Abstract
To explore the patterns of essential oil synthesis and the optimal harvesting period of citral-type Camphora officinarum Nees ex Wall (C. officinarum) at different growth stages, three varieties (C1, C2, C3) from the citral-type C. officinarum coppice were taken as research objects. During the leaf growth cycle from February 2022 to January 2023 (12 periods in total), their morphological indicators, biomass, essential oil yield, chemical components, and output were determined. As a natural monoterpenoid widely used in the food additive, pharmaceutical intermediate, and flavor/fragrance industries, citral-related research is of great significance for improving the breeding value of citral-type C. officinarum germplasm resources. The results showed that: (1) The leaf area, total biomass, and leaf-branch ratio of the three varieties all reached the maximum in Period I (late August, full fruit stage), among which the leaf biomass and total biomass of C1 were significantly higher than those of C2 and C3. (2) The essential oil yield based on dry weight of leaves (0.53–2.79%) was significantly higher than that of branches (0.48–0.7%). Citral (geranial and neral) was the main component of the essential oil; the citral contents in C1 and C2 were the highest in Period G (late June, early fruit stage) (78.38% and 71.78%, respectively), while that in C3 peaked in Period F (late May, late flowering stage) (70.46%). (3) The total essential oil yield reached the peak in Period I, with C1 being significantly higher at 20.19 ± 1.88 g/plant than C2 (13.8 ± 0.61 g/plant) and C3 (16.47 ± 0.87 g/plant). Comprehensive analysis indicated that C1 was identified as the top-performing cultivar among citral-type C. officinarum, and July–August was the optimal harvest period. During this period, both essential oil yield and citral content could be balanced to maximize economic benefits.
1. Introduction
Camphora officinarum Nees ex Wall (C. officinarum) is rich in terpenoids and is an important commercial plant [1]. C. officinarum exhibits chemical polymorphism, with common types including cis-linalool type, trans-linalool type, borneol type, camphor type, cineole type, and citral type. Citral is abundantly distributed in the vegetative tissues (roots, stems, leaves) of citral-type C. officinarum. Citral (3,7-dimethyl-2,6-octadienal) is a monoterpenoid compound, consisting of two isomers: geranial and neral, and has a generally recognized as safe (GRAS) status [2]. It serves as a crucial raw material for the synthesis of vitamin A, menthol, vitamin E, ionone, and high-grade fragrances [3] and has been listed by the U.S. Food and Drug Administration (FDA) in the list of food additives, being widely used in the pharmaceutical and cosmetics industries [4]. In addition, citral can inhibit the growth of plant and human pathogens, and exhibits anti-inflammatory, bactericidal, antioxidant, and insecticidal activities [5,6,7,8,9,10]. As chemically synthesized citral production generates high-concentration wastewater, extracting essential oils from plants aligns with the growing demand for green and natural products, underscoring its considerable commercial value [11,12].
Under coppice management systems, C. officinarum allows for mechanized harvesting of stems and leaves on an annual or biennial cycle, yielding high biomass and substantial essential oil production. Citral-type C. officinarum can contain citral as its principal component at levels up to 62.86%, establishing it as a viable industrial source of natural citral [13]. Despite this potential, research on citral-type C. officinarum remains limited. Pioneering studies selected elite chemotypes from southern China, leading to provincial certification of three cultivars after multi-year regional trials [14]. Subsequent analysis confirmed superior antioxidant capacity in these cultivars’ essential oils, attributable to citral [15]. Traditional seedling propagation causes slow germination, growth heterogeneity, and chemotypic segregation. Consequently, clonal propagation via cuttings or tissue culture is universally adopted for aromatic coppices, ensuring genetic fidelity to elite mother plants.
Essential oils containing citral are widely used in the fields of human health, food preservation, and perfumery. However, the period when the yield of plant essential oils is the highest is often inconsistent with that when the content of the primary component in essential oils reaches the peak, and there is an extremely significant difference between months [16]. Evaluating the optimal harvest time requires balancing the yield of the target compound per unit leaf weight—calculated as the product of leaf essential oil yield and the primary component’s content—to maximize economic returns [17]. In previous studies, it was found that the aldehydes increased and then stabilized with the leaf development [18]. Nonetheless, a comprehensive understanding of the metabolic patterns of citral throughout the entire growth cycle of elite chemotypes is still lacking.
Therefore, this study aimed to systematically investigate the morphological traits, biomass accumulation, essential oil yield, and chemical composition dynamics—particularly of citral—across three elite citral-type C. officinarum cultivars (C1, C2, C3) throughout a complete annual growth cycle. Using GC-MS analysis, we seek to clarify the synthesis patterns of monoterpenes and identify the optimal harvesting period that balances both high essential oil yield and citral content, thereby supporting the development of scientific cultivation practices and enhancing the commercial viability of citral-type C. officinarum.
2. Materials and Methods
2.1. Plant Materials and Treatment
The test materials were obtained from the citral-type C. officinarum coppice base of Jiangxi Provincial Engineering Research Center for Seed-Breeding and Utilization of Camphor Trees, with a total area of 5000 m2. In June 2020, cuttings of current-year branches of citral-type C. officinarum (C1, C2, C3) were collected from the cutting orchard and planted in the citral-type C. officinarum coppice base in October 2021, with a plant spacing of 1 m × 1.7 m. This study employed a detailed sampling design to track development. We monitored three clonal varieties (C1, C2, C3) across 12 key phenological stages (Table 1) from February 2022 to January 2023. At each stage, n = 3 trees per variety were sampled.

Table 1.
