Differential Biochemical Responses of Resistant and Susceptible Genotypes of Chili to Pepper Yellow Leaf Curl Thailand Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
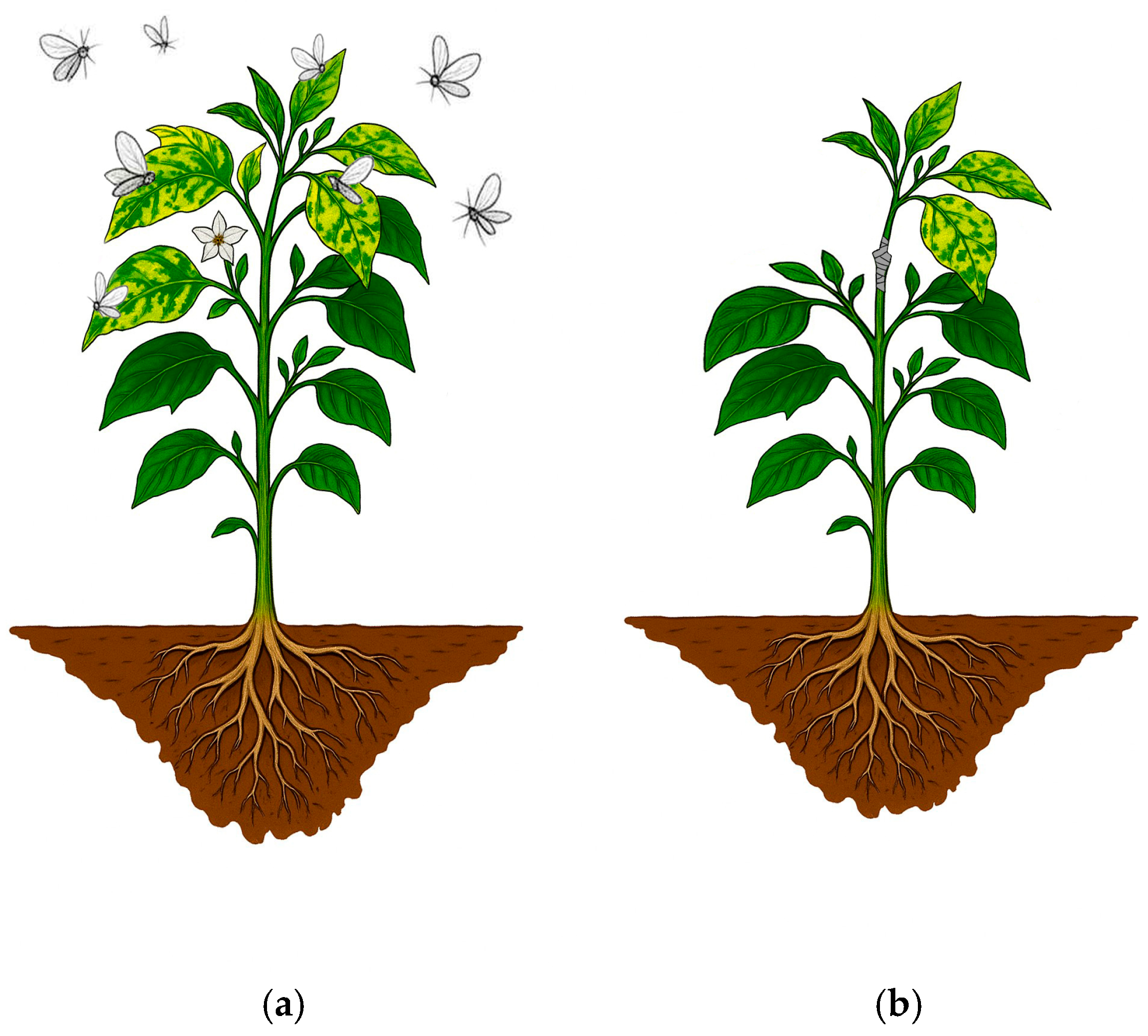
2.2. Virus Inoculation and Disease Evaluation
2.3. Determination of Total Phenolics Content (TPC)
2.4. Estimation of Peroxidase Activity (POD)
2.5. Determination of Polyphenol Oxidase Activity (PPO)
2.6. Data Analysis
3. Results
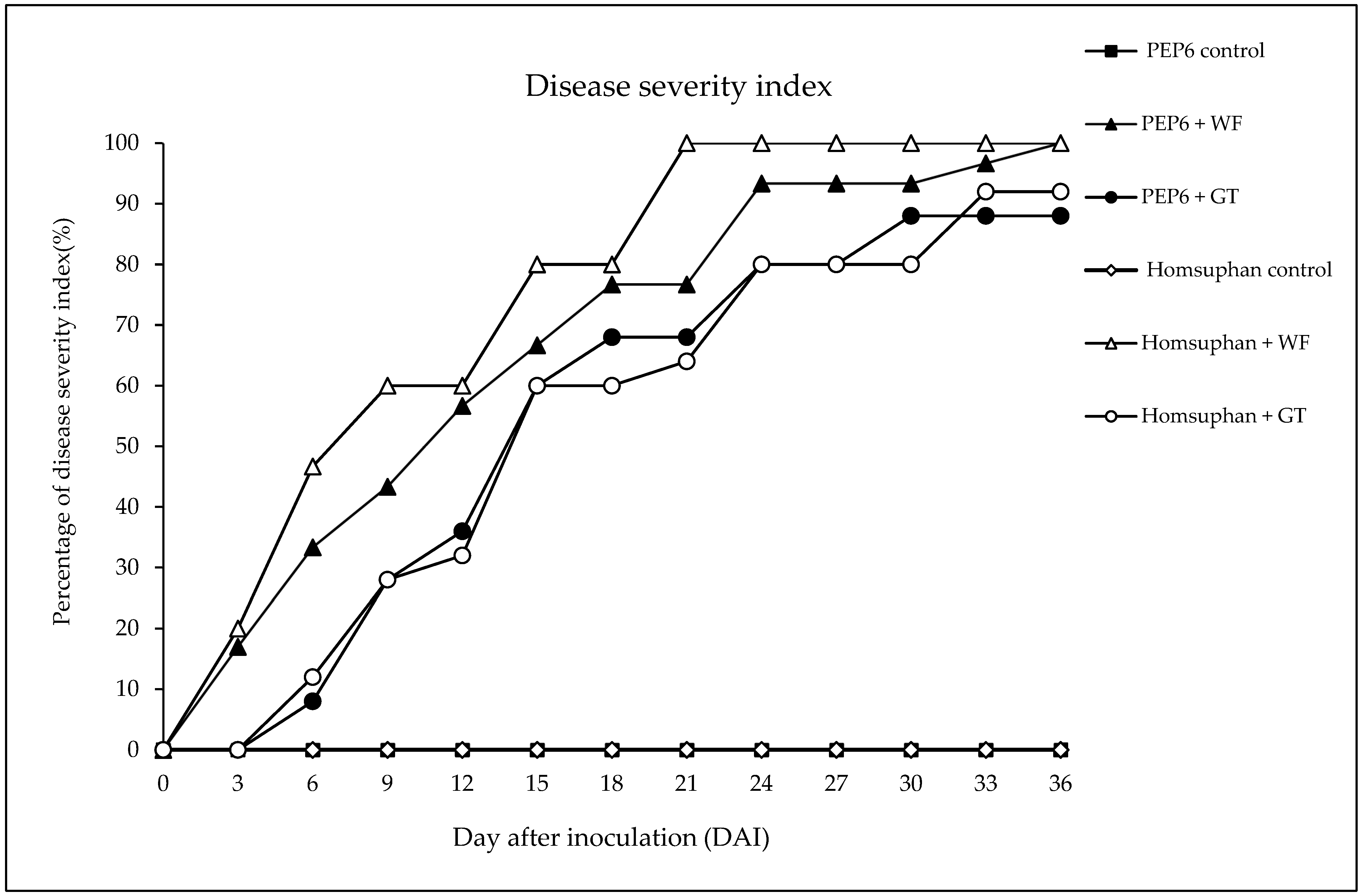
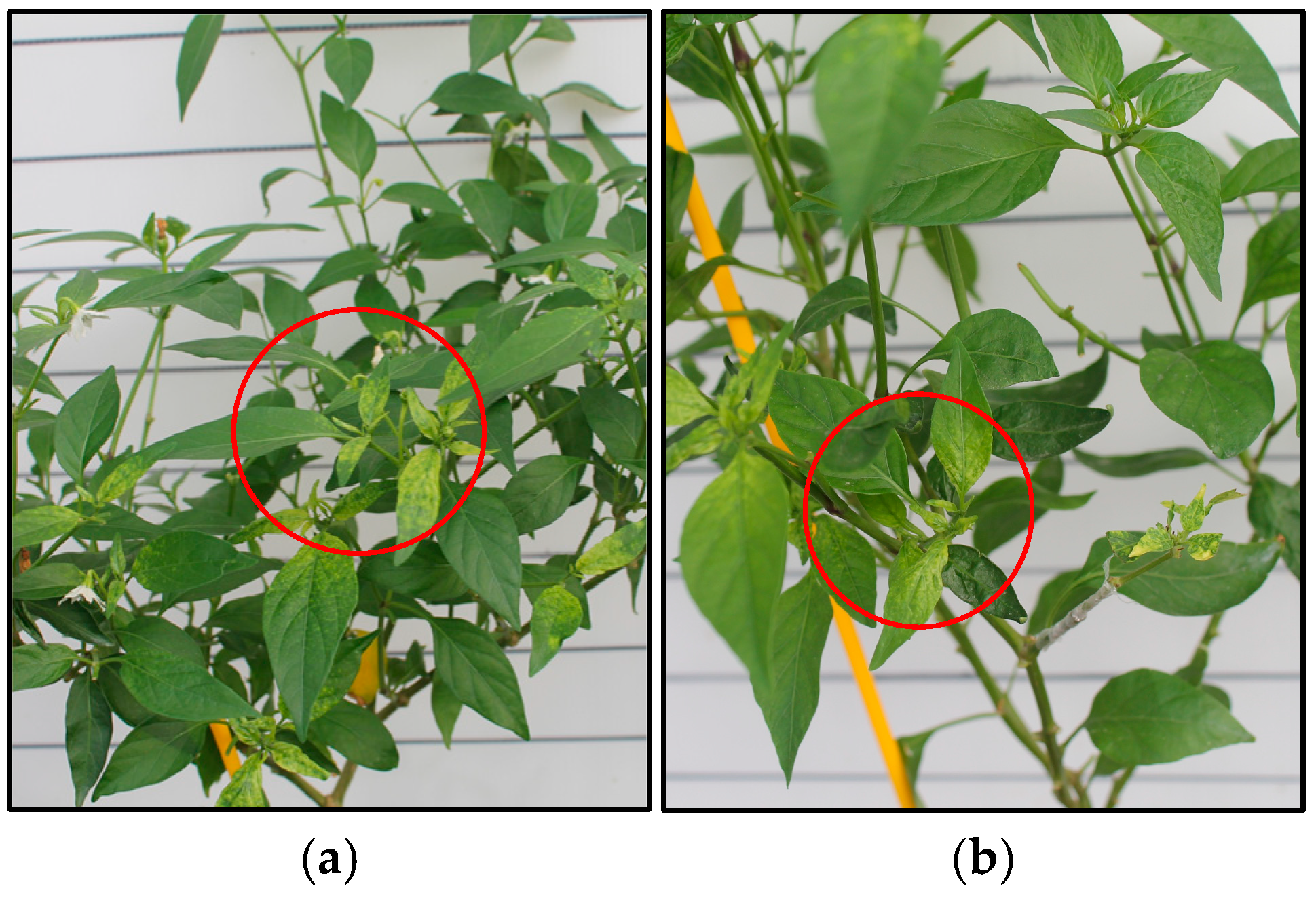
3.1. Disease Severity of PepYLCTHV
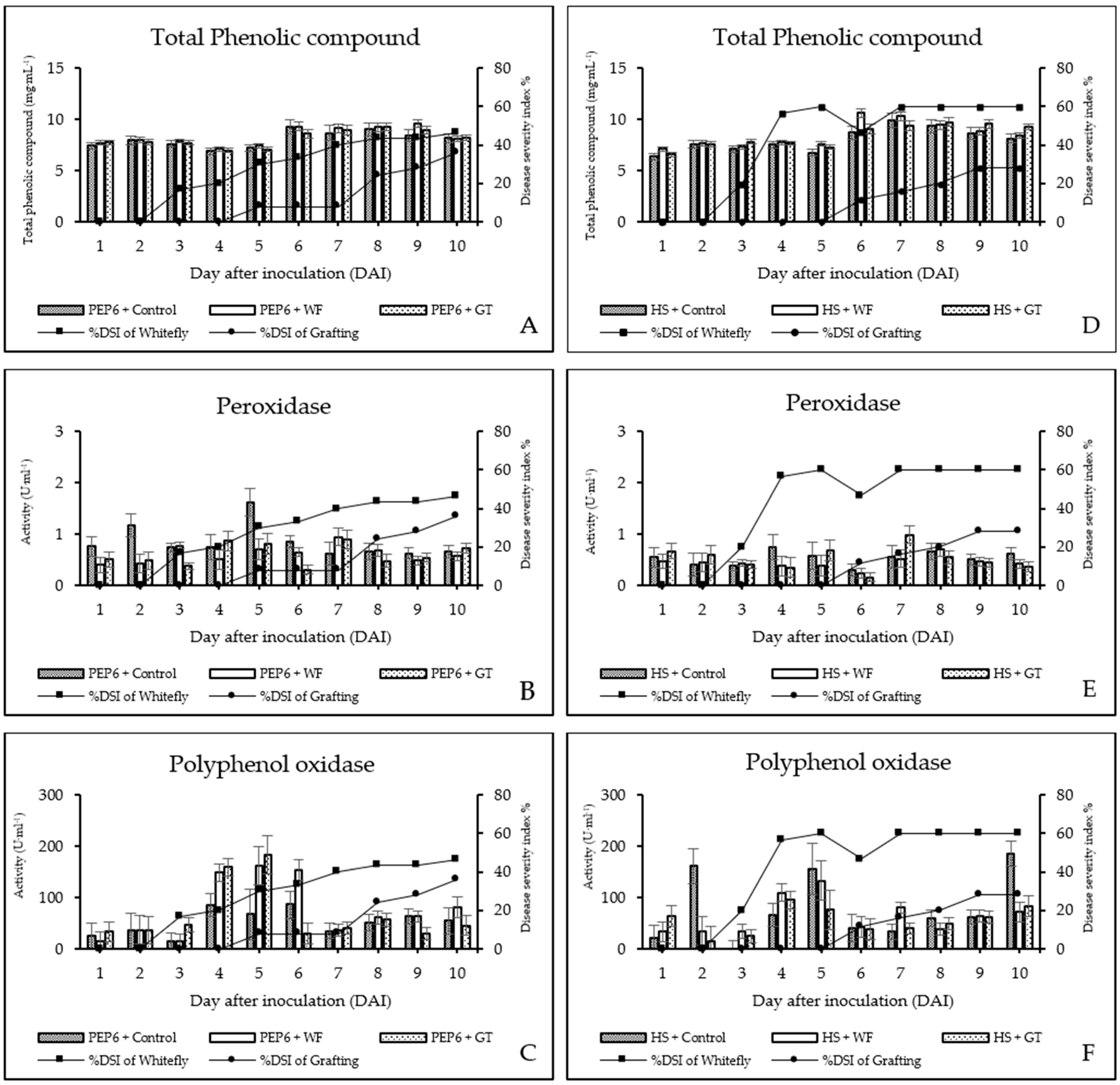
3.2. Total Phenolic Content (TPC) in Response to PepYLCTHV
3.3. Defense Enzyme Assay
3.3.1. Peroxidase (POD) Activity
3.3.2. Polyphenol Oxidase (PPO) Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization, FAO. Available online: https://www.fao.org/statistics (accessed on 4 January 2025).
- Office of Agricultural Economics. Available online: https://oae.go.th/home/article/412 (accessed on 29 June 2025).
- Wahyono, A.; Murti, R.H.; Hartono, S.; Nuringtyas, T.R.; Wijonarko, A.; Mulyantoro, M.; Firmansyah, D.; Afifuddin, A.; Purnama, I.C.G. Current Status and Complexity of Three Begomovirus Species in Pepper Plants in Lowlands and Highlands in Java Island, Indonesia. Viruses 2023, 15, 1278. [Google Scholar] [CrossRef]
- Li, Y.; Mbata, G.N.; Punnuri, S.; Simmons, A.M.; Shapiro-Ilan, D.I. Bemisia tabaci on Vegetables in the Southern United States: Incidence, Impact, and Management. Insects 2021, 12, 198. [Google Scholar] [CrossRef]
- Devendran, R.; Kumar, M.; Ghosh, D.; Yogindran, S.; Karim, M.J.; Chakraborty, S. Capsicum-infecting begomoviruses as global pathogens: Host-virus interplay, pathogenesis, and management. Trends Microbiol. 2022, 30, 170–184. [Google Scholar] [CrossRef]
