Abstract
Phospholipases (PLs) are key enzymes involved in membrane remodeling, structure, and signaling during plant stress responses. This study analyzed early and long-term transcriptional regulation of PL isoforms—PLD (CsPLDα,β,δ,γ,ζ), PLA (CsPLA2α,2β, CsPAT1), and PLC (CsNPC3, CsPI-PLC)—in Fortune mandarins exposed to thermal stress. Three conditions were evaluated: heat stress (37 °C, 90–95% relative humidity), cold stress (2 °C), and cold stress in fruit exposed to a prior heat conditioning treatment (3 days at 37 °C) that reduces chilling injury (CI). All genes—except CsNPC3—were rapidly upregulated by heat (4–12 h), suggesting a role as early heat sensors and their putative participation in the heat-induced chilling tolerance. Moreover, after transferring heat-conditioned fruit to cold, CsPLDζ, CsPI-PLC, and CsNPC3 showed marked expression increases, highlighting their relevance in such cross-adaptation. In non-conditioned fruit, CsPLDγ was strongly and consistently induced by cold and associated with CI development, as further confirmed by tissue-specific analysis comparing necrotic and healthy flavedo. Conversely, cold-induced CsPLDα, CsPLDζ, CsPLA2α, and CsNPC3 may contribute to protective responses via early signaling cascades; although CsPLA2α might contribute to CI only by 60 days. These findings highlight the isoform-specific and time-dependent regulation of PL genes under thermal stress and their potential role in citrus stress tolerance.
1. Introduction
Chilling injury (CI) causes important postharvest losses in citrus fruit and other chilling-sensitive horticultural crops of tropical and subtropical origin when they are stored at low non-freezing temperatures [1]. Different methodologies have been proposed to increase the tolerance of these crops to chilling [1,2,3]. Among them, high-temperature conditioning is very efficient in citrus fruit [4,5,6,7,8]. Among different citrus seasons, it has been shown that conditioning Fortune mandarins for 3 days at 37 °C and 90–95% relative humidity (RH) is so effective avoiding postharvest CI, manifested as peel pitting in the outer peel part (flavedo), that its effect lasts for very prolonged periods without being altered by preharvest factors, which highly influence the fruit postharvest CI severity [9,10,11,12,13]. Therefore, this heat conditioning treatment (HCT) has often been used to understand the mechanisms underlying CI and the long-term heat-induced chilling tolerance in citrus fruit [6,11,13].
Physiological and molecular studies postulate that cellular membranes are the first line of defense against temperature stress and are, therefore, the primary site for sensing temperature signals [14,15]. After sensing heat or cold temperature, a sophisticated stress-specific signal transduction pathway is rapidly initiated in plants [14]. Various components are involved in this process, including second messengers (reactive oxygen species (ROS), Ca2+) and regulators mediating temperature-responsive gene expression and protein homeostasis [14,16]. Cell membranes are also the primary sites of damage caused by chilling in fruits and plants [17,18,19]. Cold physiological adaptation involves changes in lipid metabolism and the regulation of membrane fluidity, which in turn influence the balance of ROS [18,19]. To maintain optimal membrane structure and function under low-temperature conditions, organisms adjust the lipid composition of their membranes, which is crucial in both the regulation of fluidity and signal transduction [20,21,22]. It is well known that the proportion of unsaturated fatty acids in membrane phospholipids helps counteract the rigidifying effects of cold stress [15]. Membrane fluidity also depends on the specific fatty acid composition, the structure of phospholipid head groups, and the length of fatty acid chains [17,18,19,23]. Studies investigating the possible relationship between membrane fluidity and the chilling tolerance of plants and horticultural crops are abundant. In citrus, contrasting results have been found about the importance of the degree of fatty acid unsaturation on the chilling tolerance achieved by a prior temperature conditioning treatment [6,11,24]. In particular, the high efficacy of the 3 days fruit conditioning treatment at 37 °C protecting Fortune mandarins from chilling is more likely associated with its effect on repressing genes hydrolyzing membrane lipids than on modifying the degree of fatty acid unsaturation [6,11]. In this regard, phospholipids also serve as a resource for intracellular signaling molecules (e.g., phosphatidic acid (PA), diacylglycerol (DAG), inositol-1,4,5-triphosphate (IP3), free fatty acids (FFAs), and lysophospholipids (LPLs)) when hydrolyzed by phospholipases (PLs) [25,26,27,28,29,30]. Lipid metabolism mediated by PLs constitutes a complex enzymatic network, where the products of one activity often serve as substrates for other downstream PL reactions. Moreover, each enzyme class has multiple members, which contribute to the temporal and spatial production of lipid intermediaries and to the influence of specific molecular species for selected actions [22,31]. Based on the specific location of the ester bond (they hydrolyze on the phospholipid molecule), PLs have been classified into different types (A, C, D) in plants [32,33]. In a simplified overview, phospholipases D (PLD), which shows catalytic activity on phosphatidylcholine and phosphaethanolamine producing choline/ethanolamine and the secondary messenger PA, initiates the response cascade. Downstream, phospholipases A2 (PLA2) degrade PA and other phospholipids, producing distinct FFAs and LPLs, depending on the substrate [34]. In plants, phospholipases C (PLCs) are divided into phosphoinositide-specific PLCs (PI-PLC) and non-specific PLCs (NPC), which hydrolyze common phospholipids, including phosphatidylcholine and phosphatidylethanolamine. PLCs can also act on the glycerol side of phospholipids and lead to the formation of DAG and IP3. In turn, the PLC/diacylglycerol kinase pathway may favor the bidirectional conversion of DAG into PA [22,35]. The branching complexity of this network, along with the regulation of metabolic flux through this pathway under various stress conditions, highlights the essential role of membrane phospholipid remodeling in plant adaptation to thermal stress tolerance [22]. Membrane integrity declines in response to severe or prolonged stresses or during senescence, which may lead to phospholipid catabolism. However, under mild non-lethal stress, plants can rapidly and transiently respond to produce signaling molecules derived from phospholipids able to trigger defense mechanisms, hence acquiring resistance to stress. Thus, whereas those transient and early increases in the activity of PLs, or in the expression of PL-encoding genes, may mediate defence, increases upon severe and prolonged stress exposure have a negative impact on lipid catabolism, membrane degradation, and oxidation that may end in tissue damage development [26,36].
The PL activities and the gene isoforms encoding these enzymes can be differentially regulated by specific physiological processes and/or stresses, which vary among tissues and trigger different plant defense responses [18,26]. Studies in fruits such as tomato, kiwifruit, peach, cucumber, and banana have primarily linked PLD and PLC enzymes to chilling-induced membrane hydrolysis [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. In contrast, PLA activity has been mostly associated with protective roles in the cold stress response [40,47,50,52]. This has been demonstrated by the repression of PLD and PLC activities and the induction of PLA activity following the application of various chemical treatments known to enhance cold tolerance [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. However, this apparent consensus is challenged by other studies reporting PLA induction in response to cold exposure, as well as its repression following treatment with chilling tolerance-inducing compounds [55,56,57,58]. In the case of citrus fruit, considerable research has been performed to study the regulation of PLs by biotic and abiotic stresses. The relevance of CsPLDγ in fruit abscission [59] and flavedo damage development caused by carbon starvation [36] and the interplay between CsPLA2α and CsPLDα and CsPLDβ under dehydration conditions leading to fruit senescence [60] have been demonstrated. It is also known that CsPAT1, CsPLA2α, and CsPLA2β are upregulated in the long term in developing peel lesions caused by the re-hydration of previously dehydrated citrus fruit [61]. CsPLA2β and most PLD-encoding genes are early and transiently induced as part of defense responses of oranges to carbon starvation [36,62]. Likewise, it is known that the regulation of some citrus PL-encoding genes depends on the stress severity and can be related not only to abiotic stresses but also to fungus infection [62,63]. Focusing on the effect of thermal stresses on PLs in citrus, the induction of PLD activity has been linked to the loss of membrane integrity in chilled lemons [64] and mandarins [65] following treatments with methyl jasmonate and γ-aminobutyric acid, respectively.
These contrasting findings suggest that the roles of PLs in stress responses—particularly under chilling conditions—remain controversial and may vary considerably among fruit species or even cultivars. This variability further underscores the complexity of PLs regulation and their functional diversity in plant stress physiology. In addition, most of these studies focus on the examination of the chilling effect and/or of treatments alleviating CI on the PL activities in different fruits that already manifest chilling-induced peel damage. Within the context of this work, no studies to date have addressed the role of different PLD-, PLA-, and PLC-encoding genes in the response of citrus fruit to chilling or heat stress. Furthermore, it remains unknown whether, and how, distinct PL isoforms are involved in the signaling processes triggered by heat conditioning that contribute to peel integrity and the subsequent reduction in postharvest CI in citrus.
We hypothesize that specific gene isoforms may serve as early indicators of chilling perception, participating in the initial response phase prior to the onset of CI by activating defense mechanisms against stress. In contrast, other isoforms may be differentially regulated during prolonged cold exposure, potentially contributing to cellular damage or representing downstream responses to injury, aimed at limiting the spread of tissue lesions. Furthermore, heat treatment at 37 °C may regulate PLs expression in such a way that certain isoforms play key roles in heat-induced cross-adaptation to chilling stress. Therefore, the aim of this study is to determine whether the different genes encoding PLD, PLA, and PLC, which have been previously related to biotic and/or abiotic stress in citrus fruit, can serve as early or long-term molecular markers and sensors of Fortune mandarin fruit responses to cold and heat stress or whether they participate in cross-adaptive mechanisms triggered by heat conditioning that enhance chilling tolerance during postharvest storage.