C. officinarum citral-type leaves samples at different growth periods.
Based on C. officinarum leaf developmental patterns, we monitored growth stages from leaf primordium formation to senescence, including the following: apical growth → rachis extension → marginal growth → young leaves (blade, petiole, stipule) → intercalary growth → juvenile foliage → pre-flowering leaves → pre-fruiting leaves → fruit-ripening leaves → senescent leaves. Leaf samples were collected at critical phenological nodes (detailed timelines in Table 1). To eliminate diurnal variation effects, all specimens were randomly harvested between 09:00 and 10:00 AM (30 g fresh weight per sample). Essential oils were extracted via supercritical CO2 extraction (SFE-CO2) prior to compositional analysis.
2.2. Determination Indicators and Methods
2.2.1. Determination of Morphological Indicators and Biomass
A portable leaf area meter (Beijing YaXin LiYi Technology Co., Ltd., Beijing, China) was used to measure leaf area (La), leaf length (Ll), leaf width (Lw), and leaf perimeter (Lp).
At different growth stages, three plants of each variety were randomly selected and harvested at 15 cm above the ground. The branches and leaves of the plants were separated, placed in an oven for blanching at 105 °C for 30 min to inactivate enzymes, then dried (80 °C) to constant weight. The dry weights of roots, stems, and leaves were weighed, and the total biomass of the plant and root–shoot ratio were calculated.
2.2.2. Essential Oil Extraction and Component Determination
In this experiment, essential oils were extracted by the supercritical CO2 (Applied Separations Co., Ltd., Allentown, PA, USA) method. In total, 30 g of leaves were placed in the extraction kettle, with an extraction temperature of 46 °C, an extraction pressure of 20 MPa, a CO2 flow rate of 25 L/h, and an extraction time of 90 min. The extracted essential oils were fully dissolved with dichloromethane (the concentration is 0.5 mg/mL), then centrifuged at 10,000 r/min for 3 min using a high-speed centrifuge. The supernatant was stored at 4 °C in the dark. Subsequently, a 7890B-5975C gas chromatography-mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) was used for component determination.
Mass spectrometry (MS) conditions: Electron Ionization (EI) source with an electron energy of 70 eV; transfer line temperature of 250 °C; ion source temperature of 230 °C; quadrupole temperature of 150 °C; full-scan acquisition mode with a mass range of m/z 50–650 u; and a multiplier voltage of 1200 V.
Gas chromatography (GC) conditions: A capillary column (30 m × 250 µm × 0.25 µm) was used. The column temperature program was set as follows: initial column temperature of 80 °C held for 5 min, solvent delay of 3 min, then increased to 120 °C at a rate of 2.5 °C/min and held for 1 min, followed by a further increase to 240 °C at a rate of 20 °C/min and held for 8 min. High-purity helium was used as the carrier gas with a column flow rate of 1 mL/min; injector temperature of 100 °C; injection volume of 1 µL; and split ratio of 1:20. Quantification in the experiment was performed using the area normalization method.
Identification methods for constituents were performed according to the literature [19].
2.2.3. Data Processing and Analysis
Experimental data were analyzed using SPSS 26.0 software. Differences among varieties and across growth stages were assessed using analysis of variance (ANOVA). Significant differences between means were evaluated using Duncan’s multiple comparison test. A probability value of p < 0.05 was considered statistically significant, and p < 0.01 was considered highly significant. Graphs were plotted using Origin 2023b software. Results were expressed as the mean ± standard error of three biological replicates.
3. Results
3.1. Differences in Morphological Indicators of Citral-Type C. officinarum at Different Stages
ANOVA revealed highly significant inter-stage variations (p < 0.01) in the morphometric traits of citral-yielding Cinnamomum chemotypes, including height, basal diameter, leaf dimensions (length/width), and leaf area (Table S1). The tree height and ground diameter of citral-type C. officinarum reached the maximum in Period J, and those in Periods J–L were significantly higher than those in other periods (p < 0.05), with no significant difference between Periods J and L (Figure 1). This indicates that citral-type C. officinarum entered the dormant period and stopped growing in November.
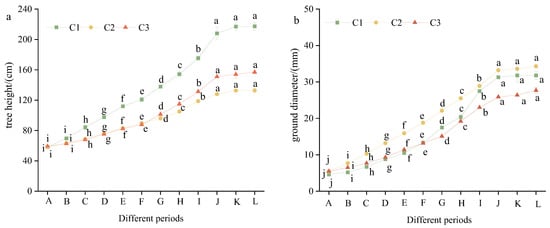
Figure 1.
The tree height and ground diameter of citral-type C. officinarum at different periods. Note: (a) tree height; (b) ground diameter. The specific sampling time of X-axis A–L is shown in Table 1. Different lowercase letters indicate significant differences in tree height and ground diameter of the same variety of citral-type C. officinarum at different growth stages, p < 0.05.
It can be seen from Figure 2a–c that the leaf length, leaf width, and leaf area of C1 increased rapidly during Periods B–C, while those in Periods A and B were significantly lower than those in other periods (p < 0.05). For C2 and C3, their leaf length, leaf width, and leaf area increased rapidly during Periods B–E, reaching the maximum in Period E, and then fluctuated during Periods E–L, with no significant difference observed among Periods E–L. This indicates that the leaves of citral-type C. officinarum mainly increased rapidly during Periods A–E, and there were differences in the leaf enlargement periods among different varieties of citral-type C. officinarum. The leaves of the three varieties reached the largest size 5–9 weeks after leaf flushing, and then gradually became leathery.
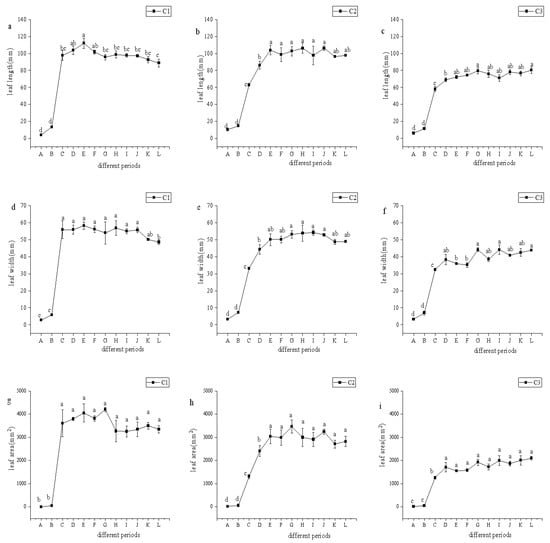
Figure 2.