- Chiemsombat, P.; Yule, S.; Srikamphung, B. Begomoviruses Associated to Pepper Yellow Leaf Curl Disease in Thailand. Open Access J. Agric. Res. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Anaya-López, J.L.; Torres-Pacheco, I.; González-Chavira, M.; Garzon-Tiznado, J.A.; Pons-Hernandez, J.L.; Guevara-González, R.G.; Muñoz-Sánchez, C.I.; Guevara-Olvera, L.; Rivera-Bustamante, R.F.; Hernández-Verdugo, S. Resistance to Geminivirus Mixed Infections in Mexican Wild Peppers. HortScience 2003, 38, 251–255. [Google Scholar] [CrossRef]
- Adluri, P.; Baldodiya, G.M.; Nath, P. Screening of Bhut Jolokia (Capsicum chinense Jacq.) Germplasm of North East India against Chilli Leaf Curl virus. Int. J. Pure Appl. Biosci. 2017, 5, 1189–1196. [Google Scholar] [CrossRef]
- Srivastava, A.; Mangal, M.; Saritha, R.K.; Kalia, P. Screening of chilli pepper (Capsicum spp.) lines for resistance to the begomoviruses causing chilli leaf curl disease in India. Crop Prot. 2017, 100, 177–185. [Google Scholar] [CrossRef]
- Sangsotkaew, Y.; Jeeatid, N.; Siri, N.; Thummabenjapone, P.; Chatchawankanphanich, O.; Phuangrat, B.; Techawongstien, S. Phenotypic responses of putative resistance chili cultivars infected by PepYLCV with viruliferous whitefly transmission. ActaHortic 2021, 1326, 181–188. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Yule, S.; Jeeatid, N.; Lin, S.-w.; Wang, Y.-w.; Lin, T.-h.; Chan, Y.-l.; Kenyon, L. A Novel Source of Resistance to Pepper yellow leaf curl Thailand virus (PepYLCThV) (Begomovirus) in Chile Pepper. HortScience 2019, 54, 2146–2149. [Google Scholar] [CrossRef]
- Kumsee, N.; Suwor, P.; Teerarak, M.; Tsai, W.-S.; Techawongstien, S.; Tarinta, T.; Kumar, S.; Jeeatid, N.; Chatchawankanphanich, O.; Kramchote, S. Improving Pepper Inbreds for Resistance to Pepper Yellow Leaf Curl Thailand Virus (PepYLCTHV) through Challenged Inoculations. Horticulturae 2024, 10, 1000. [Google Scholar] [CrossRef]
- Koeda, S.A.-O.; Onouchi, M.; Mori, N.; Pohan, N.S.; Nagano, A.A.-O.; Kesumawati, E. A recessive gene pepy-1 encoding Pelota confers resistance to begomovirus isolates of PepYLCIV and PepYLCAV in Capsicum annuum. Theor. Appl. Genet. 2021, 134, 2947–2964. [Google Scholar] [CrossRef] [PubMed]