2. Materials and Methods
2.1. Plant Material
A total of 1200 fully mature (maturity index = 9.8) Fortune mandarins (Citrus clementina Hort. Ex Tanaka x Citrus reticulata, Blanco) were harvested on 8 March at random from 12-year-old trees grafted onto Cleopatra mandarin (Citrus unshui L) planted at 4 × 5 m and grown in commercial orchards in Burriana (Castellón, Spain) under regular horticultural practices for citrus fruit. All the harvested fruits were free of defects and of similar size. After harvest at 9 a.m., they were transported to the laboratory in less than 1 h and sorted into three groups to immediately set up the experiments. The mandarins included in the first group were used to determine the effect of heating the fruit on the transcriptional regulation of the PL-encoding genes. Fruits from the second group were taken to check the CI development kinetics as well as the changes in the time course of the CsPLs transcripts in fruit stored at a chilling temperature with respect to fruit stored at a control non-chilling temperature and to fruit stored at the chilling temperature after being conditioned at high temperature to reduce CI. Lastly, fruits from the third group were employed to compare the accumulation of all PL transcripts in chilling-induced necrotic flavedo tissues and of healthy flavedo tissues from the same fruit.
2.2. The Heat Treatment
A total of 270 mandarins were used to evaluate the effect of heating the fruit at 37 °C. This temperature was selected because it is commonly used in heat conditioning treatments for citrus fruits, and heating Fortune mandarins at 37 °C has proven to be highly effective at triggering heat-induced cross-adaptation to chilling stress. Furthermore, fruit from this cultivar develops during winter, when the average maximum and mean temperatures in the orchard throughout fruit development and prior to harvest consistently remain below 24 °C and 14 °C, respectively. Fruits in the group selected for this experiment were separated into two subgroups, containing the same number of fruits. The mandarins included in the first subgroup were stored in constant darkness at 37 °C and 90–95% RH to avoid dehydration (subgroup 1), and those in the second subgroup were stored at room temperature (20 °C), and the same RH was used as a control fruit when evaluating the effect of heating. A reference temperature of 20 °C was chosen to evaluate the effect of heat, as it neither induces thermal nor chilling stress nor accelerates senescence over the 3-day experiment duration. Both subgroups included three biological replicates of 10 fruit each per sampling time. Flavedo samples were separated carefully from the whole fruit surface of freshly harvested fruit (0 h) and of fruit stored at either 37 or 20 °C for 4, 12, 24, and 72 h after harvest and were frozen and homogenized under liquid nitrogen in a coffee mill until a very fine powder was obtained. Then, they were stored at −80 °C for further analyses. The flavedo tissue was selected in this and the following experiments because CI is just manifested in this peel part in Fortune mandarins.
2.3. Storage Temperatures and Heat-Conditioning Treatment
Examination of the effect of chilling temperature on transcriptional regulation and peel damage in fruit previously conditioned, or not, at high temperature was performed with mandarins of the second group (see Section 2.1), which contained 750 fruits. They were sorted into three subgroups containing the same number of fruits, which were exposed to different thermal conditions. The three subgroups were stored for up to 60 days in darkness and 80–85% RH. Fruits in the first subgroup were stored at 2 °C (chilling temperature), and those in the second subgroup were stored at 12 °C (control non-chilling temperature). Proper airflow inside the chamber was automatically and dynamically regulated by integrated sensors, which ensured consistent air circulation and maintained the preset temperature and humidity conditions. The chilling storage temperature of 2 °C was selected to simulate an extreme chilling stress condition that is nonetheless tolerated by Fortune mandarins during prolonged storage, provided the fruit has been previously exposed to an HCT of 3 days at 37 °C. Additionally, this temperature is required in quarantine protocols for citrus fruit export to overseas markets. The mandarins of the third subgroup were stored at the chilling temperature after being exposed to a prior HCT of 3 days at 37 °C and 90–95% RH. In this experiment, 12 °C was selected as the control non-chilling temperature to minimize senescence since the duration of the experiment was 60 days. This temperature (12 °C) is higher than the critical chilling threshold for Fortune mandarins and closely matches the average mean temperature observed in the orchard during the fruit’s developmental period. To evaluate both early- and long-term responses to chilling, samples from each subgroup were taken on days 3, 10, 20, 30, 45, and 60 after fruit harvest. In each subgroup, the fruits were separated into two lots. The first one contained 3 replicates of 20 fruits each to periodically evaluate the incidence and severity of CI. In the second lot, three biological replicates containing 10 fruits each per sampling period were included. Flavedo samples for analyses were taken from the whole fruit and prepared and frozen as described above.
2.4. Differentiation Between Healthy and Chilling-Induced Necrotic Flavedo Tissues
The third group (see Section 2.1) of fruit included 180 mandarins and was used to further understand whether the accumulation of the citrus PL transcripts was related to the chilling-induced damage or was just a fruit response to cold stress. Three biological replicates of 60 mandarins stored for 45 days at 2 °C under darkness were used to have enough flavedo tissue from the necrotic (brown pitted areas, manifested only in the flavedo) and healthy (no-damaged flavedo) zones, collected from opposite sides of the same fruit. In this experiment, the healthy and necrotic flavedo tissues from the same fruit were carefully separated with a scalpel. Both types of samples were then homogenized and frozen in liquid nitrogen and kept at −80 °C for later analyses.
2.5. CI Incidence and Severity
CI is manifested as brown-pit-like depressions in the flavedo of Fortune mandarins that extend along the whole fruit area as chilling progresses. The incidence of CI was determined by trained evaluators and calculated as the percentage of fruit manifesting the disorder in three biological replicates, containing 20 fruits each, and the results are the means of values obtained in each replicate sample ± standard error. CI severity was assessed using a rating scale based on the extent of surface pitting and browning intensity. The scale ranged from 0 (no pitting, healthy fruit) to 3 (severe pitting), with intermediate scores of 1 and 2 indicating very slight and clearly visible CI symptoms, respectively. This method enables consistent differentiation between damage levels while reducing evaluator variability. The severity index was determined by adding up the products of the number of fruits in each class multiplied by its score value within the rating scale and then dividing the resulting value by the total number of fruits evaluated. The severity index was calculated in the three replicate samples of 20 fruits, and the results are the average severity values of these replicates ± standard error. The same fruits were used for determining both the CI incidence and severity at the different evaluation dates.
2.6. Total RNA Extraction and cDNA Synthesis
The total RNA was extracted and purified from 1 g of the frozen flavedo samples by following the protocol described by [66]. Briefly, the extraction was performed with 10 mL of a 200 mM Tris HCl buffer (pH 8.0), containing 1% (w/v) PVPP40, 50 mM EDTA, 400 mM NaCl, 2% (w/v) SDS, and 1% (v/v) β-mercaptoethanol. It was mixed with 5 mL of phenol at 65 °C. The extracts were washed twice with 5 mL of chloroform/isoamilic acid (24:1, v/v), and the nucleic acids precipitated with cold ethanol. TES (10 mM Tris HCl, 5 mM EDTA, 0.1% SDS, pH 7.5) was used to dissolve the pellet, and the resulting solution was precipitated overnight at 4 °C with lithium chloride (12 M). The precipitate was washed with sodium acetate (3M, pH 6.0) and resuspended in 50 µL sterile water. To remove genomic cDNA, the RNA was treated with ribonuclease-free DNAse (Applied Biosystems) by following the manufacturer’s recommendations. The RNA quality and integrity were evaluated by agarose gel electrophoresis and GelRed staining (Biotium, Fremont, CA, USA), and the total RNA concentration was quantified using a spectrophotometer. The synthesis of the first-strand cDNA was performed from 2 μg of total RNA using the ‘Maxima H Minus First Strand cDNA Synthesis Kit’ (Thermo Scientific, Waltham, MA, USA) according to the instructions of the manufacturer.
2.7. RT-qPCR Analysis of Genes Encoding the Enzymes PLD, PLA, and PLC
Primer3Plus software (https://www.primer3plus.com/index.html (accessed on 12 June 2025)) was used to design the specific primer pairs of genes encoding PLD (CsPLDα, CsPLD β, CsPLDδ, CsPLDγ, and CsPLDζ), PLA (CsPLA2α, CsPLA2β, and CsPAT1), and PLC (CsNPC3 and CsPI-PLC). Their forward and reverse sequences are shown in Supplementary Table S1. The optimal annealing temperature and primer concentrations (forward and reverse) were optimized for each primer pair based on achieving efficiency values as close as possible to 2. The primer pair’s specificity for its target gene was assessed through melting curve analysis, where a single, well-defined melting peak was required to continue with the expression analyses. Data normalization was performed using CsACT and CsTUB genes (Supplementary Table S1), whose stability across all samples in the experimental design was confirmed using geNorm software implemented in R. The amplification of cDNA for gene expression determination was monitored for 10 s at 95 °C, followed by 5 s at the annealing temperature and 10 s at 72 °C. To that end, SYBR Green 1 Master (Roche Diagnostics, Barcelona, Spain) and a LyghCycler480 System (Roche Diagnostic) were used [66]. The ‘Relative Expression Software Tool’ (REST, https://www.gene-quantification.de/rest-paper.html (accessed on 5 June 2025)) was also used for gene expression analysis. The gene expression levels for all samples were referred to those obtained in the flavedo at fruit harvest and expressed as relative gene expression. All values shown for each sample and experiment were obtained from the mean of three biological replicates, with two analytical technical determinations.
