depth (cm) | Soil Chemical Property | |||||
|---|---|---|---|---|---|---|
| pH | Total Salt Content (g·kg−1) | Organic Matter (g·kg−1) | Hydrolyzable Nitrogen (mg·kg−1) | Available Potassium (mg·kg−1) | Available Phosphorus (mg·kg−1) | |
| 0–20 | 8.22 | 0.59 | 32.00 | 198.00 | 640.00 | 555.00 |
| 20–40 | 8.22 | 0.65 | 25.30 | 171.00 | 585.00 | 297.00 |
| Harvest Time | Variety | ||||||
|---|---|---|---|---|---|---|---|
| N1 | N7 | 5-6 | 8-9 | 9-2 | 14-02 | N1H | |
| Berry length (mm) | |||||||
| III | 6.18 ± 0.86 Bf | 6.39 ± 0.51 Ce | 9.20 ± 0.76 Cb | 7.40 ± 0.76 Cd | 8.27 ± 0.77 Cc | 10.03 ± 1.20 Ca | 5.45 ± 0.67 Cg |
| IV | 18.59 ± 1.33 Af | 20.46 ± 1.53 Ae | 27.98 ± 1.96 Ba | 20.90 ± 2.40 Bd | 24.76 ± 3.53 Bc | 26.54 ± 3.43 Bb | 18.62 ± 1.94 Bf |
| V | 18.55 ± 1.74 Ag | 19.65 ± 1.61 Be | 28.03 ± 3.22 Aa | 22.09 ± 1.82 Ad | 25.8 ± 33.32 Ac | 27.10 ± 2.67 Ab | 18.70 ± 1.71 Af |
| Berry width (mm) | |||||||
| III | 3.40 ± 0.35 Ce | 3.69 ± 0.24 Cc | 4.04 ± 0.41 Cb | 3.63 ± 0.29 Cd | 4.05 ± 0.41 Cb | 4.52 ± 0.52 Ca | 3.06 ± 0.44 Cf |
| IV | 10.03 ± 1.07 Bg | 11.27 ± 0.83 Bd | 11.58 ± 1.27 Bc | 10.40 ± 1.13 Be | 12.66 ± 1.65 Ba | 11.85 ± 1.36 Bb | 9.75 ± 1.14 Bf |
| V | 10.65 ± 1.04 Af | 11.17 ± 0.58 Ad | 11.93 ± 0.98 Ac | 11.04 ± 0.91 Ae | 12.92 ± 1.04 Aa | 12.22 ± 1.04 Ab | 10.31 ± 1.01 Ag |
| FSI | |||||||
| III | 1.82 ± 0.14 Bd | 1.76 ± 0.16 Be | 2.26 ± 0.21 Ca | 2.02 ± 0.17 Ac | 2.05 ± 0.23 Ac | 2.12 ± 0.20 Bb | 1.78 ± 0.16 Ce |
| IV | 1.89 ± 0.15 Ae | 1.84 ± 0.13 Ae | 2.43 ± 0.29 Aa | 2.04 ± 0.14 Ac | 1.99 ± 0.34 Bcd | 2.24 ± 0.20 Ab | 1.94 ± 0.21 Ad |
| V | 1.73 ± 0.10 Cd | 1.75 ± 0.12 Bd | 2.33 ± 0.22 Ba | 2.02 ± 0.24 Ac | 2.00 ± 0.18 Bc | 2.22 ± 0.22 Ab | 1.83 ± 0.18 Bd |
| Fruit weight (g) | |||||||
| III | 0.64 ± 0.25 Ce | 0.99 ± 0.16 Cc | 1.01 ± 0.84 Cc | 0.78 ± 0.26 Bd | 1.17 ± 0.10 Cb | 1.39 ± 0.15 Ca | 0. 42 ± 0.21 Cf |
| IV | 0.74 ± 0.15 Be | 1.06 ± 0.16 Bc | 1.43 ± 0.20 Ba | 0.90 ± 0.10 Ad | 1.25 ± 0.15 Bb | 1.45 ± 0.10 Ba | 0. 58 ± 0.12 Bf |
| V | 0.85 ± 0.14 Af | 1.20 ± 0.12 Ad | 1.53 ± 0.11 Ab | 0.90 ± 0.14 Ae | 1.36 ± 0.12 Ac | 1.80 ± 0.10 Aa | 0.84 ± 0.14 Af |