The leaf parameters of citral-type C. officinarum at different periods. Note: (a) C1 leaf length; (b) C2 leaf length; (c) C3 leaf length; (d) C1 leaf width; (e) C2 leaf width; (f) C3 leaf width; (g) C1 leaf area; (h) C2 leaf area; (i) C3 leaf area. The specific sampling time of X-axis A–L is shown in Table 1. Different lowercase letters indicate significant differences in leaf morphology parameters at different growth stages, p < 0.05.
Analysis of variance on the biomass and leaf-branch ratio of different varieties of citral-type C. officinarum at different stages showed that there were extremely significant differences in branch biomass, leaf biomass, total leaf-branch biomass, and leaf-branch ratio under the effects of variety, stage, and their interaction (p < 0.01) (Table S2).
Foliar biomass dynamics in all three chemotypes followed a unimodal pattern across ontogeny, with progressive accumulation from leaf primordia expansion (A) to maximal yield at peak fruiting (I) (p < 0.05 vs. earlier phases) and, thereafter, declining with senescence. Subsequently, the total leaf biomass gradually decreased from Period J (fruit ripening stage) to Period K (fruit drop stage), and then to Period L (leaf abscission stage). In the same growth period, the leaf biomass of C1 was significantly higher than that of C2 and C3 (Figure 3a–c). It can be seen from Figure 3d,e that the branch biomass of C1, C2, and C3 gradually increased in different growth periods, with significant differences between periods (p < 0.05).
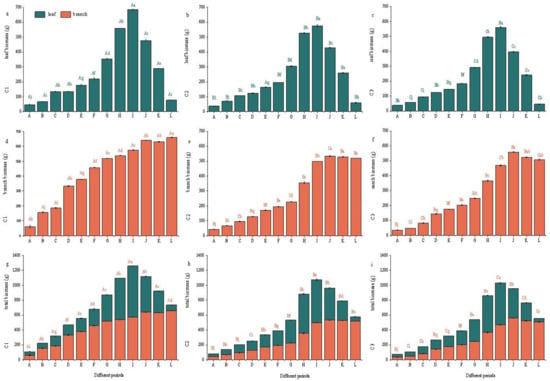
Figure 3.
The biomass of citral-type C. officinarum at different periods. Note: (a) C1 leaf biomass; (b) C2 leaf biomass; (c) C3 leaf biomass; (d) C1 branch biomass; (e) C2 branch biomass; (f) C3 branch biomass; (g) C1 total biomass; (h) C2 total biomass; (i) C3 total biomass. The sampling time of X-axis A–L is shown in Table 1. Different uppercase letters indicate significant differences in biomass among different varieties at the same period, while different lowercase letters indicate significant differences in biomass among the same variety at different periods, p < 0.05.
The variation pattern of total leaf–branch biomass was consistent with that of leaf biomass. The biomass in Period I was significantly higher than that in other growth periods (p < 0.05), and there were significant differences in total biomass among different varieties. Among them, C1 had the largest total biomass, reaching 1258.8 g at Period I (full fruit stage), which was significantly higher than the total leaf–branch biomass of C2 (1073.7 g) and C3 (1029.6 g) at the full fruit stage (Figure 3g–i). This indicates that the maximum leaf–branch biomass can be obtained by harvesting at Period I.
Leaf-to-stem biomass ratios (LSR) in citral-type Cinnamomum chemotypes C1–C3 exhibited bimodal distribution patterns, with peaks occurring during Stage C (April) and Stages H–I (July–August). Notably, C1 achieved significantly higher LSR at Stage I than other periods (p < 0.05), whereas C2 and C3 peaked at Stage H. Minimal LSR values occurred at Stage L, coinciding with leaf abscission and reduced foliar biomass, while stems reached annual maximum growth. The LSR at Stage L was significantly lower than all preceding stages (p < 0.01) (Figure 4).
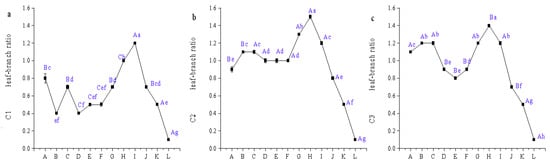
Figure 4.
The leaf–branch ratio of citral-type C. officinarum at different periods. Note: (a) C1 leaf–branch ratio; (b) C2 leaf–branch ratio; (c) C3 leaf–branch ratio. The sampling time of X-axis A–L is shown in Table 1. Different uppercase letters indicate significant differences in leaf–branch ratio among different varieties at the same period, while different lowercase letters indicate significant differences in leaf–branch ratio among the same variety at different periods, p < 0.05.
3.2. Differences in Essential Oil Indicators of Citral-Type C. officinarum at Different Stages
3.2.1. Oil Yield of Citral-Type C. officinarum at Different Stages
Leaf essential oil yields (DW) differed significantly across cultivars and developmental stages for leaves (p < 0.01, two-way ANOVA). In contrast, branch yields demonstrated non-significant temporal dynamics (p > 0.05) (Table S3). A unimodal essential oil accumulation pattern was observed: Yields showed progressive (though fluctuating) gains from leaf initiation (A) through mid-development (F), maximized during late growth to maturation (F–I), then declined precipitously through senescence (I–L) (p < 0.05) (Figure 5). The oil yield based on the dry weight of branches changed little, and the analysis of variance showed no significant difference; thus, no multiple comparison analysis was conducted for it.