2.8. Statistical Analysis
Statgraphics Plus 4.0 software (Manugistics, Inc., Rockville, MD, USA) was used to perform the statistical analyses. The results shown were obtained from three biological replicated samples and are expressed as the mean values ± standard error. Normality and homoscedasticity of the data were tested to ensure that a parametric analysis could be applied. An ANOVA analysis was then performed to compare, for the same storage period, the gene expression values of the HCT and the non-HCT fruit stored at 2 °C, and Tukey’s test was used to determine significant differences (p < 0.05). The same analyses were performed to compare values between the non-HCT fruit stored at 2 and 12 °C to investigate the effect of cold stress per se and values between fruit exposed at 20 and 37 °C to examine the heat effect on the transcriptional regulation of each gene.
3. Results
3.1. Effect of Heat, Chilling, and of Conditioning Fortune Mandarins for 3 Days at 37 °C to Reduce the Chilling-Induced Damage on the Regulation of the PLD-Encoding Genes
The CsPLD-encoding genes were differentially regulated by heating the fruit at 37 °C at an RH of 90–95% (Figure 1). The expression levels of CsPLDα (Figure 1A), CsPLDδ (Figure 1C), CsPLDγ (Figure 1D), and CsPLDζ (Figure 1E) were transiently upregulated at very short-term heat fruit exposition (4 h) with respect to their control fruit maintained for the same period at room temperature (20 °C) (Figure 1). The highest fold-change expression levels induced at 37 °C for any PLD were observed for CsPLDγ (Figure 1D), showing about 4.7-fold by 4 h and still 2-fold higher transcript levels in the heated than the non-heated fruit by 12 h, whereas greater expression levels were not found at 37 °C after 4 h for the other genes (Figure 1A,C,E).
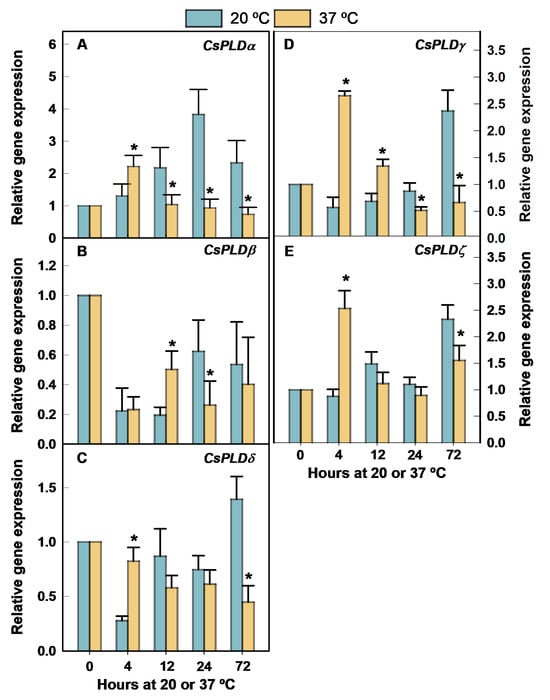
Figure 1.
Changes in the expression of the genes encoding PLD in the flavedo of Fortune mandarins heated at 37 °C (yellow bars) with respect to the control fruit maintained at 20 °C (blue bars). The standard errors of the calculated mean values are indicated by the error interval. The asterisk indicates significant differences (p < 0.05) between the heated and the control fruit for the same storage period. (A) CsPLDα, (B) CsPLDβ, (C) CsPLDδ, (D) CsPLDγ, (E) CsPLDζ.
In fact, the accumulation of all these transcripts was higher at 20 °C than at 37 °C by the end of the heat treatment (Figure 1A,C–E). CsPLDβ expression sharply declined after fruit detachment in both fruit stored at 20 °C and 37 °C, but the accumulation of this transcript was transiently higher at 37 °C than at 20 °C by 12 h (Figure 1B). Thereafter, no significant differences in the expression of this gene were found between fruit stored at 20 and 37 °C.
Most CsPLD-encoding genes were regulated at 2 °C, both in the HCT and the non-treated fruit (Figure 2). In this experiment, 12 °C was selected as the control non-chilling temperature to delay senescence because the fruits were stored for very prolonged periods (60 days) to determine both the early- and the long-term responses to chilling in the HCT and the non-treated chilled fruit. As expected, CI did not develop at 12 °C during the 60 days that the experiment lasted (Supplementary Figure S1). Likewise, no CI was detected for up to 60 days at 2 °C in the HCT fruit, despite CI developing by 20 days in the non-conditioned fruit and affecting 93% of the fruit by 60 days (Supplementary Figure S1A).
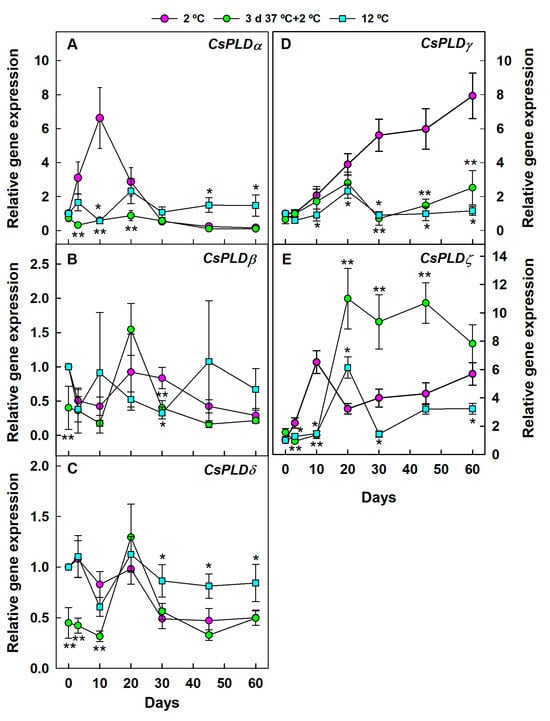
Figure 2.
Changes in the expression of the genes encoding PLD in the flavedo of Fortune mandarins stored at 2 °C (chilling temperature) (pink circle) and 12 °C (blue square) (control non-chilling temperature) and in the flavedo of fruit exposed to a heat conditioning treatment (HCT) of 3 days at 37 °C and then stored at 2 °C (green circle). The standard errors of the calculated mean values are indicated by the error interval. Significant differences (p < 0.05) between the HCT and the non-HCT fruit stored at 2 °C for the same storage period are indicated by two asterisks (**), whereas one asterisk (*) corresponds to comparisons between 2 and 12 °C in the non-HCT fruit. (A) CsPLDα, (B) CsPLDβ, (C) CsPLDδ, (D) CsPLDγ, (E) CsPLDζ.
Some PLD gene isoforms were transiently upregulated at early cold exposure in the non-HCT fruit but downregulated after prolonged cold storage, whereas the expression of CsPLDγ (Figure 2D) continuously increased at 2 °C with CI (Supplementary Figure S1) in the non-HCT fruit with respect to the control fruit stored at 12 °C (Figure 2). Among the PLD-encoding genes upregulated at early cold temperature exposure in the non-HCT fruit (10 days, before CI development), we found CsPLDα (Figure 2A) and CsPLDζ (Figure 2E), whose expressions increased by about 6-fold with respect to fruit stored at 12 °C or to the cold stored fruit exposed to the prior HCT. Such an increase was transient. In the long term (45–60 days), CsPLDα expression was significantly higher at 12 °C than at 2 °C, and there were no significant differences in its expression between the HCT and the non-HCT fruit stored at 2 °C (Figure 2A). In contrast, CsPLDζ expression was still higher at 2 °C than at 12 °C after long-term storage. The HCT markedly favored the accumulation of this transcript during cold storage (Figure 2E) while avoiding CI (Figure S1), which highlights the relevance of CsPLDζ in the heat-induced cross-adaption to chilling rather than being related to damage development. Conversely, the HCT avoided the chilling-induced upregulation of CsPLDγ (Figure 2D). No clear effect of the different thermal conditions was observed for CsPLDβ (Figure 2B). At long-term storage, the behavior of CsPLDδ (Figure 2C) was similar to that of CsPLDα (Figure 2A) because its expression was higher at 12 °C than at 2 °C in either the HCT or the non-HCT fruit. No relevant differences in the accumulation of CsPLDδ transcript were found between both temperatures at short-term storage, although their expression levels in the cold-stored fruit were repressed by the prior HCT.
3.2. The PLA-Encoding Genes Are Differentially Regulated by Heat, Chilling, and the HCT That Promotes Heat-Induced Cross-Adaptation to Chilling
Heating the fruit at 37 °C had a marked effect on the regulation of all PLA-encoding genes (Figure 3). There is a noticeable heat effect on CsPLA2β because the accumulation of this transcript was constantly higher at 37 °C than at 20 °C after harvest (Figure 3B).
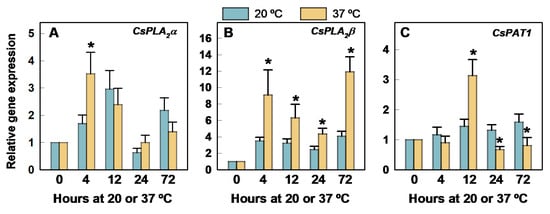
Figure 3.
Changes in the expression of the genes encoding PLA in the flavedo of Fortune mandarins heated at 37 °C (yellow bars) with respect to the control fruit maintained at 20 °C (blue bars). The standard errors of the calculated mean values are indicated by the error interval. The asterisk indicates significant differences (p < 0.05) between the heated and the control fruit for the same storage period. (A) CsPLA2α, (B) CsPLA2β, (C) CsPAT1.
Its expression sharply increased at 37 °C by 4 h. Thereafter, it decreased and then increased again after long-term exposure (3 days) at 37 °C (Figure 3B). The heat-induced upregulation of CsPLA2α (Figure 3A) and CsPAT1 (Figure 3C) was transient and occurred at 4 and 12 h, respectively. No significant differences were found in CsPLA2α at any other period, but the expression of CsPAT1 was lower at 37 °C than at 20 °C after 12 h.