| Harvest Time | Variety | ||||||
|---|---|---|---|---|---|---|---|
| N1 | N7 | 5-6 | 8-9 | 9-2 | 14-02 | N1H | |
| Polysaccharides (mg·g−1) | |||||||
| I | - | 54.3 ± 1.25 Bc | 64.6 ± 1.27 Aa | 61.4 ± 1.18 Ab | - | - | - |
| II | 51.0 ± 1.14 Bc | 53.8 ± 1.07 Bb | 61.0 ± 1.26 Ba | 62.8 ± 1.15 Aa | 40.4 ± 1.05 Ce | 44.6 ± 1.13 Bd | 43.3 ± 2.11 Cd |
| III | 42.7 ± 2.14 Cd | 50.3 ± 1.07 Cc | 50.1 ± 1.07 Dc | 54.4 ± 1.05 Bb | 54.1 ± 2.10 Bb | 54.5 ± 2.91 Ab | 62.0 ± 2.17 Aa |
| IV | 54.9 ± 1.09 Ade | 58.1 ± 1.17 Abc | 60.4 ± 1.12 Bb | 42.2 ± 1.02 Cf | 67.1 ± 1.26 Aa | 53.8 ± 1.13 Ae | 56.9 ± 0.92 Bcd |
| V | 54.1 ± 1.07 Ac | 53.0 ± 1.19 Bc | 52.8 ± 2.35 Cc | 62.5 ± 1.21 Ab | 65.5 ± 2.21 Aa | 42.1 ± 1.25 Be | 45.0 ± 1.15 Cd |
| VI | 29.7 ± 0.07 Da | 19.9 ± 0.04 Dc | 17.6 ± 0.08 Ed | - | - | 17.0 ± 0.05 Cd | 27.6 ± 0.09 Db |
| Flavonoids (mg·g−1) | |||||||
| I | - | 3.14 ± 0.61 CDb | 9.53 ± 0.74 Ca | 3.96 ± 1.09 Cb | - | - | - |
| II | 15.54 ± 1.80 Ab | 6.71 ± 1.23 Bf | 12.74 ± 2.23 Ac | 10.03 ± 1.58 Ad | 9.58 ± 1.56 Ad | 16.30 ± 1.32 Aa | 7.97 ± 1.09 Ce |
| III | 11.66 ± 0.87 Bb | 6.99 ± 1.31 Bc | 7.04 ± 1.33 Dc | 7.38 ± 1.19 Bc | 11.42 ± 1.14 Ab | 16.08 ± 1.58 Aa | 14.31 ± 1.07 Bab |
| IV | 4.32 ± 1.25 Dc | 3.61 ± 1.15 Cc | 3.34 ± 0.61 Ec | 4.03 ± 0.56 Cc | 3.45 ± 1.02 Bc | 6.55 ± 1.16 Cb | 16.54 ± 1.44 Aa |
| V | 6.98 ± 0.95 Ca | 2.09 ± 1.20 Dd | 6.58 ± 1.27 Da | 1.56 ± 1.06 De | 5.13 ± 1.19 Bb | 2.43 ± 1.04 Dd | 4.23 ± 1.16 Dc |
| VI | 7.76 ± 0.45 Cc | 10.41 ± 1.33 Ab | 11.01 ± 0.45 Bb | - | - | 8.35 ± 0.61 Bc | 17.58 ± 1.19 Aa |
| Carotenoids (mg·g−1) | |||||||
| I | - | 1.01 ± 0.06 Ba | 0.61 ± 0.02 Cc | 0.73 ± 0.04 Ab | - | - | - |
| II | 0.53 ± 0.02 Cc | 0.25 ± 0.05 Ed | 0.73 ± 0.04 ABb | 0.54 ± 0.08 Dc | 0.77 ± 0.08 Ba | 0.15 ± 0.01 Ef | 0.20 ± 0.06 Ae |
| III | 0.87 ± 0.05 Bb | 1.32 ± 0.06 Aa | 0.73 ± 0.05 Ac | 0.60 ± 0.03 Cd | 0.43 ± 0.06 Ce | 0.71 ± 0.01 Dc | 0.13 ± 0.02 Df |
| IV | 0.35 ± 0.02 Df | 0.54 ± 0.09 De | 0.71 ± 0.02 Bc | 0.67 ± 0.08 Bd | 0.90 ± 0.03 Ab | 1.81 ± 0.09 Aa | 0.11 ± 0.08 Eg |
