Protein Hydrolysates Modulate Quality Traits of Tomato Fruit Under Salt Stress by Regulating the Expression Patterns of Genes Related to Sugar Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection and Characterization of Protein Hydrolysates
2.2. Experimental Site and Design, Plant Material, and Growth Conditions
2.3. Biostimulant Application
2.4. Nutrient Solution Application
2.5. Measurements and Harvesting
2.6. Analysis of Fruit Quality
2.7. Analysis of Total Nitrogen, Minerals, and Organic Acids
2.8. Determination of Chlorophyll and Carotenoids
2.9. Determination of Proline, Total Proteins and Malondialdehyde
2.10. RNA Extraction and Gene Expression Analysis
2.11. Statistical Analysis
3. Results
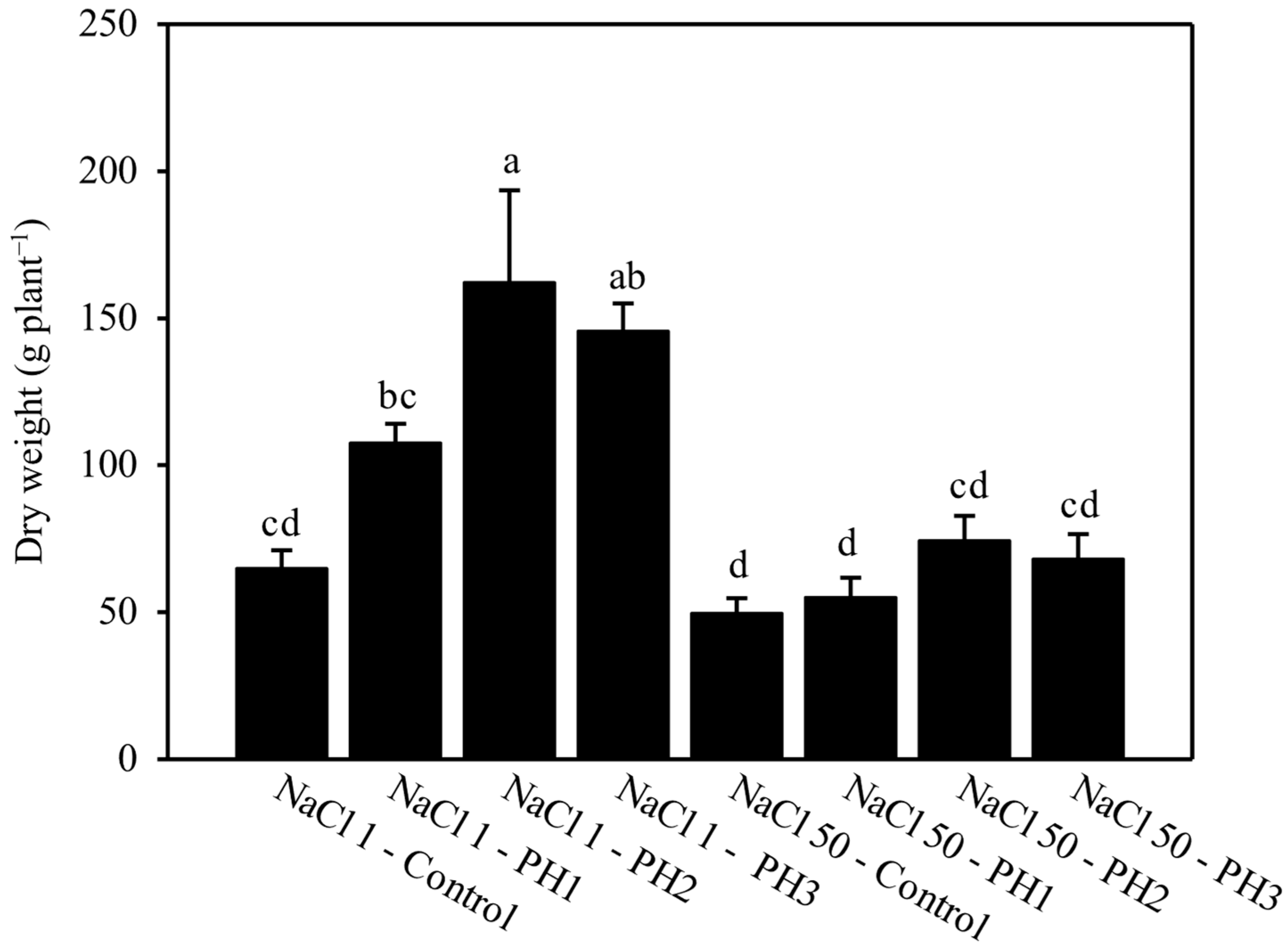
3.1. Effect of Salinity and PH on Plant Biomass and Its Partitioning
3.2. Effect of Salinity and PH on Fruit Yield and Yield Components
3.3. Effects of Salinity and PH on Leaf Mineral Composition
3.4. Effect of Salinity and PH on Fruit Quality
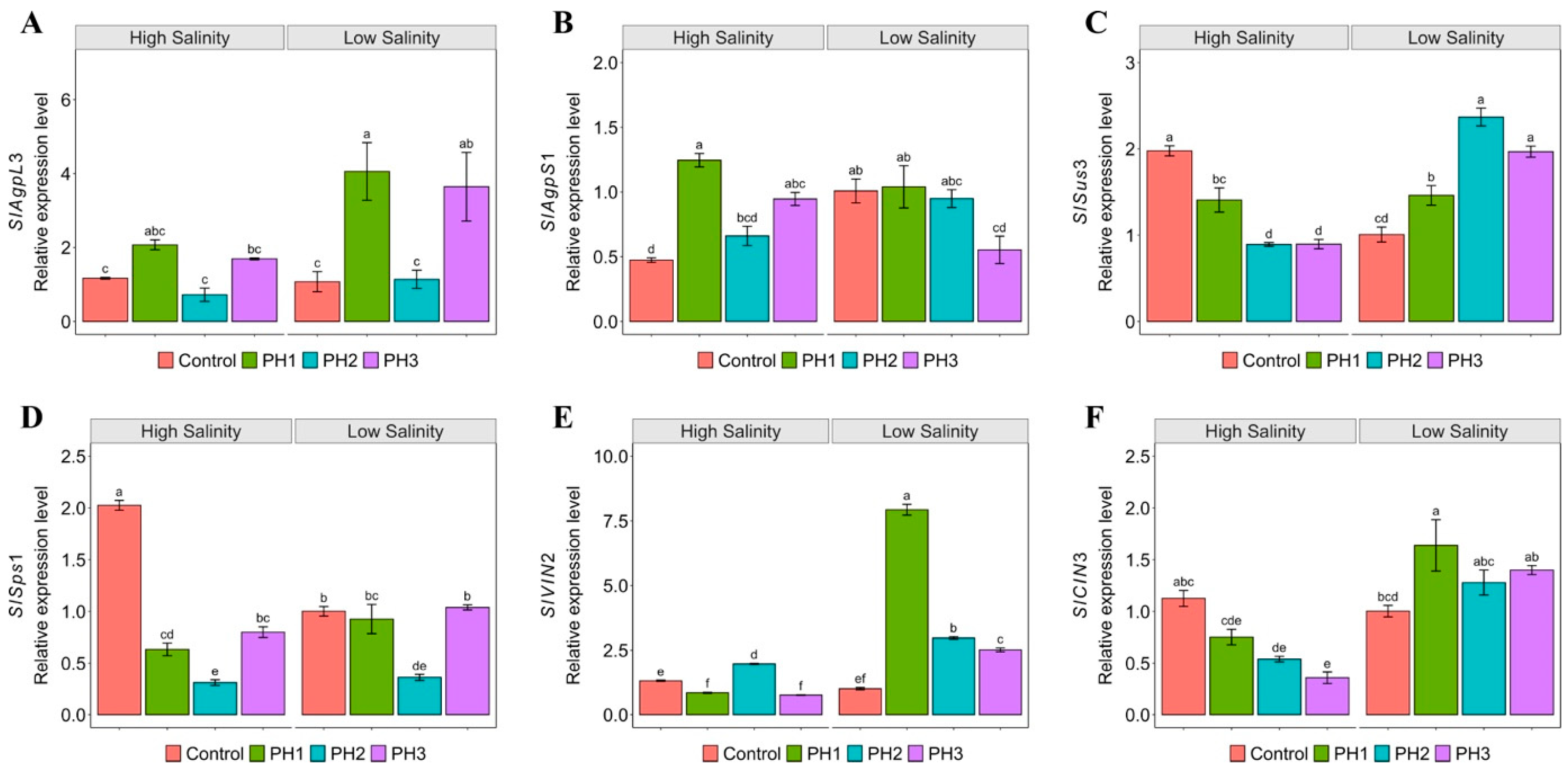
3.5. Effect of Salinity and PH on Modulation of Sugar Metabolism-Related Gene Expression
4. Discussion
4.1. Influence of Salinity and PH on Biomass Partitioning: Interpretation
4.2. Implications of Salinity and PH on Fruit Production
4.3. Implications of Salinity and PH on Leaf Mineral Nutrition
4.4. Understanding the Effects of Salinity and PH on Fruit Quality
4.5. Salinity and PH Influence on Sugar Metabolism-Related Gene Expression: Insights
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Ali, A.A.M.; Ben Romdhane, W.; Tarroum, M.; Al-Dakhil, M.; Al-Doss, A.; Alsadon, A.A.; Hassairi, A. Analysis of salinity tolerance in tomato introgression lines based on morpho-physiological and molecular traits. Plants 2021, 10, 2594. [Google Scholar] [CrossRef]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- D’Amato, R.; Del Buono, D. Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy 2021, 11, 1755. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L.). Sci. Hortic. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Salt stress differentially affects the primary and secondary metabolism of peppers (Capsicum annuum L.) according to the genotype, fruit part, and salinity level. Plants 2022, 11, 853. [Google Scholar] [CrossRef]
- Li, W.; Lu, X.; Li, J. The effect of organic nutrient solution on flavor in ripe cherry tomato fruit—Transcriptome and metabolomic analyses. Environ. Exp. Bot. 2022, 194, 104721. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and physiological responses of tomato plants grown in different soilless culture systems with saline water under greenhouse conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Lorenzo, P. Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in greenhouses in Mediterranean climatic conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- Musabyisoni, A.; Waweru, B.; Nishizawa, T. Effect of salt stress by ‘onsen’ water on plant growth and fruit quality of tomato cv. Reika in pot soil. Adv. Hortic. Sci. 2021, 35, 383–388. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, S.; Dai, Y.; Zhang, Z.; Senge, M. Combined treatment of salinity stress and fruit thinning effect on tomato. Front. Nutr. 2022, 9, 857977. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato responses to salinity stress: From morphological traits to genetic changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef]
- D’Amico, M.L.; Izzo, R.; Tognoni, F.; Pardossi, A.; Navari, F. Application of diluted sea water to soilless culture of tomato (Lycopersicon esculentum Mill.): Effects on plant growth, yield, fruit quality and antioxidant capacity. J. Food Agric. Environ. 2003, 1, 112–116. [Google Scholar]
- Zushi, K.; Matsuzoe, N. Metabolic profile of organoleptic and health-promoting qualities in two tomato cultivars subjected to salt stress and their interactions using correlation network analysis. Sci. Hortic. 2015, 184, 8–17. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional tomato varieties improve fruit quality without affecting fruit yield under moderate salt stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Matsuzoe, N.; Kitano, M. Developmental and tissue-specific changes in oxidative parameters and antioxidant systems in tomato fruits grown under salt stress. Sci. Hortic. 2009, 122, 362–368. [Google Scholar] [CrossRef]
- Dere, S.; Kusvuran, S.; Dasgan, H.Y. Does drought increase the antioxidant nutrient capacity of tomatoes? Int. J. Food Sci. Technol. 2022, 57, 6633–6645. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, X.; Gong, B.; Yan, Y.; Shi, Q. Proteomics and metabolomics analysis of tomato fruit at different maturity stages and under salt treatment. Food Chem. 2020, 311, 126009. [Google Scholar] [CrossRef]
- Moles, T.M.; de Brito Francisco, R.; Mariotti, L.; Pompeiano, A.; Lupini, A.; Incrocci, L.; Carmassi, G.; Scartazza, A.; Pistelli, L.; Guglielminetti, L. Salinity in autumn-winter season and fruit quality of tomato landraces. Front. Plant Sci. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Bidel, L.P.R.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2013, 37, 273–289. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of salt stress on tomato fruit antioxidant systems depends on fruit development stage. Physiol. Mol. Biol. Plants 2013, 20, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Li, T.L.; Jing, J. Effects of tomato fruit under Na+ -salt and Cl− -salt stresses on sucrose metabolism. Afr. J. Agric. Res. 2010, 5, 2227–2231. [Google Scholar]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef]