The three genes encoding PLA were transiently but markedly upregulated by 20 days in fruit stored at 12 °C with respect to the HCT and the non-HCT fruit stored at 2 °C (Figure 4). Immediately after fruit detachment (3 days), the accumulation of CsPLA2α (Figure 4A) and CsPLA2β (Figure 4B) was higher at 2 °C than at 12 °C. Thereafter, expression levels of both genes were similar or higher at 12 °C than at 2 °C, with the exception of CsPLA2α transcript accumulations at 10 and 60 days, which were about 2-fold higher in the non-HCT fruit than at 12 °C. These responses to cold were repressed by the previous HCT (Figure 4A). Interestingly, the expression of this gene barely changed for up to 60 days at 2 °C in the HCT mandarins (Figure 4A). The HCT also initially repressed CsPAT1 in the cold-stored fruit (Figure 4C). Until day 30, the expression of this gene was noticeably higher in fruit kept at 12 °C than in the HCT fruit stored at 2 °C, which, in any case, developed CI (Supplementary Figure S1).
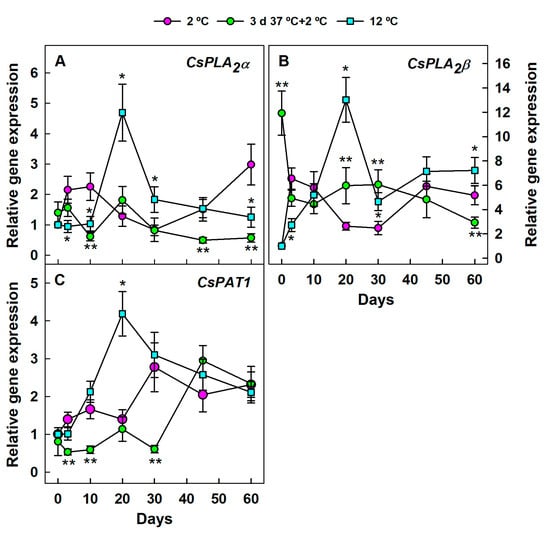
Figure 4.
Changes in the expression of the genes encoding PLA in the flavedo of Fortune mandarins stored at 2 °C (chilling temperature) (pink circle) and 12 °C (blue square) (control non-chilling temperature) and in the flavedo of fruit exposed to a heat conditioning treatment (HCT) of 3 days at 37 °C and then stored at 2 °C (green circle). The standard errors of the calculated mean values are indicated by the error interval. Significant differences (p < 0.05) between the HCT and the non-HCT fruit stored at 2 °C for the same storage period are indicated by two asterisks (**), whereas one asterisk (*) corresponds to comparisons between 2 and 12 °C in the non-HCT fruit. (A) CsPLA2α, (B) CsPLA2β, (C) CsPAT1.
3.3. Regulation of PLC-Encoding Genes by Heat, Chilling, and the HCT That Reduces Chilling Injury
The expression of CsNPC3 transiently and markedly increased by 12 h at 20 °C (Figure 5A). Heating the fruit at 37 °C had a noticeable effect on reducing this increase and the one occurring by 72 h with respect to fruit held at 20 °C (Figure 5A). In contrast, the expression of CsPI-PLC barely changed through storage at 20 °C, but this gene was markedly upregulated by 37 °C exposure with respect to 20 °C (Figure 5B).
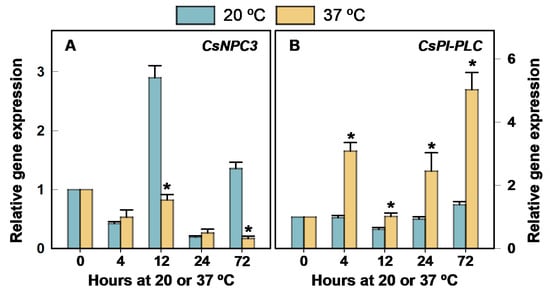
Figure 5.
Changes in the expression of the genes encoding PLC in the flavedo of Fortune mandarins heated at 37 °C (yellow bars) with respect to the control fruit maintained at 20 °C (blue bars). The standard errors of the calculated mean values are indicated by the error interval. The asterisk indicates significant differences (p < 0.05) between the heated and the control fruit for the same storage period. (A) CsNPC3, (B) CsPI-PLC.
Despite the effect of fruit heating on downregulating CsNPC3 (Figure 5A), exposing the fruit to the HCT sharply increased the transcript accumulation with respect to the non-HCT fruit stored at 2 °C by 10 and 20 days cold storage (Figure 6A). In turn, the expression of this gene was upregulated in the non-HCT fruit at early stages of cold stress with respect to the fruit stored at 12 °C. At this control non-chilling temperature, CsNPC3 expression barely changed for up to 60 days.
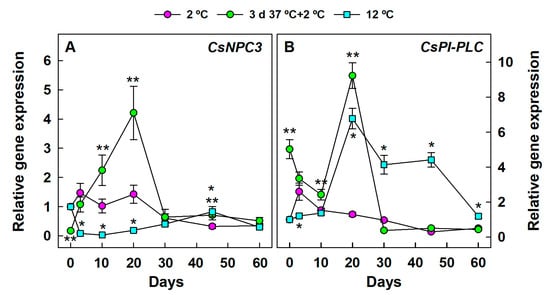
Figure 6.
Changes in the expression of the genes encoding PLC in the flavedo of Fortune mandarins stored at 2 °C (chilling temperature) (pink circle) and 12 °C (blue square) (control non-chilling temperature) and in the flavedo of fruit exposed to a heat conditioning treatment (HCT) of 3 days at 37 °C and then stored at 2 °C (green circle). The standard errors of the calculated mean values are indicated by the error interval. Significant differences (p < 0.05) between the HCT and the non-HCT fruit stored at 2 °C for the same storage period are indicated by two asterisks (**), whereas one asterisk (*) corresponds to comparisons between 2 and 12 °C in the non-HCT fruit. (A) CsNPC3, (B) CsPI-PLC.
The differences found in CsNPC3 expression when comparing the effect of the three thermal conditions selected in this study were lost or were of little relevance after 20 days (Figure 6A). The effect of cold stress on CsPI-PLC differed from that observed in CsNPC3 (Figure 6A) because the expression of this gene was lower at 2 °C than at 12 °C in the non-HCT fruit for a long storage period (Figure 6B).
In fact, the accumulation of CsPI-PLC markedly increased in mandarins stored at 12 °C. It declined after 45 days and tended to decrease for up to 60 days at 2 °C. As in the case of CsNPC3, exposing the fruit to the HCT favored the transient accumulation of CsPI-PLC at the early stages of fruit exposition to the chilling temperature (2 °C). After 20 days, no relevant differences in gene expression between the HCT and the non-HCT fruit were observed at 2 °C, and gene expressions in these samples were noticeably lower than in fruit held at 12 °C (Figure 6B).
3.4. Relative Expression Levels of PL-Encoding Genes in Necrotic and Non-Necrotic Flavedo Tissues of Chilled Fruit
To know whether the regulation of the different PL-encoding genes was related to damage caused by chilling in the flavedo or alternatively was associated with the response of the fruit to cope with chilling stress, the expression levels of all genes were compared in flavedo tissues showing necrotic lesions and in flavedo tissues taken from the healthy part of the same fruit stored at 2 °C. All transcripts were detectable in both the healthy and the necrotic tissues (Figure 7). Only the expression of CsPLDγ was positively regulated in the chilling-induced necrotic tissues with respect to the healthy area of the same fruit.
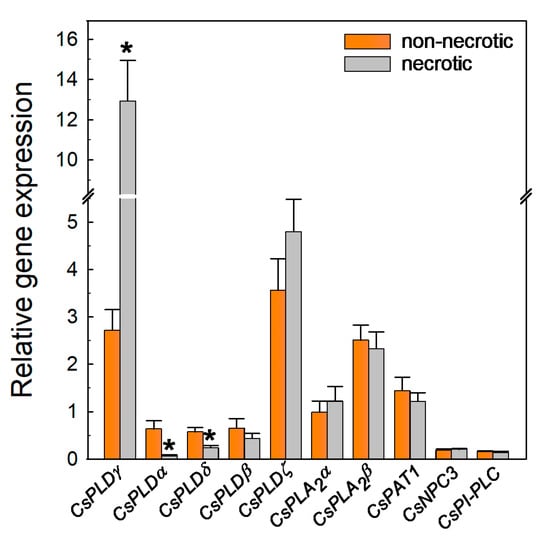
Figure 7.
Relative expression levels of genes encoding PLD, PLA, and PLC in the non-necrotic (orange bars) and necrotic (gray bars) tissues of mandarins stored for 45 days at 2 °C with respect to freshly harvested fruit. The standard errors of the calculated mean values are indicated by the error interval. Significant differences (p < 0.05) between the non-necrotic and necrotic tissues are indicated by the asterisks (*).
The accumulation of this transcript was at least 4-fold higher in the necrotic areas than in the non-necrotic healthy flavedo (Figure 7). Conversely, CsPLDα and CsPLDδ were less expressed in the necrotic tissues (Figure 7). The results also revealed that there were no significant differences in the expression of any of the three PLA and the two PLC-encoding genes, or that of CsPLDβ and CsPLDζ, between the chilling-induced necrotic and the healthy tissues of the same cold-stored fruit (Figure 7).