| V | 0.89 ± 0.08 Bb | 0.78 ± 0.05 Cc | 0.57 ± 0.04 Dd | 0.46 ± 0.06 Ee | 0.77 ± 0.03 Bc | 1.77 ± 0.07 Ba | 0.16 ± 0.01 Cf |
| VI | 0.95 ± 0.04 Ab | 1.03 ± 0.49 Ba | 0.37 ± 0.07 Ec | - | - | 0.93 ± 0.06 Cb | 0.19 ± 0.09 Bd |
| Variety | Harvest Time | Ci | Ranking Order | ||
|---|---|---|---|---|---|
| N1 | III | 0.2512 | 0.1133 | 0.3108 | 20 |
| IV | 0.2397 | 0.1291 | 0.3501 | 16 | |
| V | 0.2134 | 0.1842 | 0.4633 | 7 | |
| N7 | III | 0.2669 | 0.1011 | 0.2748 | 21 |
| IV | 0.2466 | 0.1299 | 0.3449 | 18 | |
| V | 0.2384 | 0.1325 | 0.3572 | 15 | |
| 5-6 | III | 0.2356 | 0.1340 | 0.3626 | 13 |
| IV | 0.1898 | 0.2019 | 0.5155 | 2 | |
| V | 0.1743 | 0.2340 | 0.5732 | 1 | |
| 8-9 | III | 0.2338 | 0.1132 | 0.3262 | 19 |
| IV | 0.2159 | 0.1552 | 0.4182 | 12 | |
| V | 0.2088 | 0.1917 | 0.4786 | 3 | |
| 9-2 | III | 0.2354 | 0.1317 | 0.3588 | 14 |
| IV | 0.2154 | 0.1690 | 0.4396 | 9 | |
| V | 0.2004 | 0.1780 | 0.4705 | 5 | |
| 14-02 | III | 0.2222 | 0.1609 | 0.4199 | 11 |
| IV | 0.2237 | 0.1825 | 0.4492 | 8 | |
| V | 0.2182 | 0.1986 | 0.4764 | 4 | |
| N1H | III | 0.2538 | 0.1941 | 0.4334 | 10 |
| IV | 0.2144 | 0.1860 | 0.4645 | 6 | |
| V | 0.2469 | 0.1317 | 0.3479 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wan, R.; Shi, Z.; Yang, L.; Fang, S. Phenotypic and Phytochemical Variations in Wolfberry Varieties and Their Harvest Times. Horticulturae 2025, 11, 1138. https://doi.org/10.3390/horticulturae11091138
Zhang Y, Wan R, Shi Z, Yang L, Fang S. Phenotypic and Phytochemical Variations in Wolfberry Varieties and Their Harvest Times. Horticulturae. 2025; 11(9):1138. https://doi.org/10.3390/horticulturae11091138
Chicago/Turabian StyleZhang, Yiyuan, Ru Wan, Zhigang Shi, Libin Yang, and Shengzuo Fang. 2025. "Phenotypic and Phytochemical Variations in Wolfberry Varieties and Their Harvest Times" Horticulturae 11, no. 9: 1138. https://doi.org/10.3390/horticulturae11091138
APA StyleZhang, Y., Wan, R., Shi, Z., Yang, L., & Fang, S. (2025). Phenotypic and Phytochemical Variations in Wolfberry Varieties and Their Harvest Times. Horticulturae, 11(9), 1138. https://doi.org/10.3390/horticulturae11091138