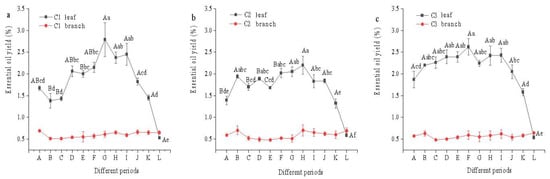
Figure 5.
Oil yields from leaves and branches of citral-type C. officinarum at different periods. Note: (a) C1 Oil yields from leaves and branches; (b) C2 Oil yields from leaves and branches; (c) C3 Oil yields from leaves and branches. The sampling time of X-axis A–L is shown in Table 1. Different uppercase letters indicate significant differences in leaf oil yield among different varieties at the same period, while different lowercase letters indicate significant differences in leaf oil yield among the same variety at different periods, p < 0.05. The ANOVA of the branch oil yield (DW) was not significant; therefore, multiple comparisons were not conducted.
The oil yield based on the dry weight of leaves ranged from 0.53% to 2.79%, and that of branches ranged from 0.48% to 0.7%. Paired comparisons were conducted on the mean values of oil yield based on the dry weight of branches and leaves of the three varieties of citral-type C. officinarum at different growth periods. The results of the t-test showed that the oil yield based on the dry weight of leaves in C1, C2, and C3 was significantly higher than that of branches (p < 0.01) (Table S4).
3.2.2. Chemical Components of Citral-Type C. officinarum at Different Stages
Chemical profiling identified 30–38 compounds in C1, 30–40 in C2, and 26–38 in C3 essential oils across developmental stages. The compositional richness varied among phenophases, indicating substantial metabolic divergence during ontogeny (Tables S5–S7).
All identified compounds accounted for 91.04–97.04% of the total essential oil content, which could be classified into monoterpene olefins, oxygenated monoterpenes, sesquiterpene olefins, and oxygenated sesquiterpenes. Among them, oxygenated monoterpenes accounted for the highest proportion, and the seven main components with the highest contents were geranial, neral, isoneal, isogeranial, nerol, citronellal, and geraniol. These seven main components accounted for more than 80% of the total essential oil content; for example, in Period G, these seven components in C1 accounted for 88% of the total essential oil, indicating that these seven compounds constitute the majority of the essential oil.
A specific analysis of the main secondary metabolites in C. officinarum essential oil at each growth stage revealed that the main component of leaf essential oil in C1, C2, and C3 was citral (geranial and neral), but their contents differed significantly. C1 and C2 had the highest citral content in Period G (early fruit stage), being 78.38% and 71.78% respectively, while their citral contents were low in Period A (bud stage), being 48.28% and 33.28% respectively (Table 2 and Table 3). C3 had the highest citral content of 70.46% in Period F (late flowering stage) and a low content of 42.34% in Period A (bud stage) (Table 4). Citral showed the fastest growth rate during Periods A–C (1–5 weeks after leaf flushing).

Table 2.
Essential oil composition of C. officinarum-JX/NC/001 (C1) leaves at different periods.

Table 3.
Essential oil composition of C. officinarum-JX/NC/002 (C2) leaves at different periods.

Table 4.
Essential oil composition of C. officinarum-GX/ZS/003 (C3) leaves at different periods.
The contents of citronellal in the leaf essential oils of C1, C2, and C3 were significantly higher in Periods J, K, and L than those in other periods (p < 0.05). The citral/citronellal ratio in Periods J, K, and L was significantly lower than that in other periods, while the vigorous growth periods had a higher citral/citronellal concentration ratio. As shown in Table 2, Table 3 and Table 4, the citral/citronellal concentration ratio of C1 was the highest in Periods C–F (171.19–202.39); that of C2 was the highest in Periods F–G (136.48–138.04); and that of C3 was the highest in Periods F–G (148.15–163.86). The conversion rate of citral to citronellal was relatively low, which indicated that the citral content was the highest in the vigorous growth Period G. It is thus inferred that with the growth and abscission of leaves, citral gradually accumulated and converted to citronellal before leaf abscission (Table 2, Table 3 and Table 4).
The contents of geranial and neral in the leaf essential oils of C1, C2, and C3 all showed a “first increased and then decreased” trend with leaf growth. The geranial/neral ratio in C1 and C3 showed a gradual increase: it was significantly lower in the A tender bud stage than in the leaf growth stage (p < 0.05), and significantly higher in the L leaf abscission stage than in other stages (p < 0.05). The change of the geranial/neral ratio in the C2 in leaf growth stages was more complex, but on the whole, the ratio in the A tender bud stage was also significantly lower than in other stages (p < 0.05). It is thus inferred that more neral is synthesized in citral-type C. officinarum during the tender bud stage, and as the leaves grow, neral is converted into geranial. The fluctuating changes of the geranial/neral ratio in C2 at different growth stages indicate that neral and geranial interconvert in C. officinarum (Table 2, Table 3 and Table 4).
Several compounds related to citral monoterpene synthesis were analyzed separately, and the conversion pathway of several main metabolites in citral-type C. officinarum was deduced from a metabolic perspective (Figure 6). Phosphatase catalyzes geranyl diphosphate to synthesize geraniol. In the later stage, geraniol is reduced to geranial, which is then converted into neral by isomerase; meanwhile, geranial can be converted into citronellal by reductase.
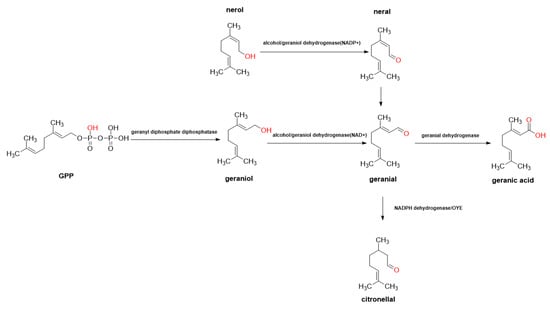
Figure 6.
Citral metabolism pathway diagram.