4. Discussion
The present work provides the first comprehensive characterization of the heat-induced PL transcriptional regulatory network in fruits. It shows that heat differentially regulates a relevant set of genes encoding PLD (Figure 1), PLA (Figure 3), and PLC (Figure 5) in citrus fruit. The CsNPC3 gene was downregulated after heating Fortune mandarins, but CsPI-PLC and all the CsPLDs and CsPLAs isoforms were transiently upregulated after very short-term fruit exposure to 37 °C. In most cases (CsPLDα, CsPLDδ, CsPLDγ, CsPLDζ, CsPLA2α, CsPLA2β, and CsPI-PLC), such an increase was induced in just 4 h, which preceded that of CsPAT1 (12 h). Therefore, these genes are part of the early response of citrus fruit to heat and good sensors of this stress. The PL type and isoform-dependent response to the heat treatment might be partially explained by their differences in Ca2+ requirement for PLD activity, catalytic and regulatory properties, specific preferences for phospholipid substrates, cofactor requirements, subcellular distribution, structure, substrate selectivity, and/or reaction conditions [26,67]. In addition, this general early upregulation of CsPLDs suggests their role in phospholipid remodeling and membrane fluidity for the heat thermotolerance of citrus fruit but also their participation in the production of different lipid derivatives and signaling messengers aiming to protect the fruit against heat stress [26,32,68,69]. Among these, PA, DAG, and FFAs are particularly noteworthy, with FFAs acting upstream in the oxylipin pathway leading to jasmonate synthesis (Hou et al., 2016, [22]). This agrees with previous findings indicating the involvement of the PLD enzyme in heat tolerance in grape berries [70]. Among these early heat sensor genes, only the accumulation of CsPLA2β and CsPI-PLC transcripts increased again after long-term storage at 37 °C, which suggests that they might be further good molecular markers of prolonged heat exposure. Still, these 72 h of heat treatment did not provoke heat damage but increased the fruit’s tolerance to the following chilling exposure. These results, together with the high efficacy of the HCT reducing CI in Fortune mandarins, suggest the participation of the heat-induced CsPLs in the activation of downstream specific-defence signaling pathways involved in the citrus fruit adaptation to heat and to subsequent chilling stress in the HCT fruit. The expression of CsPLDs tended to increase after 4 h at 20 °C. Such increases were repressed by 12, 24, or 72 h at 37 °C, depending on the PLD isoform (Figure 1). In this regard, it is relevant to mention that abscisic acid (ABA) can act upstream of PLDs in stressed citrus fruit regulating stress signals [60], that this hormone decreases after 12 h at 37 °C in Fortune mandarin [12], and that PLD and PA are mediators of the ABA signal in plant responses to abiotic stresses, such as drought and salinity [4,30]. Therefore, an interesting possibility is that ABA can mediate the long-term heat effect on CsPLDs in citrus fruit. This hypothetical connection warrants further research.
PLD activity often increases with CI initiation in fruit [39,40,42,46,49,50,51,52,56], including lemons [64] and mandarins [65]. Studies on the effect of HCTs that reduce CI on PLD are scarce, although it has been demonstrated that heat conditioning reduces the cold-induced rises in PLD activity in litchi [48] and both the activity and the expression of a PLD-encoding gene in cucumber [45], tomato [43], and pineapple [47]. These results are not completely in line with our analyses showing that chilling may positively or negatively regulate distinct citrus CsPLDs, which agrees with findings in bell pepper fruit [55], and that such regulation varies with the chilling duration and is differentially altered by an HCT that drastically reduces CI. Specifically, it is worth highlighting the marked effect of HCT on CsPLDζ. This gene is required for auxin response mediated by PA in Arabidopsis [26,71]. However, CsPLDζ was upregulated in the HCT Fortune mandarin stored at 2 °C (Figure 2E) with respect to the non-HCT fruits, whereas the auxin levels were reduced by the treatment after long-term storage [12]. Moreover, during fruit heating at 37 °C, auxin only increased by 72 h [12] when the expression of the gene was repressed (Figure 1E), further suggesting that the regulation of CsPLDζ by HCT in Fortune mandarins is not involved in the auxin response. Other roles for this gene in ROS signaling and phosphate starvation have been proposed in plants [26,72], although no evidence for its involvement in such processes can be deduced from the reports in citrus fruit. On the other hand, CsPLDζ was also upregulated by 60 days at 2 °C with respect to 12 °C in the non-HCT fruit (Figure 2E), although its expression did not differ significantly between the necrotic and the non-necrotic tissues of the same fruit at 2 °C by 45 days (Figure 7). This finding supports the relevance of this gene in the long-term heat-induced chilling tolerance in citrus fruit. Therefore, the global results indicate that the CsPLDζ cold-induced accumulation might be a defense mechanism in citrus fruit aiming to delay CI propagation rather than the cause of peel damage. Conversely, the HCT had a marked effect on repressing the continuous chilling-induced CsPLDγ rise (Figure 2D), which suggests the involvement of this gene in CI damage propagation. This result contrasts with the upregulation of a PLDγ by a hexanal treatment that reduced chilling-induced browning in nectarines [54]. Similarly, although oxidative stress is involved in CI development of citrus fruit [11], PLDγ conferred increased resistance to aluminum stress in Arabidopsis and reduced oxidative damage and lipid peroxidation [73], further providing controversial roles for this gene across species. Our results indicate, however, that CsPLDγ expression was much higher in the chilling-induced necrotic than in the non-necrotic tissues of the same fruit (Figure 7). Therefore, the repression of this gene could contribute to preventing the peel damage development in the HCT Fortune fruit, hence reducing CI. A plausible explanation is that this gene would also contribute to PA production, which, at early stress, may participate in the generation of stress defense response, including ROS signaling, but under severe, prolonged stress, an excess of PA would have a negative impact on promoting increasing lipid catabolism and membrane oxidative damage [30,73,74]. The CsPLDα (Figure 2A) and CsPLDδ (Figure 2C) genes were initially repressed at 2 °C by the HCT (before CI developed in the non-HCT fruit), and their expression was similar in the HCT and the non-HCT fruit after long-term cold storage. In addition, their expression was higher in the non-necrotic than in the chilling-induced necrotic tissues of the same fruit stored under cold temperature and in fruit stored at 12 °C than at 2 °C for prolonged periods (Figure 7). It is noteworthy that findings in cold-stressed Arabidopsis indicate that PLDα1 was involved in the hydrolysis of structural phospholipids and ROS production [20,75,76] and that oxidative stress participates in CI development in citrus fruit [11]. In addition, the repression of PLDα was related to the efficacy of a heat and a glycine betaine treatment that reduced CI in tomato [43] and peach [49], respectively. It is also noteworthy that CaPLDα4 was related to the degradation of membrane lipids and that an excess in PA accumulation could further generate ROS, contributing to membrane damage, hydroperoxides, and free radicals in bell peppers [42,77], but PLD-encoding genes were both up- and downregulated by chilling stress in this fruit crop [55]. In turn, PLDδ and PA interact with protein G to transduce hydrogen peroxide signals [30,36,43]. It is, therefore, reasonable to infer that these genes would not be involved in CI development in citrus fruit but could be rather related to triggering early and transitory downstream signals aiming to prevent peel damage in chilled citrus fruits. In this regard, the lack of short-term CsPLDα and CsPLDδ induction at 2 °C in the HCT Fortune mandarins might be due to other protective mechanisms initiated during the prior 3 days HCT, which would reduce the requirement of the chilling-induced CsPLDα and CsPLDδ accumulation to cope with chilling stress in the HCT fruit. Last, a comparison of the three temperature regimes on CsPLDβ, which has not been localized at a plasma membrane [67], suggests that this gene is not relevant in the susceptibility of citrus fruit to chilling (Figure 2B), in contrast to melon fruit, where the expression of a PLDβ gene was related to the efficacy of n-butanol decreasing CI [53]. On the other hand, the results indicate that CsPLDα and CsPLDζ are involved in the early chilling defense responses of non-HCT fruit. Their activity may lead to the formation of PA and DAG, as well as alterations in FFAs. Notably, changes in FFAs may also contribute to downstream defense mechanisms, such as the synthesis of methyl jasmonate—a compound known to increase in response to cold stress in Fortune mandarin [12].
The putative involvement of PLA in fruit CI development has been less studied, and contrasting results have been found among different fruits [40,52,55,56,57]. Inconsistent differences among PLA isoforms’ regulation were found here between 2 and 12 °C in the non-HCT mandarins (Figure 4). Indeed, while CsPLA2α increased at short- (up to 10 days) and long-term storage (60 days) at 2 °C, as compared to 12 °C, CsPLA2β transcript levels were higher in the chilled fruit only at very early storage periods (3 days), and CsPAT1 was not induced by cold at any time during the whole experiment (Figure 4). These results indicate the lower relevance of PLA-encoding genes as compared to CsPLDγ, whose expression markedly and continuously increased with CI in the response of citrus fruit to chilling. Nevertheless, the elevated expression of PLA-encoding genes during the early stages of cold exposure in non-HCT fruit suggests their potential involvement in the chilling defense response. This may occur through the generation of LPLs and changes in the pool of FFA composition. This is in concordance with the fact that there were no significant differences in the expression of any PLA-encoding genes between the healthy and necrotic tissues taken from fruit stored for 45 days at 2 °C (Figure 7). The overall results obtained when comparing CsPLA transcriptional changes under the three thermal regimes suggest a link between the CsPLA2α isoform and chilling-induced damage after very prolonged cold storage (60 days), as the expression of this gene markedly increased at 2 °C with respect to 12 °C in the non-HCT fruit by 60 days but not in the HCT fruit. These results align with those showing that PLA activity increases with CI in bananas [56] and cucumbers [57]. Interestingly, the expression of the three CsPLA-encoding genes transiently, but markedly, increased by 20 days at 12 °C. Such an increase might be due to starvation caused by fruit detachment that would occur faster at 12 °C than at 2 °C, although only CsPLA2β has been related to this stress in citrus fruit [36]. The three genes were also upregulated at a non-chilling temperature (20 °C) in Navelate oranges during the re-hydration of previously dehydrated fruit [61]. However, this upregulation was linked to non-chilling peel pitting development, while Fortune mandarins did not develop damage at 12 °C. As far as we know, there is only one work examining the effect of a heat pretreatment on CI and PLA regulation in fruit [47], and it suggested that heat preserves membrane function by inducing three PLA2-encoding genes (Aco0061, Aco1304, and Aco9892) in cold-stored pineapples. The results found here show that the HCT had no relevant effect on increasing the expression of any selected CsPLA encoding gene in the cold-stored citrus fruits (Figure 4). However, the three CsPLAs were upregulated at some point during the HCT (Figure 3) and, therefore, we cannot discard their participation in the heat-induced chilling tolerance in citrus fruit.