- Yin, Y.-G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) fruits in an ABA- and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Liang, G.; Li, Y.; Wang, P.; Jiao, S.; Wang, H.; Mao, J.; Chen, B. VaAPL1 promotes starch synthesis to constantly contribute to soluble sugar accumulation, improving low temperature tolerance in Arabidopsis and tomato. Front. Plant Sci. 2022, 13, 920424. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Sadhukhan, S. Imperative role of trehalose metabolism and trehalose-6-phosphate signaling on salt stress responses in plants. Physiol. Plant. 2022, 174, e13647. [Google Scholar] [CrossRef]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2018, 38, 449–462. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Biostimulative effect of amino acids on the enzymatic and metabolic response of two Capsicum annuum L. cultivars grown under salt stress. Sci. Hortic. 2023, 309, 111713. [Google Scholar] [CrossRef]
- Zhang, L.; Freschi, G.; Rouphael, Y.; De Pascale, S.; Lucini, L. The differential modulation of secondary metabolism induced by a protein hydrolysate and a seaweed extract in tomato plants under salinity. Front. Plant Sci. 2023, 13, 1072782. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Tallarita, A.; Vecchietti, L.; Cozzolino, E.; Sekara, A.; Golubkina, N.; Mirabella, M.; Cuciniello, A.; Maiello, R.; Cenvinzo, V.; Troncone, R. Yield, quality, and antioxidants of greenhouse grown ‘miniplum’ tomato as affected by biostimulant treatment duration in southern Italy. Acta Hortic. 2023, 1375, 393–400. [Google Scholar] [CrossRef]
- Zuluaga, M.Y.A.; Monterisi, S.; Rouphael, Y.; Colla, G.; Lucini, L.; Cesco, S.; Pii, Y. Different vegetal protein hydrolysates distinctively alleviate salinity stress in vegetable crops: A case study on tomato and lettuce. Front. Plant Sci. 2023, 14, 1077140. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Calone, R.; Rodríguez-Heredia, R.; Asaff-Torres, A.; Boscaiu, M.; Ibáñez-Asensio, S.; Moreno-Ramón, H.; Vicente, O. Use of a biostimulant to mitigate the effects of excess salinity in soil and irrigation water in tomato plants. Plants 2023, 12, 1190. [Google Scholar] [CrossRef]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the environmental impact of chemically-produced protein hydrolysate from leather waste vs. enzymatically-produced protein hydrolysate from legume grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar application of different vegetal-derived protein hydrolysates distinctively modulates tomato root development and metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Maas, E.; Hoffman, G. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Norrie, J.; Graham, M.E.D.; Gosselin, A. Potential evapotranspiration as a means of predicting irrigation timing in greenhouse tomatoes grown in peat bags. J. Am. Soc. Hortic. Sci. 1994, 119, 163–168. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. The influence of drip irrigation or subirrigation on zucchini squash grown in closed-loop substrate culture with high and low nutrient solution concentrations. HortScience 2009, 44, 306–311. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Oxid. Antioxid. Part A 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; Agronomy Monograph 9: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Petropoulos, S.A.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. Dataset on the organic acids, sulphate, total nitrogen and total chlorophyll contents of two lettuce cultivars grown hydroponically using nutrient solutions of variable macrocation ratios. Data Brief 2020, 29, 105135. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Foliar application of protein hydrolysates on baby-leaf spinach grown at different N levels. Agronomy 2021, 12, 36. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.-J. Effects of plant-derived protein hydrolysates on yield, quality, and nitrogen use efficiency of greenhouse grown lettuce and tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2012, 364, 145–158. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Grieve, C.; Grattan, S. Mineral nutrient acquisition and response by plants grown in saline environments. In Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 1999; pp. 203–229. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Desoky, E.-S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Amr, A.; Raie, W. Tomato components and quality parameters. A Review. Jordan J. Agric. Sci. 2022, 18, 199–220. [Google Scholar] [CrossRef]
- Dobrin, A.; Nedelu, A.; Bujor, O.; Mot, A.; Zugravu, M.; Badulescu, L. Nutritional quality parameters of the fresh red tomato varieties cultivated in organic system. Sci. Papers. Ser. B Hortic. 2019, LXIII, 439–443. [Google Scholar]
- Agius, C.; von Tucher, S.; Rozhon, W. The effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2018, 8, e00162. [Google Scholar] [CrossRef]
- Cardarelli, M.; Ceccarelli, A.V.; El Nakhel, C.; Rouphael, Y.; Salehi, H.; Ganugi, P.; Zhang, L.; Luigi, L.; Pii, Y.; Choi, S. Foliar applications of a Malvaceae-derived protein hydrolysate and its fractions differentially modulate yield and functional traits of tomato under optimal and suboptimal nitrogen application. J. Sci. Food Agric. 2024, 104, 7603–7616. [Google Scholar] [CrossRef]
- Salamone, P.R.; Greene, T.W.; Kavakli, I.H.; Okita, T.W. Isolation and characterization of a higher plant ADP-glucose pyrophosphorylase small subunit homotetramer. FEBS Lett. 2000, 482, 113–118. [Google Scholar] [CrossRef]
- Wang, F.; Smith, A.G.; Brenner, M.L. Isolation and sequencing of tomato fruit sucrose synthase cDNA. Plant Physiol. 1993, 103–104, 1463–1464. [Google Scholar] [CrossRef]