3.2.3. Essential Oil Production of Citral-Type C. officinarum
Varieties, developmental stages, and their interaction exerted highly significant effects on both leaf essential oil yield and total oil yield (p < 0.01). While variety and stage significantly impacted branch oil yield (p < 0.01), the variety × stage interaction showed non-significant effects (p > 0.05) (Table S8).
The production of essential oil is mainly determined by the essential oil yields of roots, branches, leaves, and biomass. However, in actual production, the management mode for fragrant C. officinarum is coppice cutting, and roots are not dug up for oil extraction. Therefore, this study only tested and determined the essential oil yields of branches and leaves of citral-type C. officinarum. A paired t-test was conducted on the essential oil yields of branches and leaves in the coppice of three varieties (C1, C2, C3) of citral-type C. officinarum, and the results showed that the leaf essential oil yield was significantly higher than that of branches (p < 0.01) (Table S9). This indicates that the total essential oil yield mainly comes from leaves; especially in Period I, the leaf essential oil yield accounted for 83.1% (C1), 76.5% (C2), and 82.0% (C3) of the total essential oil yield, respectively.
The leaf essential oil yields of the three varieties of citral-type C. officinarum all showed a trend of “first increasing and then decreasing” in different growth periods. From Period A (the first week of leaf flushing, bud stage) to Period I (fruit enlargement stage), the leaf essential oil yield gradually increased, and the leaf essential oil yield at Period I (full fruit stage) was significantly higher than that in other growth periods (p < 0.05). Subsequently, the leaf essential oil yield gradually decreased from Period J (fruit ripening stage) to Period K (fruit drop stage), and then to Period L (leaf abscission stage) (Figure 7a–c). It can be seen from Figure 7d,e that, in different growth periods, the branch essential oil yield of the same variety of citral-type C. officinarum gradually increased from Periods A–H, with significant differences observed among Periods A–H (p < 0.05). During Periods I–L, the branch essential oil yield no longer increased, with no significant difference among these periods. A comparative analysis of different varieties in the same period showed that in Periods A–G, the branch essential oil yield of C1 was significantly higher than that of C2 and C3 (p < 0.05), while there was no significant difference in branch essential oil yield among C1, C2, and C3 in Periods H–K.
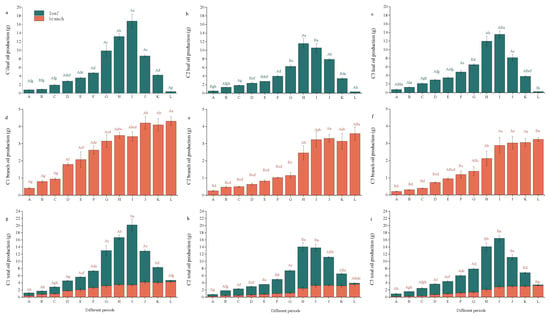
Figure 7.
The essential oil production of citral-type C. officinarum at different periods. Note: (a) C1 leaf production; (b) C2 leaf production; (c) C3 leaf production; (d) C1 branch production; (e) C2 branch production; (f) C3 branch production; (g) C1 total production; (h) C2 total production; (i) C3 total production. The sampling time of X-axis A–L is shown in Table 1. Different uppercase letters indicate significant differences in oil production among different varieties at the same period, while different lowercase letters indicate significant differences in oil production among the same variety at different periods, p < 0.05.
The variation pattern of total essential oil yield was consistent with that of leaf essential oil yield. The essential oil yield in Period I was significantly higher than that in other growth periods (p < 0.05), and there were significant differences in total essential oil yield among different varieties. Among them, C1 had the highest total essential oil yield, reaching 20.19 ± 1.88 g/plant in Period I (full fruit stage, late August), which was significantly higher than that of C2 (13.8 ± 0.61 g/plant) and C3 (16.47 ± 0.87 g/plant) in the full fruit stage (Figure 7g–i). This indicates that Period I (late August), the full fruit stage, is the optimal harvesting period.
4. Discussion
4.1. Differences in Biomass and Essential Oil Yield at Different Periods
The morphological and biomass traits of citral-type C. officinarum exhibited clear developmental trends, with leaf expansion occurring predominantly during the early growth phases (Periods B–E), followed by stabilization. Total biomass and leaf-to-stem ratio peaked in Period I (late August), consistent with the culmination of vegetative growth prior to senescence. Notably, essential oil yield derived from leaves (0.53–2.79% DW) significantly exceeded that from branches (0.48–0.7% DW), indicating that leaves are the primary organ for essential oil accumulation in aromatic plants. Therefore, regulating the leaf-to-branch ratio is of crucial importance.
In this study, the leaf-branch ratio of citral-type C. officinarum C1 in Period I was significantly higher than that in other periods, while that of C2 and C3 in Period H was significantly higher than that in other periods, with the highest leaf oil yield [20]. The higher the leaf quantity, the higher the oil yield. There are significant differences in the oil yield of citral-type camphor across different periods, showing a trend of “first increasing and then decreasing”. The oil yield fluctuated and increased during Periods A–F, reaching a maximum of 2.79% in Period G, and then dropped sharply during Periods I–L. Among these, Periods A–F correspond to the vigorous growth period of camphor (March–May), which is also a critical period for biomass accumulation. During this period, new leaves develop from germination to reaching their maximum leaf area. As temperatures rise, photosynthesis becomes more intense; plants collect energy through photosynthesis to produce several carbohydrates, which are then used in plant metabolism, including primary metabolism and secondary metabolism [21]. Primary metabolites further contribute to the production of secondary metabolites [22]. New leaves usually accumulate metabolites more rapidly than old leaves [23]. Since essential oils are secondary metabolites of plants [24], the combination of photosynthesis and secondary metabolism optimizes the production efficiency of terpenoids and contributes to the formation of essential oils [25]. Their synthesis is accelerated during this period. The oil yield is the highest during Periods G–I (July–August). The high temperature and drought in summer stimulate the synthesis of secondary metabolites [26]. Meanwhile, after the previous rapid vegetative growth, the substrates for synthesizing secondary metabolites increase, and the synthesis of secondary metabolites is enhanced; thus, this period is the optimal period for the utilization of essential oils. During Periods I–J (September–October), nutrients are preferentially allocated to the structural growth of stems and roots, while the substrates for synthesizing secondary metabolites decrease, leading to a gradual reduction in the content of secondary metabolites [27]. During Periods K–L (November of the current year to January of the following year), the fruits of C. officinarum gradually mature and fall off. Substances produced by photosynthesis are mainly used for reproductive growth, leaves gradually senesce and abscise, the vital activities of the tree decrease, and the oil yield shows a gradual downward trend. On one hand, primary metabolism accumulates the material basis for secondary metabolism; on the other hand, primary metabolism obtains energy from plants [28]. When intermediate products of primary metabolism accumulate to a certain extent, secondary metabolism reaches its peak under enzymatic action.