The results further suggested, for the first time, that the genes encoding PI-PLC and NPC (Figure 6) participate in the heat cross-adaption to chilling in fruits. CsNPC3 expression was downregulated during the HCT, while CsPI-PLC expression increased notably under the same conditions (Figure 5). In contrast, both CsPLC isoforms exhibited a sharp and transient upregulation in cold-stressed HCT fruit at 10 and 20 days, with only CsNPC3 showing increased expression at a later stage (45 days) (Figure 6). These results are consistent with studies in transgenic Arabidopsis plants, which demonstrated that among the various members of the NPC family, only NPC1—responsible for hydrolyzing phosphatidylcholine into DAG—was directly involved in heat tolerance [78]. Additionally, findings in model plants highlight the importance of PI-PLC family genes in heat stress responses, as their activation leads to increased levels of intracellular IP3 and Ca2+, both of which play crucial roles in signal transduction [79,80]. Therefore, these genes are not likely involved in the chilling-induced peel damage in citrus fruit, which is further supported by the similar expression levels found between the healthy and the necrotic tissues of the same chilled fruit (Figure 7). The transient activation (0–20 days) of CsNPC3 at 2 °C with respect to 12 °C was much lower in the non-HCT than in the HCT fruit and, therefore, it should be an early citrus fruit protective response to cope with chilling stress rather than contributing to damage development. Therefore, although PLC activation has often been related to chilling-induced membrane hydrolysis and damage in fruits [37,44,50,52], the overall results found herein better agree with the role of PLCs acting as catalyzers of the production of specific signaling messengers (DAG and IP3) in the defence of plants against stresses [27]. This also fits with findings showing that nitric oxide reduces CI and increases PI-PLC activity in kiwifruit [41] and that chemical treatments that reduce CI may both down- and upregulate PLC-encoding genes in peach [81].
The overall results demonstrate that thermal stress significantly impacts phospholipid metabolism, encouraging further research to characterize global changes in metabolite accumulation through a lipidomic approach. The findings suggest potential applications to enhance the chilling tolerance of citrus fruit, thereby extending the storability of chilling-sensitive cultivars and optimizing the HCT. Special attention should be paid to industrial treatments increasing DAG contents since HCT enhances the expression of CsPLDζ, CsNPC3, and CsPI-PLC in chilled fruit. These treatments may allow for a reduction in the duration or temperature required for HCT to achieve very long-term chilling tolerance—an important consideration, as such reductions can otherwise compromise treatment efficacy [9]. Similarly, the role of PA and treatments that promote its synthesis during early chilling stress should be investigated, along with inhibitors of CsPLDγ, which appears to be a key gene involved in CI development. Ultimately, understanding the involvement of key PL genes in thermal stress responses may contribute to the development of new genetic resources through biotechnological approaches aimed at improving citrus fruit tolerance to chilling, both in pre- and postharvest conditions.
5. Conclusions
Heat stress significantly affects cell membranes in Fortune mandarins by altering phospholipid metabolism, leading to early and transient upregulation of genes encoding PLD, PLA, and PLC enzymes. These transcriptional changes may result in modifications to membrane lipid composition and the generation of second messengers and lipid derivatives, including PA, DAG, FFAs, and IP3, produced by the five CsPLD isoforms and the CsPI-PLC analyzed in this study. Additionally, LPLs and FFAs are likely generated through the activity of CsPLA2α, CsPLA2β, and CsPAT1. Notably, variations in FFA composition may also support downstream stress responses, such as the biosynthesis of methyl jasmonate via PLA2-derived oxylipins, which increase under cold stress in Fortune mandarins. These molecular changes, triggered by a non-lethal HCT (3 days at 37 °C, 90–95% RH), may enhance fruit resilience to subsequent cold stress, contributing to heat-induced cross-adaptation to CI. The increased expression of CsPLDζ, CsNPC3, and CsPI-PLC in chilled fruit previously subjected to HCT, along with the potential bidirectional conversion of DAG into PA, further underscores the importance of these genes and the accumulation of these metabolites in conferring chilling tolerance. Moreover, the early (3–10 days) responses of CsPLDα, CsPLDζ, CsPLA2α, and CsNPC3 to chilling in non-conditioned fruit—potentially involving PA, DAG, LPLs, and changes in FFAs—suggest their role in initiating protective signaling cascades. Finally, the induction of CsPLDγ in non-conditioned fruit and the observed differences in transcript levels between healthy and necrotic tissues highlight the relevance of this gene in CI development. Its contribution to PA production may be beneficial during early stress responses, including ROS signaling, whereas under prolonged stress exposure, it may exacerbate lipid catabolism and oxidative membrane damage.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11091126/s1, Figure S1: Percentage (S1A) and CI index (S1B) of Fortune mandarins showing CI (rating scale from 0 to 3) stored at a chilling (2 °C) and a non-chilling temperature (12 °C) and in fruit stored at 2 °C after being exposed to a heat conditioning treatment (HCT) of 3 days at 37 °C. The asterisk indicates, for the same storage period, significantly (p < 0.05) higher levels in non-HCT fruit stored at 2 °C as compared to the HCT fruit kept at the same temperature or the fruit stored immediately after fruit harvesting at the control non-chilling temperature (12 °C). Table S1: Primers used for RT-qPCR analyses.
Author Contributions
Conceptualization, M.T.L. and F.A.; methodology, P.R., R.S. and M.T.L.; formal analysis, P.R., R.S. and M.T.L.; investigation, M.T.L., F.A., P.R. and R.S.; resources, M.T.L. and F.A.; writing—original draft, M.T.L.; writing—review and editing, M.T.L., P.R., F.A. and R.S.; supervision, M.T.L.; project administration, M.T.L.; funding acquisition, M.T.L. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Projects AGL2009-11969 (MCIU/AEI/FEDER) and 2020AEPP135 (MCIU/CSIC). The award of the Spanish government MCIN/AEI to the IATA-CSIC as Center of Excellence Accreditation Severo Ochoa (CEX2021-001189-S/MCIN/AEI/10.13039/501100011033) is also acknowledged.