- Koeda, S.A.-O.; Mori, N.; Horiuchi, R.; Watanabe, C.; Nagano, A.A.-O.; Shiragane, H. PepYLCIV and PepYLCAV resistance gene Pepy-2 encodes DFDGD-Class RNA-dependent RNA polymerase in Capsicum. Theor. Appl. Genet. 2022, 135, 2437–2452. [Google Scholar] [CrossRef]
- Kingkampang, H.; Teerarak, M.; Kramchote, S.; Techawongstien, S.; Suwor, P. Phenols and peroxidase activity in Pepper yellow leaf curl Thailand virus (PepYLCThV) resistant and susceptible chili (Capsicum annuum L.) genotypes. Int. J. Agric. Technol. 2020, 16, 845–854. [Google Scholar]
- Saijo, Y.; Loo, E.P.-i. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds and Their Role in Disease Resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Rai, V.; Jaiswal, N.; Kumar, S.; Singh, S.; Kumar, R.; Singh, M.; Rai, A. Response of total phenols and peroxidase activity in Chilli exposed to pepper leaf curl virus disease. Veg. Sci. 2010, 37, 78–80. [Google Scholar]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Lapidot, M.; Friedmann, M. Breeding for resistance to whitefly-transmitted geminiviruses. Ann. Appl. Biol. 2002, 140, 109–127. [Google Scholar] [CrossRef]
- Polston, J.E.; Anderson, P.K. The Emergence Of Whitefly-Transmitted Geminiviruses in Tomato in the Western Hemisphere. Plant Dis. 1997, 81, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, S.; Fawer, M.S.; Maier, G.; Takata, K.; Ritter, G. Enzyme assays for the phenolic content of natural juices. J. Agric. Food Chem. 1994, 42, 1824–1828. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Suwor, P.; Masirayanan, T.; Khingkumpungk, H.; Tsai, W.S.; Saetiew, K.; Techawongstien, S.; Kumar, S.; Kramchote, S. Chili (Capsicum annuum L.) genotypes resistant to Pepper yellow leaf curl Thailand virus (PepYLCTHV). J. Plant Prot. Res. 2021, 61, 377–383. [Google Scholar] [CrossRef]