Previous studies have found that the four chemotypes of C. officinarum (linalool, eucalyptol, camphor, and borneol) have the highest oil yield and main component content in July [29]. Some studies have shown that the essential oil yield and linalool ratio are the highest from May to July [21]. Non-Lauraceae plants also follow a similar pattern in oil yield. In rose plants, the secondary metabolism that produces aromatic substances is controlled by primary metabolism. When the flower yield is high, primary metabolism synthesis is vigorous, resulting in a relative insufficiency of substrates for secondary metabolism synthesis, thereby affecting the synthesis of essential oils [30]. In Rosmarinus officinalis (rosemary), the oil yield of essential oils and the content of main component monoterpenes fluctuate throughout the year, with higher secondary metabolite content in summer [31]. The accumulation of essential oils in leaves at different growth and development stages is closely related to the leaves’ own growth and development. Studies have shown that high temperature and strong light in summer promote the release of monoterpenes, which scavenge ROS, protect photosynthesis, and improve the heat tolerance of plants [26].
4.2. Differences in Essential Oil Components and Total Yield at Different Periods
While leaf morphology stabilized after Period E, citral accumulation continued to increase, reaching its peak in Period G for C1 and C2, and Period F for C3. This asynchrony suggests that essential oil synthesis persists beyond leaf morphological maturation. Leaves are the basis of secondary metabolic activities; the accumulation of essential oils lags behind the morphological changes of leaves, and the rapid accumulation period of main essential oil components also lags behind the period of rapid leaf shape change [18]. Seven main components were identified in citral-type C. officinarum C1, C2, and C3: geranial, neral, isoneal, isogeranial, nerol, citronellal, and geraniol, accounting for more than 80% of the total essential oil content. This differs from the chemical components of C. officinarum reported in the literature, such as linalool, camphor, borneol, and 1,8-cineole [32,33,34]. This indicates that test conditions such as different varieties, cultivation methods, growth environment conditions (soil and climate), and different sampled tissues and organs all affect the synthesis and accumulation of chemical components in C. officinarum [35,36,37].
Citral content varied significantly among varieties and stages, with maxima of 78.38% (C1, Period G), 71.78% (C2, Period G), and 70.46% (C3, Period F). The decline in citral and concurrent increase in citronellal during late stages (J–L) suggest possible enzymatic conversion during leaf senescence, as indicated by declining citral/citronellal ratios. Total essential oil yield peaked in Period I (late August) for all varieties, with C1 yielding significantly more (20.19 ± 1.88 g/plant) than C2 and C3. This period balances high citral content with maximal biomass, making it agronomically optimal for harvest. July–August harvests allow sufficient time for new shoots to lignify before winter, reducing cold damage and ensuring sustainable coppice management. Alternatively, harvest in late October–November—though yielding less oil—may be preferable in colder regions to avoid frost damage to new growth.
4.3. Limitations and Environmental Context
The generalizability of the findings in this study must be considered in conjunction with the environmental context of the research year. The 2022 growing season in Nanchang was unusually hot and dry, with record-high temperatures in March, July, and August, and rainfall below the long-term average. While such climatic conditions may have promoted essential oil synthesis, the role of monoterpenes in C. officinarum’s resistance to high-temperature stress reveals that monoterpenes can enhance the plant’s high-temperature stress tolerance by regulating the expression of genes related to photosynthesis, non-enzymatic antioxidant formation, membrane lipid metabolism, primary metabolism, and heat shock proteins [38]. The high-temperature weather in 2022 may have led to a significant increase in monoterpene content, resulting in certain variability in the study results, which cannot fully represent the conditions of typical years. Such climate anomalies indicate that multi-year studies are necessary to incorporate the impacts of inter-annual climatic variations.
Other limitations include the focus on only three cultivars, the lack of controlled irrigation or temperature treatments, and the single-location design. Future work should expand to multiple sites and longer timelines to validate these patterns under diverse conditions. Controlled experiments manipulating water and temperature would help clarify their roles in citral biosynthesis and accumulation.
Despite these limitations, this study provides a robust initial framework for understanding growth-metabolism dynamics in citral-type C. officinarum and offers practical guidance for optimal harvest timing.
5. Conclusions
This study systematically determined and analyzed the morphological indices, biomass, essential oil yield, chemical composition, and yield of three elite varieties (C1, C2, C3) from the citral-type C. officinarum coppice base in Jiangxi Province across 12 growth periods from February 2022 to January 2023. It clarified the monoterpene metabolism patterns of citral-type C. officinarum and the optimal cultivation and harvesting strategies. The main conclusions are as follows: (1) Leaf length, leaf width, and leaf area all increased rapidly during Periods B–C (3–5 weeks after leaf flushing), with the growth rate slowing down during Periods C–E (5–9 weeks after leaf flushing), and remained stable after Period F (12 weeks after leaf flushing). The total biomass of branches and leaves and the leaf–branch ratio showed a similar trend, peaking at Period I (late August). (2) The leaf dry weight oil yield across different growth periods ranged from 0.53% to 2.79%, which was higher than the branch dry weight oil yield (0.48–0.7%). The citral content in different growth periods was 48.28–78.38% for C1, 33.28–71.78% for C2, and 42.34–70.46% for C3, with the highest content observed at Period G. The total essential oil yield of C1, C2, and C3 all reached the peak at Period I, being 20.19 ± 1.88 g/plant, 13.8 ± 0.61 g/plant, and 16.47 ± 0.87 g/plant respectively, and ANOVA showed that C1 was significantly higher than C2 and C3.