Data Availability Statement
Data are contained within the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABA | Abscisic acid |
| CI | Chilling injury |
| DAG | Diacylglycerol |
| FFA | Free fatty acid |
| HCT | Heat conditioning treatment |
| IP3 | Inositol-1,4,5-triphosphate |
| LPL | Lysophospholipid |
| NPC | Non-specific PLC |
| PA | Phosphatidic acid |
| PI-PLC | Phosphoinositide-specific PLC |
| PL | Phospholipase |
| PLA | Phospholipase A |
| PLC | Phospholipase C |
| PLD | Phospholipase D |
References
- Sevillano, L.; Sanchez-Ballest, M.T.; Romojaro, F.; Flores, F.B. Physiological, Hormonal and Molecular Mechanisms Regulating Chilling Injury in Horticultural Species. Postharvest Technologies Applied to Reduce Its Impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Mulas, M.; Schirra, M. The Effect of Heat Conditioning Treatments on the Postharvest Quality of Horticultural Crops. Stewart Postharvest Rev. 2007, 3, 1–6. [Google Scholar] [CrossRef]
- Valenzuela, J.L.; Manzano, S.; Palma, F.; Carvajal, F.; Garrido, D.; Jamilena, M. Oxidative Stress Associated with Chilling Injury in Immature Fruit: Postharvest Technological and Biotechnological Solutions. Int. J. Mol. Sci. 2017, 18, 1467. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tellez, M.A.; Lafuente, M.T. Effect of High Temperature Conditioning on Ethylene, Phenylalanine Ammonia-Lyase, Peroxidase and Polyphenol Oxidase Activities in Flavedo of Chilled ‘Fortune’ Mandarin Fruit. J. Plant Physiol. 1997, 150, 674–678. [Google Scholar] [CrossRef]
- Moreno, A.S.; Margarit, E.; Morales, L.; Montecchiarini, M.; Bello, F.; Vázquez, D.; Tripodi, K.E.J.; Podestá, F.E. Immediate- and Long-Term Proteomic Responses of Epicarp from Two Heat Conditioned Tangor Cultivars Stored at Low Temperature Differing in Their Susceptibility to Infection. Postharvest Biol. Technol. 2020, 161, 111091. [Google Scholar] [CrossRef]
- Mulas, M.; Lafuente, M.T.; Zacarias, L. Postharvest Temperature Conditioning and Chilling Effects on Flavedo Lipid Composition of ‘Fortune’ Mandarin. Proc. Int. Soc. Citricult. 1997, 2, 1132–1135. [Google Scholar]
- Rodov, V.; Ben-Yehoshua, S.; Albagli, R.; Fang, D.Q. Reducing Chilling Injury and Decay of Stored Citrus Fruit by Hot Water Dips. Postharvest Biol. Technol. 1995, 5, 119–127. [Google Scholar] [CrossRef]
- Schirra, M.; Mulas, M.; Fadda, A.; Cauli, E. Cold Quarantine Responses of Blood Oranges to Postharvest Hot Water and Hot Air Treatments. Postharvest Biol. Technol. 2004, 31, 191–200. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Zacarias, L.; Perez-Amador, M.A.; Carbonell, J.; Lafuente, M.T. Polyamine Content and Chilling Susceptibility Are Affected by Seasonal Changes in Temperature and by Conditioning Temperature in Cold-Stored ‘Fortune’ Mandarin Fruit. Physiol. Plant 2000, 108, 140–146. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Martínez-Téllez, M.A.; Zacarías, L. Abscisic Acid in the Response of ‘Fortune’ Mandarins to Chilling. Effect of Maturity and High-Temperature Conditioning. J. Sci. Food Agric. 1997, 73, 494–502. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Establés-Ortíz, B.; González-Candelas, L. Insights into the Molecular Events That Regulate Heat-Induced Chilling Tolerance in Citrus Fruits. Front. Plant Sci. 2017, 8, 262778. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Romero, P. Hormone Profiling and Heat-Induced Tolerance to Cold Stress in Citrus Fruit. Postharvest Biol. Technol. 2022, 194, 112088. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Lluch, Y.; Gosalbes, M.J.; Zacarias, L.; Granell, A.; Lafuente, M.T. A Survey of Genes Differentially Expressed during Long-Term Heat-Induced Chilling Tolerance in Citrus Fruit. Planta 2003, 218, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, S. Surviving and Thriving: How Plants Perceive and Respond to Temperature Stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yao, L.; Wang, X.; Zhang, Y.; Zhang, G.; Li, X. Mechanisms for Cell Survival during Abiotic Stress: Focusing on Plasma Membrane. Stress Biol. 2025, 5, 1. [Google Scholar] [CrossRef]
- Wei, X.; Liu, S.; Sun, C.; Xie, G.; Wang, L. Convergence and Divergence: Signal Perception and Transduction Mechanisms of Cold Stress in Arabidopsis and Rice. Plants 2021, 10, 1864. [Google Scholar] [CrossRef]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling Injury. A Review of Possible Mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Liang, S.M.; Kuang, J.F.; Ji, S.J.; Chen, Q.F.; Deng, W.; Min, T.; Shan, W.; Chen, J.Y.; Lu, W.J. The Membrane Lipid Metabolism in Horticultural Products Suffering Chilling Injury. Food Qual. Saf. 2020, 4, 9–14. [Google Scholar] [CrossRef]
- Wu, G.; Baumeister, R.; Heimbucher, T. Molecular Mechanisms of Lipid-Based Metabolic Adaptation Strategies in Response to Cold. Cells 2023, 12, 1353. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses: Role of phospholipase dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Murphy, A.; Schulz, B.; Peer, W. The Plant Plasma Membrane, 1st ed.; Springer: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid Signalling in Plant Responses to Abiotic Stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and Functions of Membrane Lipid Remodeling in Plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef]
- Schirra, M.; Cohen, E. Long-Term Storage of ‘Olinda’ Oranges under Chilling and Intermittent Warming Temperatures. Postharvest Biol. Technol. 1999, 16, 63–69. [Google Scholar] [CrossRef]
- Alferez, F. The Role of Lipid Metabolism and Signalling during Postharvest Treatment and Storage of Horticultural Crops. Stewart Postharvest Rev. 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant Phospholipases D and C and Their Diverse Functions in Stress Responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Meijer, H.J.G.; Munnik, T. Phospholipid-Based Signaling in Plants. Annu. Rev. Plant Biol. 2003, 54, 265–306. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.B. Phospholipid-Derived Signaling Mediated by Phospholipase A in Plants. Trends Plant Sci. 2004, 9, 229–235. [Google Scholar] [CrossRef]
- Wang, X. Plant Phospholipases. Annu. Rev. Plant Biol. 2001, 52, 211–231. [Google Scholar] [CrossRef]
- Li, J.; Wang, X. Phospholipase D and Phosphatidic Acid in Plant Immunity. Plant Sci. 2019, 279, 45–50. [Google Scholar] [CrossRef]
- Wang, X.; Chapman, K.D. Lipid Signaling in Plants. Front. Plant Sci. 2013, 4, 57100. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.E.; Fidelio, G.D. Secretory Phospholipases A2 in Plants. Front. Plant Sci. 2019, 10, 451927. [Google Scholar] [CrossRef]
- Kirik, A.; Mudgett, M.B. SOBER1 Phospholipase Activity Suppresses Phosphatidic Acid Accumulation and Plant Immunity in Response to Bacterial Effector AvrBsT. Proc. Natl. Acad. Sci. USA 2009, 106, 20532–20537. [Google Scholar] [CrossRef] [PubMed]
- Munnik, T.; Laxalt, A.M. Measuring PLD Activity in vivo. In Plant Lipid Signaling Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1009, pp. 219–231. [Google Scholar] [CrossRef]
- Romero, P.; Alférez, F.; Lafuente, M.T. Involvement of Phospholipases and Sucrose in Carbon Starvation-Induced Non-Chilling Peel Pitting in Citrus Fruit. Postharvest Biol. Technol. 2020, 169, 111295. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and Biochemical Mechanisms Regulating Chilling Tolerance in Fruits and Vegetables under Postharvest Salicylates and Jasmonates Treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Khorsandi, O.; Mohayeji, M. Alleviation of Postharvest Chilling Injury of Tomato Fruit by Salicylic Acid Treatment. J. Food Sci. Technol. 2014, 51, 2815–2820. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asle-Mohammadi, Z.; Ebrahimi, A.; Razavi, F. Exogenous Dopamine Application Ameliorates Chilling Injury and Preserves Quality of Kiwifruit during Cold Storage. Sci. Rep. 2025, 15, 2894. [Google Scholar] [CrossRef]
- Huang, D.; Tian, W.; Feng, J.; Zhu, S. Interaction between Nitric Oxide and Storage Temperature on Sphingolipid Metabolism of Postharvest Peach Fruit. Plant Physiol. Biochem. 2020, 151, 60–68. [Google Scholar] [CrossRef]
- Jiao, C. IP3 Mediates NO-Enhanced Chilling Tolerance in Postharvest Kiwifruit. Postharvest Biol. Technol. 2021, 176, 111463. [Google Scholar] [CrossRef]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in Membrane Lipid Composition and Function Accompanying Chilling Injury in Bell Peppers. Plant Cell Physiol. 2018, 59, 167–178. [Google Scholar] [CrossRef]
- Li, Z.; Palmer, W.M.; Martin, A.P.; Wang, R.; Rainsford, F.; Jin, Y.; Patrick, J.W.; Yang, Y.; Ruan, Y.L. High Invertase Activity in Tomato Reproductive Organs Correlates with Enhanced Sucrose Import into, and Heat Tolerance of, Young Fruit. J. Exp. Bot. 2012, 63, 1155–1166. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khosravi-Nejad, F.; Hatamnia, A.A.; Sheikhakbari Mehr, R. Impact of Postharvest Exogenous γ-Aminobutyric Acid Treatment on Cucumber Fruit in Response to Chilling Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Mao, L.; Pang, H.; Wang, G.; Zhu, C. Phospholipase D and Lipoxygenase Activity of Cucumber Fruit in Response to Chilling Stress. Postharvest Biol. Technol. 2007, 44, 42–47. [Google Scholar] [CrossRef]