- Cohen, R.; Pivonia, S.; Burger, Y.; Edelstein, M.; Gamliel, A.; Katan, J. Toward Integrated Management of Monosporascus Wilt of Melons in Israel. Plant Dis. 2000, 84, 496–505. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Fang, Q. Studies on Antivirus Disease Mechanism of Grafted Seedless Watermelon. Anhui Nongxueyuan Xuebao = J. Anhui Agric. Coll. 2002, 29, 336–339. [Google Scholar]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting From a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Anhui Nongxueyuan Xuebao 2021, 11, 621999. [Google Scholar] [CrossRef]
- Kappagantu, M.; Brandon, M.; Tamukong, Y.B.; Culver, J.A.-O. Rootstock-induced scion resistance against tobacco mosaic virus is associated with the induction of defence-related transcripts and graft-transmissible mRNAs. Mol. Plant Pathol. 2023, 24, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.; Ruiz, J.; Romero, L. Role of grafting in horticultural plants under stress condition. J. Food Agric. Environ. 2003, 1, 70–74. [Google Scholar]
- Jones, D.R. Plant Viruses Transmitted by Whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Czosnek, H.; Hariton-Shalev, A.; Sobol, I.; Gorovits, R.; Ghanim, M. The Incredible Journey of Begomoviruses in Their Whitefly Vector. Viruses 2017, 9, 273. [Google Scholar] [CrossRef]
- Senanayake, D.M.J.B.; Varma, A.; Mandal, B. Virus–vector Relationships, Host Range, Detection and Sequence Comparison of Chilli leaf curl virus Associated with an Epidemic of Leaf Curl Disease of Chilli in Jodhpur, India. J. Phytopathol. 2012, 160, 146–155. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X.; Hassell, R.; Thies, J. Defense Mechanisms Involved in Disease Resistance of Grafted Vegetables. HortScience 2012, 47, 164–170. [Google Scholar] [CrossRef]
- Li, D.; Li, H.-Y.; Zhang, J.-R.; Wu, Y.-J.; Zhao, S.-X.; Liu, S.-S.; Pan, L.-L. Plant resistance against whitefly and its engineering. Anhui Nongxueyuan Xuebao 2023, 14, 1232735. [Google Scholar] [CrossRef] [PubMed]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Duan, X.; Liu, F.; Bi, H.; Ai, X. Grafting Enhances Bacterial Wilt Resistance in Peppers. Agriculture 2022, 12, 583. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dunand, C. Performing the paradoxical: How plant peroxidases modify the cell wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Bhavani Shankar, A.V.; Jindal, P.C. Biochemicle resistance of grape genotypes against anthracnose. Indian J. Agric. Res. 2001, 35, 44–47. [Google Scholar]
- Li, Y.; Gu, Y.; Li, J.; Xu, M.; Wei, Q.; Wang, Y. Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front. Microbiol. 2015, 6, 883. [Google Scholar] [CrossRef]
- Chaudhary, G.; Singh, D.; Sharma, M. Effect of chemical elicitors on the differential expression pattern of PR genes in susceptible and resistant cultivars of tomato against bacterial wilt disease caused by Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2021, 116, 101689. [Google Scholar] [CrossRef]

| Score | Symptoms | Disease Incidence (%) | Disease Response |
|---|---|---|---|
| 0 | No visible symptoms on leaves | 0% | HR; highly resistant |
| 1 | Curling and clearing of upper leaves | 0–5% | R; resistant |
| 2 | Curling and clearing of leaves, and swelling of veins | 6–25% | MR; moderately resistant |
| 3 | Curling, puckering, and yellowing of leaves and swelling of veins | 26–50% | MS; moderately susceptible |
| 4 | Leaf curling, stunted plant growth, and blistering of internodes | 51–75% | S; susceptible |
| 5 | Curling and deformed small leaves, stunted plant growth without flowering | >75% | HS; highly susceptible |
| S.O.V | DF | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | ||
| Variety (V) | 1 | 4.47 ** | 0.10 ns | 1360.51 ns | 0.43 ns | 0.28 ns | 7255.43 ns | 0.47 ns | 0.31 ns | 201.16 ns | 2.21 ns | 0.30 ns | 10,451.42 * | 0.001 ns | 1.51 ** | 1572.66 ns |
| Inoculation (I) | 2 | 0.43 ** | 0.11 ns | 1907.78 ns | 0.057 ns | 0.23 ns | 10,667.86 ns | 0.23 ns | 0.12 ** | 1578.17 ns | 0.06 ns | 0.17 ns | 6413.72 * | 0.47 ns | 0.57 ns | 2454.84 ns |