This study recommends that growers and the camphor oil industry prioritize the cultivation of the C1 cultivar and plan harvests for the late summer period (July–August). This practice balances the highest oil production with superior citral content, maximizing economic profitability per unit area. The findings provide a scientifically-grounded and practical strategy for enhancing the efficiency and commercial value of citral-type camphor production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11091125/s1, Table S1: ANOVA results of morphological parameters citral-type C. officinarum at different stages; Table S2: ANOVA results for main effects and interactions of variety and period on biomass indicators; Table S3: ANOVA results for main effects and interactions of variety and period on essential oil yield; Table S4: Comparison branch oil yield mean and leaf oil yield mean of citral-type C. officinarum (t-test); Table S5: Essential oils composition of the C. officinarum-JX/NC/001 (C1) leaves (% ± SD); Table S6: Essential oils composition of the C. officinarum-JX/NC/002 (C2) leaves (% ± SD); Table S7: Essential oils composition of the C. officinarum-GX/ZS/003 (C3) leaves (% ± SD); Table S8: ANOVA results for main effects and interactions of variety and period on essential oil production; Table S9: Comparison branch oil production mean and leaf oil production mean of citral-type C. officinarum.
Author Contributions
Conceptualization, Q.L., and Z.J.; Methodology, J.J.; Formal analysis and investigation, L.H., R.Z., and Y.L.; Writing—original draft preparation, L.H. and Q.L.; Writing—review and editing, Q.L. and J.J.; Funding acquisition, J.J.; Resources, B.Z. and Z.X.; Supervision, Z.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the General Project of Jiangxi Provincial Natural Science Foundation (20252BAC240643), Doctoral Research Startup Program at Jiangxi University of Water Resources and Electric Power (2024kyqd059), Department of Education of Jiangxi Province (GJJ2401405), Jiangxi Innovation Training Programme for University Students (S202411319007), Key Research and Development Program of Jiangxi Province (20252BCF320003).
Data Availability Statement
Data supporting the findings of this work were available within the paper and its Supplementary Information Files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, R.; Su, P.; Jin, B.; Guo, J.; Tian, M.; Mao, L.; Tang, J.; Chen, T.; Lai, C.; Zeng, W.; et al. Molecular Cloning and Functional Identification of a High-Efficiency (+)-Borneol Dehydrogenase from Cinnamomum camphora (L.) Presl. Plant Physiol. Biochem. 2021, 158, 363–371. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Maternal Reproductive Toxicity of Some Essential Oils and Their Constituents. Int. J. Mol. Sci. 2021, 22, 2380. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, H.; Wen, Z.; Zhang, B.; Chen, G. Toward the Efficient Synthesis of Pseudoionone from Citral in a Continuous-Flow Microreactor. Ind. Eng. Chem. Res. 2018, 57, 11288–11298. [Google Scholar] [CrossRef]
- Hirai, M.; Ota, Y.; Ito, M. Diversity in Principal Constituents of Plants with a Lemony Scent and the Predominance of Citral. J. Nat. Med. 2022, 76, 254–258. [Google Scholar] [CrossRef]
- Chaouki, W.; Leger, D.Y.; Liagre, B.; Beneytout, J.-L.; Hmamouchi, M. Citral Inhibits Cell Proliferation and Induces Apoptosis and Cell Cycle Arrest in MCF-7 Cells. Fund. Clin. Pharmacol. 2009, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; Bezerra, A.P.d.B.; de Sousa, J.P.; Guerra, F.Q.S.; de Lima, E.O. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid.-Based Compl. Alt. 2014, 2014, 378280. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-C.; Yang, T.-S.; Chou, J.-C.; Chen, J.; Lee, S.-C.; Kuo, Y.-H.; Ho, C.-L.; Chao, L.K.-P. Anti-Inflammatory Activity of Neral and Geranial Isolated from Fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
- Sharma, S.; Habib, S.; Sahu, D.; Gupta, J. Chemical Properties and Therapeutic Potential of Citral, a Monoterpene Isolated from Lemongrass. Med. Chem. 2021, 17, 2–12. [Google Scholar] [CrossRef]
- dos Santos e Silva, G.; de Jesus Marques, J.N.; Linhares, E.P.M.; Bonora, C.M.; Costa, E.T.; Saraiva, M.F. Review of Anticancer Activity of Monoterpenoids: Geraniol, Nerol, Geranial and Neral. Chem. Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- da Silva Júnior, A.Q.; da Silva, D.S.; Figueiredo, P.L.B.; Sarrazin, S.L.F.; Bouillet, L.E.M.; de Oliveira, R.B.; Maia, J.G.S.; Mourão, R.H.V. Seasonal and Circadian Evaluation of a Citral-Chemotype from Lippia alba Essential Oil Displaying Antibacterial Activity. Biochem. Syst. Ecol. 2019, 85, 35–42. [Google Scholar] [CrossRef]
- Southwell, I. Backhousia Citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral. Foods 2021, 10, 1596. [Google Scholar] [CrossRef]