- Rui, H.; Cao, S.; Shang, H.; Jin, P.; Wang, K.; Zheng, Y. Effects of Heat Treatment on Internal Browning and Membrane Fatty Acid in Loquat Fruit in Response to Chilling Stress. J. Sci. Food Agric. 2010, 90, 1557–1561. [Google Scholar] [CrossRef]
- Song, K.; Gu, H.; Golding, J.B.; Pristijono, P.; Hou, X.; Zhang, L.; Hong, K.; Yao, Q.; Zhang, X. Insight into the Physiological and Molecular Mechanisms of Hot Air Treatment Which Reduce Internal Browning in Winter-Harvested Pineapples. Postharvest Biol. Technol. 2022, 194, 112066. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Nagendra Prasad, K.; You, X.; Li, L.; Liao, F.; Peng, H.; He, X.; Li, Z.; Zhang, Y. Membrane Deterioration, Enzymatic Browning and Oxidative Stress in Fresh Fruits of Three Litchi Cultivars during Six-Day Storage. Sci. Hortic. 2012, 148, 97–103. [Google Scholar] [CrossRef]
- Wang, L.; Bokhary, S.U.F.; Xie, B.; Hu, S.; Jin, P.; Zheng, Y. Biochemical and Molecular Effects of Glycine Betaine Treatment on Membrane Fatty Acid Metabolism in Cold Stored Peaches. Postharvest Biol. Technol. 2019, 154, 58–69. [Google Scholar] [CrossRef]
- Xie, P.; Yang, Y.; Gong, D.; Li, Y.; Wang, Y.; Li, Y.; Prusky, D.; Bi, Y. Spraying L-Phenylalanine during Fruit Development Alleviates Chilling Injury in Harvested Muskmelons by Regulating Membrane Lipid Metabolism. Postharvest Biol. Technol. 2024, 211, 112858. [Google Scholar] [CrossRef]
- Xu, D.; Lam, S.M.; Zuo, J.; Yuan, S.; Lv, J.; Shi, J.; Gao, L.; Chen, B.; Sui, Y.; Shui, G.; et al. Lipidomics Reveals the Difference of Membrane Lipid Catabolism between Chilling Injury Sensitive and Non-Sensitive Green Bell Pepper in Response to Chilling. Postharvest Biol. Technol. 2021, 182, 111714. [Google Scholar] [CrossRef]
- Xu, P.; Huber, D.J.; Gong, D.; Yun, Z.; Pan, Y.; Jiang, Y.; Zhang, Z. Amelioration of Chilling Injury in ‘Guifei’ Mango Fruit by Melatonin Is Associated with Regulation of Lipid Metabolic Enzymes and Remodeling of Lipidome. Postharvest Biol. Technol. 2023, 198, 112233. [Google Scholar] [CrossRef]
- Huang, S.; Bi, Y.; Li, H.; Liu, C.; Wang, X.; Wang, X.; Lei, Y.; Zhang, Q.; Wang, J. Reduction of Membrane Lipid Metabolism in Postharvest Hami Melon Fruits by N-Butanol to Mitigate Chilling Injury and the Cloning of Phospholipase D-β Gene. Foods 2023, 12, 1904. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; El Kayal, W.; Sullivan, J.A.; Paliyath, G.; Jayasankar, S. Pre-Harvest Application of Hexanal Formulation Enhances Shelf Life and Quality of ‘Fantasia’ Nectarines by Regulating Membrane and Cell Wall Catabolism-Associated Genes. Sci. Hortic. 2018, 229, 117–124. [Google Scholar] [CrossRef]
- Kong, X.M.; Zhou, Q.; Luo, F.; Wei, B.D.; Wang, Y.; Sun, H.J.; Zhao, Y.B.; Ji, S.J. Transcriptome Analysis of Harvested Bell Peppers (Capsicum annuum L.) in Response to Cold Stress. Plant Physiol. Biochem. 2019, 139, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, H.; Lin, H.T.; Lin, M.S.; Wang, H.; Wei, W.; Chen, J.Y.; Lu, W.J.; Shao, X.F.; Fan, Z.Q. The Metabolism of Membrane Lipid Participates in the Occurrence of Chilling Injury in Cold-Stored Banana Fruit. Food Res. Int. 2023, 173, 113415. [Google Scholar] [CrossRef]
- Lu, X.; Yin, F.; Liu, C.; Liang, Y.; Song, M.; Shang, F.; Liu, Y.; Shuai, L. Nitric Oxide Alleviates Chilling Injury in Cucumber (Cucumis sativus L.) Fruit by Regulating Membrane Lipid and Energy Metabolism. Int. J. Food Prop. 2023, 26, 1047–1061. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, Y.; Watkins, C.B.; Zuo, J.; Liu, H.; Wang, Y.; Wang, Z.; Ma, L.; He, H.; Hu, L. Transcriptomic and Metabolomic Analysis of Quality Deterioration of Postharvest Okra Fruit at Different Storage Temperatures. Postharvest Biol. Technol. 2024, 218, 113146. [Google Scholar] [CrossRef]
- Malladi, A.; Burns, J.K. CsPLDα1 and CsPLDγ1 are Differentially Induced during Leaf and Fruit Abscission and Diurnally Regulated in Citrus sinensis. J. Exp. Bot. 2008, 59, 3729–3739. [Google Scholar] [CrossRef]
- Romero, P.; Gandía, M.; Alférez, F. Interplay between ABA and Phospholipases A2 and D in the Response of Citrus Fruit to Postharvest Dehydration. Plant Physiol. Biochem. 2013, 70, 287–294. [Google Scholar] [CrossRef]
- Alferez, F.; Lluch, Y.; Burns, J.K. Phospholipase A2 and Postharvest Peel Pitting in Citrus Fruit. Postharvest Biol. Technol. 2008, 49, 69–76. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Ballester, A.R.; Holland, N.; Cerveró, J.; Romero, P. Interrelation between ABA and Phospholipases D, C and A2 in Early Responses of Citrus Fruit to Penicillium digitatum Infection. Postharvest Biol. Technol. 2021, 175, 111475. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T.; Alférez, F. A Transcriptional Approach to Unravel the Connection between Phospholipases A2 and D and ABA Signal in Citrus under Water Stress. Plant Physiol. Biochem. 2014, 80, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, S.; Li, Y.; Huang, Z.; Li, J.; Xiong, B.; Zhang, M.; Sun, G.; Wang, Z. Pre- or Post-Harvest Treatment with MeJA Improves Post-Harvest Storage of Lemon Fruit by Stimulating the Antioxidant System and Alleviating Chilling Injury. Plants 2022, 11, 2840. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Nawaz, A.; Naz, S.; Ejaz, S.; Shah, A.A.; Haider, M.W. The Combined Application of Arabic Gum Coating and γ-Aminobutyric Acid Mitigates Chilling Injury and Maintains Eating Quality of ‘Kinnow’ Mandarin Fruits. Int. J. Biol. Macromol. 2023, 236, 123966. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Rodrigo, M.J.; Alférez, F.; Ballester, A.R.; González-Candelas, L.; Zacarías, L.; Lafuente, M.T. Unravelling Molecular Responses to Moderate Dehydration in Harvested Fruit of Sweet Orange (Citrus sinensis L. Osbeck) Using a Fruit-Specific ABA-Deficient Mutant. J. Exp. Bot. 2012, 63, 2753–2767. [Google Scholar] [CrossRef]
- Fan, L.; Zheng, S.; Cui, D.; Wang, X. Subcellular Distribution and Tissue Expression of Phospholipase Dα, Dβ, and Dγ in Arabidopsis. Plant Physiol. 1999, 119, 1371–1378. [Google Scholar] [CrossRef]
- Mishkind, M.; Vermeer, J.E.M.; Darwish, E.; Munnik, T. Heat Stress Activates Phospholipase D and Triggers PIP2 Accumulation at the Plasma Membrane and Nucleus. Plant J. 2009, 60, 10–21. [Google Scholar] [CrossRef]
- Horváth, I.; Glatz, A.; Nakamoto, H.; Mishkind, M.L.; Munnik, T.; Saidi, Y.; Goloubinoff, P.; Harwood, J.L.; Vigh, L. Heat Shock Response in Photosynthetic Organisms: Membrane and Lipid Connections. Prog. Lipid Res. 2012, 51, 208–220. [Google Scholar] [CrossRef]
- Wan, S.B.; Tian, L.; Tian, R.R.; Pan, Q.H.; Zhan, J.C.; Wen, P.F.; Chen, J.Y.; Zhang, P.; Wang, W.; Huang, W.D. Involvement of Phospholipase D in the Low Temperature Acclimation-Induced Thermotolerance in Grape Berry. Plant Physiol. Biochem. 2009, 47, 504–510. [Google Scholar] [CrossRef]
- Li, G.; Xue, H.W. Arabidopsis PLDζ2 Regulates Vesicle Trafficking and Is Required for Auxin Response. Plant Cell 2007, 19, 281–295. [Google Scholar] [CrossRef]
- Su, Y.; Li, M.; Guo, L.; Wang, X. Different Effects of Phospholipase Dζ2 and Non-Specific Phospholipase C4 on Lipid Remodeling and Root Hair Growth in Arabidopsis Response to Phosphate Deficiency. Plant J. 2018, 94, 315–326. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Bedair, M.; Welti, R.; Sumner, L.; Baxter, I.; Wang, X. Suppression of Phospholipase Dγs Confers Increased Aluminum Resistance in Arabidopsis thaliana. PLoS ONE 2011, 6, e28086. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hong, Y.; Wang, X. Phospholipase D- and Phosphatidic Acid-Mediated Signaling in Plants. Biochem. Biophys. Acta 2009, 1791, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Cui, D.; Wang, X. Phospholipase D and Phosphatidic Acid-Mediated Generation of Superoxide in Arabidopsis. Plant Physiol. 2001, 126, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Rajashekar, C.B.; Zhou, H.E.; Zhang, Y.; Li, W.; Wang, X. Suppression of Phospholipase Dα1 Induces Freezing Tolerance in Arabidopsis: Response of Cold-Responsive Genes and Osmolyte Accumulation. J. Plant Physiol. 2006, 163, 916–926. [Google Scholar] [CrossRef]
- Kong, X.M.; Zhou, Q.; Zhou, X.; Wei, B.D.; Ji, S.J. Transcription Factor CaNAC1 Regulates Low-Temperature-Induced Phospholipid Degradation in Green Bell Pepper. J. Exp. Bot. 2020, 71, 1078–1091. [Google Scholar] [CrossRef]
- Krčková, Z.; Brouzdová, J.; Daněk, M.; Kocourková, D.; Rainteau, D.; Ruelland, E.; Valentová, O.; Pejchar, P.; Martinec, J. Arabidopsis Non-Specific Phospholipase C1: Characterization and Its Involvement in Response to Heat Stress. Front. Plant Sci. 2015, 6, 165719. [Google Scholar] [CrossRef]
- Zheng, S.Z.; Liu, Y.L.; Li, B.; Shang, Z.L.; Zhou, R.G.; Sun, D.Y. Phosphoinositide-Specific Phospholipase C9 is Involved in the Thermotolerance of Arabidopsis. Plant J. 2012, 69, 689–700. [Google Scholar] [CrossRef]
- Gao, K.; Liu, Y.L.; Li, B.; Zhou, R.G.; Sun, D.Y.; Zheng, S.Z. Arabidopsis Thaliana Phosphoinositide-Specific Phospholipase C Isoform 3 (AtPLC3) and AtPLC9 Have an Additive Effect on Thermotolerance. Plant Cell Physiol. 2014, 55, 1873–1883. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.L.; Chen, S. Genome-Wide Identification of Phospholipase C Related to Chilling Injury in Peach Fruit. J. Plant Biochem. Biotechnol. 2021, 30, 452–461. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).