| V × I | 2 | 0.22 ns | 0.07 ns | 605.56 ns | 0.012 ns | 0.41 ns | 10,998.32 ns | 0.31 ns | 0.11 * | 1113.60 ns | 0.002 ns | 0.15 ns | 948.52 ns | 0.19 ns | 0.41 ns | 17,894.02 ns |
| Error | 18 | 0.27 | 0.105 | 1917.76 | 0.33 | 0.151 | 3631.36 | 0.19 | 0.023 | 900.32 | 0.19 | 0.18 | 1456.95 | 0.21 | 0.213 | 7218.01 |
| C.V.% | 7.21 | 60.01 | 130.87 | 7.43 | 69.39 | 128.39 | 5.71 | 29.16 | 118.56 | 5.95 | 73.15 | 32.76 | 6.21 | 60.73 | 64.13 | |
| S.O.V | DF | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | ||||||||||
| TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | ||
| Variety (V) | 1 | 0.77 ns | 0.87 ** | 14,602.07 * | 4.34 ns | 0.10 ns | 1120.38 ns | 0.52 ns | 0.004 ns | 305.72 ns | 0.005 ns | 0.04 ns | 735.53 ns | 1.13 ns | 0.20 ns | 17,238.78 ** |
| Inoculation (I) | 2 | 3.53 * | 0.24 ** | 10,165.28 * | 0.75 ns | 0.26 ns | 1343.69 ns | 0.14 ns | 0.09 ns | 58.56 ns | 0.86 ns | 0.02 ns | 959.05 ns | 0.80 ns | 0.04 ns | 6109.27 ns |
| V × I | 2 | 1.35 ns | 0.08 ns | 8760.68 * | 0.43 ns | 0.17 ns | 1579.69 ns | 0.06 ns | 0.003 ns | 445.41 ns | 1.17 ns | 0.005 ns | 793.08 ns | 0.62 ns | 0.06 ns | 9122.83 * |
| Error | 18 | 0.92 | 0.041 | 2124.79 | 1.03 | 0.15 | 637.66 | 0.82 | 0.076 | 715.30 | 0.71 | 0.037 | 650.53 | 0.36 | 0.042 | 1888.01 |
| C.V.% | 10.27 | 51.92 | 69.88 | 10.76 | 49.65 | 54.82 | 9.61 | 44.46 | 50.70 | 9.26 | 37.72 | 44.80 | 7.08 | 37.26 | 52.92 | |
| Varieties | Day-1 | Day-2 | Day-3 | Day-4 | Day-5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | |
| PEP6 (V1) | 7.68 ± 0.17 a | 0.53 ± 0.09 | 24.70 ± 12.5 | 7.89 ± 0.18 | 0.63 ± 0.11 | 36.63 ± 17.20 | 7.77 ± 0.13 | 0.62 ± 0.04 | 25.75 ± 7.88 | 7.03 ± 0.14 | 0.71 ± 0.12 | 131.73 ± 109.01 a | 7.25 ± 0.14 | 0.96 ± 0.13 | 137.73 ± 87.14 |
| Homsuphan (V2) | 6.77 ± 0.17 b | 0.56 ± 0.09 | 39.59 ± 12.5 | 7.63 ± 0.18 | 0.50 ± 0.11 | 71.02 ± 17.20 | 7.50 ± 0.13 | 0.42 ± 0.04 | 20.02 ± 2.16 | 7.69 ± 0.14 | 0.45 ± 0.12 | 90.47 ± 67.74 b | 7.34 ± 0.14 | 0.50 ± 0.13 | 121.72 ± 71.13 |