- Yang, S.-A.; Jeon, S.-K.; Lee, E.-J.; Shim, C.-H.; Lee, I.-S. Comparative Study of the Chemical Composition and Antioxidant Activity of Six Essential Oils and Their Components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Zhang, B.; Ling, Q.; Xiao, Z.; Xiong, Y.; Lu, X.; Wang, Y.; Zhao, J.; Xiao, C.; Zhang, J.; Huang, G.; et al. Leaf Essential Oil Diversity of Camphora Fabr. in China. Ind. Crops Prod. 2025, 226, 120635. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, C.; Xiao, Z.; Zhang, H.; Cao, M.; Liu, Y.; Jin, Z. Chemical Constituents and Chemotypes of Fresh Leaf Essential Oil of Wild Species Belonging to Sect. Camphor (Trew.) Meissn. in Southeastern China. J. Essent. Oil Bear. Plants 2019, 22, 1115–1122. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical Composition and Antioxidant Activity of the Essential Oils of Citral-Rich Chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef] [PubMed]
- de Sá Filho, J.C.F.; Nizio, D.A.d.C.; de Oliveira, A.M.S.; Alves, M.F.; de Oliveira, R.C.; Luz, J.M.Q.; Nogueira, P.C.d.L.; Arrigoni-Blank, M.d.F.; Blank, A.F. Geographic Location and Seasonality Affect the Chemical Composition of Essential Oils of Lippia alba Accessions. Ind. Crops Prod. 2022, 188, 115602. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Huang, R.; Liang, H.; Xu, C.; Wu, H. Variations in Essential Oil Yields and Compositions of Cinnamomum cassia Leaves at Different Developmental Stages. Ind. Crops Prod. 2013, 47, 92–101. [Google Scholar] [CrossRef]
- Zhang, B.; Ling, Q.; Xiao, Z.; Zhong, Q.; Zhao, R.; Jin, Z. Variations in Essential Oil Compositions and Changes in Oil Cells during Leaf Development of Citral Chemotype of Camphora officinarum Nees Ex Wall. Horticulturae 2024, 10, 597. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Liu, Q.; Zhang, J.; Xiao, C.; Jin, Z.; Liu, Y. Identification of Key Genes Controlling Monoterpene Biosynthesis of Citral-Type Cinnamomum bodinieri Levl. Based on Transcriptome and Metabolite Profiling. BMC Genom. 2024, 25, 540. [Google Scholar] [CrossRef]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.M.; Murphy, A.; Peer, W.A. Fundamentals of Plant Physiology; Oxford University Press: Oxford, UK, 2024; ISBN 978-0-19-761416-7. [Google Scholar] [CrossRef]
- Wahid, A. Physiological Implications of Metabolite Biosynthesis for Net Assimilation and Heat-Stress Tolerance of Sugarcane (Saccharum officinarum) Sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, H.; Wen, S.; Qiu, F.; Liu, X. Effects of Harvest Season and Storage Time on the Essential Oil of the Linalool Chemotype of Cinnamomum camphora. J. Essent. Oil Bear. Plants 2019, 22, 1379–1385. [Google Scholar] [CrossRef]
- Hou, C.; Cai, Y.; Yao, J.; Xie, P.; He, B.; Lian, H.; Wang, Y.; Zhong, Y.; Li, B.; Wang, M.; et al. Decoding the Chemodiversity Blueprint: Chromosome-Scale Genome Assembly Unveils Photosynthesis-Terpenoid Coordination in Cinnamomum burmanni through Genomic and miRNA Regulatory Networks. Plant Sci. 2025, 360, 112733. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, B.; Ying, B.; Zhou, L.; Zhang, R. Monoterpene Emissions Contribute to Thermotolerance in Cinnamomum camphora. Trees 2017, 31, 1759–1771. [Google Scholar] [CrossRef]
- Sikron-Persi, N.; Granot, G.; Batushansky, A.; Toubiana, D.; Grafi, G.; Fait, A. Mass Spectrometry-Based Metabolite Profiling Reveals Functional Seasonal Shifts in the Metabolome of Zygophyllum dumosum Boiss and Its Relation to Environmental Conditions. Planta 2023, 258, 10. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Luo, Q.; Tian, Z.; Zheng, T.; Xu, S.; Ma, Y.; Zou, S.; Zuo, Z. Terpenoid Composition and Antioxidant Activity of Extracts from Four Chemotypes of Cinnamomum camphora and Their Main Antioxidant Agents. Biofuel. Bioprod. Bior. 2022, 16, 510–522. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; Önder, D.; Mutlucan, M. Investigation of Phenological, Primary and Secondary Metabolites Changes during Flower Developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Chahine, S.; Pintore, G.; Chessa, M. Seasonal Variation of Essential Oil in Rosmarinus officinalis Leaves in Sardinia. Nat. Prod. Commun. 2019, 14, 1934578X19864005. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.-S.; Park, S.-H.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef]
- Stubbs, B.J.; Specht, A.; Brushett, D. The Essential Oil of Cinnamomum camphora (L.) Nees and Eberm.—Variation in Oil Composition throughout the Tree in Two Chemotypes from Eastern Australia. J. Essent. Oil Res. 2004, 16, 200–205. [Google Scholar] [CrossRef]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical Composition of Essential Oils from Cinnamomum camphora and Their Insecticidal Activity against the Stored Product Pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.; Lu, X.; Xia, G. Evaluation of Growth Adaptation of Cinnamomum camphora Seedlings in Ionic Rare Earth Tailings Environment. Sci. Rep. 2023, 13, 16910. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, P.; Wu, Z.; Liu, Q.; Zhong, Y.; Liu, T.; Zheng, Y.; Yu, F. Colchicine-Induced Chromosome Doubling in Cinnamomum camphora and Its Effect on Morphological and Terpenoid Storage Structures. Ind. Crops Prod. 2025, 233, 121338. [Google Scholar] [CrossRef]
- Zuo, Z.; Weraduwage, S.M.; Huang, T.; Sharkey, T.D. How Volatile Isoprenoids Improve Plant Thermotolerance. Trends Plant Sci. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).