| F-test | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | ns |
| Treatment | |||||||||||||||
| Control (B1) | 6.89 ± 0.26 b | 0.65 ± 0.13 | 23.61 ± 17.88 | 7.78 ± 0.29 ab | 0.80 ± 0.16 | 98.61 ± 24.60 | 7.37 ± 0.22 b | 0.56 ± 0.06 ab | 7.14 ± 12.25 | 7.24 ± 0.22 b | 0.74 ± 0.17 | 75.97 ± 15.58 b | 7.01 ± 0.23 b | 1.10 ± 0.19 | 111.85 ± 34.68 |
| Whitefly (B2) | 7.42 ± 0.17 a | 0.43 ± 0.10 | 24.05 ± 13.85 | 7.83 ± 0.18 ab | 0.44 ± 0.12 | 36.47 ± 19.06 | 7.60 ± 0.14 ab | 0.61 ± 0.05 a | 25.37 ± 9.49 | 7.43 ± 0.14 a | 0.45 ± 0.13 | 129.45 ± 12.07 a | 7.52 ± 0.14 a | 0.55 ± 0.15 | 147.58 ± 26.87 |
| Grafting (B3) | 7.17 ± 0.17 ab | 0.59 ± 0.10 | 48.78 ± 13.85 | 7.68 b± 0.18 | 0.54 ± 0.12 | 26.40 ± 19.06 | 7.76 ± 0.14 a | 0.40 ± 0.05 b | 36.15 ± 9.49 | 7.34 ± 0.14 ab | 0.61 ± 0.13 | 127.88 ± 12.07 a | 7.19 ± 0.14 b | 0.76 ± 0.15 | 129.73 ± 26.87 |
| F-test | ** | ns | ns | ** | ns | ns | ** | ** | ns | ** | ns | * | ** | ns | ns |
| Varieties | Day-6 | Day-7 | Day-8 | Day-9 | Day-10 | ||||||||||
| TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | TPC | POD | PPO | |
| PEP6 (V1) | 9.03 ± 0.30 | 0.57 ± 0.06 a | 90.09 ± 62.64 a | 9.00 ± 0.32 | 0.85 ± 0.11 | 37.77 ± 22.73 | 9.24 ± 0.29 | 0.60 ± 0.08 | 56.66 ± 40.73 | 9.13 ± 0.27 | 0.54 ± 0.06 | 52.28 ± 37.09 | 8.17 ± 0.19 | 0.65 ± 0.06 | 60.71 ± 34.84 b |
| Homsuphan (V2) | 9.67 ± 0.30 | 0.22 ± 0.06 b | 41.31 ± 13.86 b | 9.86 ± 0.32 | 0.71 ± 0.11 | 51.28 ± 36.24 | 9.57 ± 0.29 | 0.64 ± 0.08 | 49.60 ± 33.67 | 9.12 ± 0.27 | 0.47 ± 0.06 | 63.2 ± 48.04 | 8.77 ± 0.19 | 0.45 ± 0.06 | 113.72 ± 87.85 a |
| F-test | ns | ** | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ** |
| Treatment | |||||||||||||||
| Control (B1) | 8.99 ± 0.48 | 0.58 ± 0.08 a | 63.94 ± 18.82 ab | 9.29 ± 0.51 | 0.59 ± 0.16 | 34.50 ± 10.31 | 9.20 ± 0.45 | 0.67 ± 0.11 | 55.53 ± 10.92 | 8.53 ± 0.42 | 0.57 ± 0.08 | 63.06 ± 10.41 | 8.61 ± 0.3 | 0.64 ± 0.08 | 120.42 ± 17.74 |
| Whitefly (B2) | 9.90 ± 0.30 | 0.45 ± 0.06 a | 98.43 ± 14.58 a | 9.72 ± 0.32 | 0.74 ± 0.12 | 58.60 ± 7.99 | 9.38 ± 0.29 | 0.70 ± 0.09 | 50.23 ± 8.46 | 9.24 ± 0.27 | 0.48 ± 0.06 | 64.12 ± 8.07 | 8.29 ± 0.19 | 0.49 ± 0.07 | 76.83 ± 13.74 |
| Grafting (B3) | 8.85 ± 0.30 | 0.24 ± 0.06 b | 34.72 ± 14.58 b | 9.20 ± 0.32 | 0.95 ± 0.12 | 40.47 ± 7.99 | 9.51 ± 0.29 | 0.52 ± 0.09 | 53.62 ± 8.46 | 9.24 ± 0.27 | 0.49 ± 0.06 | 46.08 ± 8.07 | 8.77 ± 0.19 | 0.55 ± 0.07 | 64.40 ± 13.74 |
| F-test | ns | ** | * | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueangkhong, M.; Suwor, P.; Techawongstien, S.; Teerarak, M.; Tsai, W.-S.; Tarinta, T.; Kumar, S.; Jeeatid, N.; Chatchawankanphanich, O.; Kramchote, S. Differential Biochemical Responses of Resistant and Susceptible Genotypes of Chili to Pepper Yellow Leaf Curl Thailand Virus. Horticulturae 2025, 11, 1124. https://doi.org/10.3390/horticulturae11091124
Mueangkhong M, Suwor P, Techawongstien S, Teerarak M, Tsai W-S, Tarinta T, Kumar S, Jeeatid N, Chatchawankanphanich O, Kramchote S. Differential Biochemical Responses of Resistant and Susceptible Genotypes of Chili to Pepper Yellow Leaf Curl Thailand Virus. Horticulturae. 2025; 11(9):1124. https://doi.org/10.3390/horticulturae11091124
Chicago/Turabian StyleMueangkhong, Manthana, Patcharaporn Suwor, Suchila Techawongstien, Montinee Teerarak, Wen-Shi Tsai, Tanyarat Tarinta, Sanjeet Kumar, Nakarin Jeeatid, Orawan Chatchawankanphanich, and Somsak Kramchote. 2025. "Differential Biochemical Responses of Resistant and Susceptible Genotypes of Chili to Pepper Yellow Leaf Curl Thailand Virus" Horticulturae 11, no. 9: 1124. https://doi.org/10.3390/horticulturae11091124
APA StyleMueangkhong, M., Suwor, P., Techawongstien, S., Teerarak, M., Tsai, W.-S., Tarinta, T., Kumar, S., Jeeatid, N., Chatchawankanphanich, O., & Kramchote, S. (2025). Differential Biochemical Responses of Resistant and Susceptible Genotypes of Chili to Pepper Yellow Leaf Curl Thailand Virus. Horticulturae, 11(9), 1124. https://doi.org/10.3390/horticulturae11091124