- Boris, K.V.; Ryzhova, N.N.; Skryabin, K.G. Analysis of Sus2 gene polymorphism in tomato varieties and related wild species. Dokl. Biochem. Biophys. 2010, 433, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Goren, S.; Huber, S.C.; Granot, D. Comparison of a novel tomato sucrose synthase, SlSUS4, with previously described SlSUS isoforms reveals distinct sequence features and differential expression patterns in association with stem maturation. Planta 2011, 233, 1011–1023. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Yang, J.; Li, H.; Ma, B.; Zhang, K.; Zhang, Y.; Cheng, L.; Ma, F.; Li, M. Heterologous expression of the apple hexose transporter MdHT2.2 altered sugar concentration with increasing cell wall invertase activity in tomato fruit. Plant Biotechnol. J. 2020, 18, 540–552. [Google Scholar] [CrossRef]
- Castleden, C.K.; Aoki, N.; Gillespie, V.J.; MacRae, E.A.; Quick, W.P.; Buchner, P.; Foyer, C.H.; Furbank, R.T.; Lunn, J.E. Evolution and function of the sucrose-phosphate synthase gene families in wheat and other grasses. Plant Physiol. 2004, 135, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Kos, A.; Mytych, J.; Suski, S.; Magoń, J.; Ochodzki, P.; Zebrowski, J. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus × giganteus and Zea mays at low temperature. Planta 2020, 252, 23. [Google Scholar] [CrossRef]
- Suwa, R.; Fujimaki, S.; Suzui, N.; Kawachi, N.; Ishii, S.; Sakamoto, K.; Nguyen, N.T.; Saneoka, H.; Mohapatra, P.K.; Moghaieb, R.E. Use of positron-emitting tracer imaging system for measuring the effect of salinity on temporal and spatial distribution of 11C tracer and coupling between source and sink organs. Plant Sci. 2008, 175, 210–216. [Google Scholar] [CrossRef]
- Monterisi, S.; Zhang, L.; Garcia-Perez, P.; Zuluaga, M.Y.A.; Ciriello, M.; El-Nakhel, C.; Buffagni, V.; Cardarelli, M.; Colla, G.; Rouphael, Y. Integrated multi-omic approach reveals the effect of a Graminaceae-derived biostimulant and its lighter fraction on salt-stressed lettuce plants. Sci. Rep. 2024, 14, 10710. [Google Scholar] [CrossRef]
- Barbosa, A.C.O.; Rocha, D.S.; Silva, G.C.B.; Santos, M.G.M.; Camillo, L.R.; de Oliveira, P.H.G.A.; Cavalari, A.A.; Costa, M.G.C. Dynamics of the sucrose metabolism and related gene expression in tomato fruits under water deficit. Physiol. Mol. Biol. Plants 2023, 29, 159–172. [Google Scholar] [CrossRef] [PubMed]
| Source of Variance | Yield (kg Plant −1) | Marketable Fruit Number (n. Plant−1) | Marketable Fruit Mean Weight (g Fruit−1) | |
|---|---|---|---|---|
| Total | Marketable | |||
| NaCl level (mM) | ||||
| 1 | 2.66 ± 0.06 a | 2.11 ± 0.06 a | 149.18 ± 4.01 a | 14.15 ± 0.32 a |
| 50 | 1.34 ± 0.03 b | 1.18 ± 0.03 b | 107.17 ± 2.21 b | 11.02 ± 0.27 b |
| Biostimulant (B) | ||||
| Control | 1.95 ± 0.15 b | 1.50 ± 0.09 b | 119.89 ± 4.43 b | 12.28 ± 0.49 |
| PH1 | 2.22 ± 0.19 a | 1.84 ± 0.17 a | 136.48 ± 6.96 a | 12.98 ± 0.60 |
| PH2 | 1.96 ± 0.17 b | 1.50 ± 0.12 b | 119.60 ± 5.57 b | 12.51 ± 0.56 |
| PH3 | 1.97 ± 0.16 b | 1.72 ± 0.14 ab | 131.70 ± 7.77 ab | 12.53 ± 0.60 |
| NaCl × B | ||||
| NaCl 1 × Control | 2.56 ± 0.09 | 1.79 ± 0.08 c | 132.67 ± 5.44 | 13.53 ± 0.57 |
| NaCl 1 × PH1 | 2.89 ± 0.10 | 2.45 ± 0.10 a | 165.70 ± 5.37 | 14.76 ± 0.61 |
| NaCl 1 × PH2 | 2.63 ± 0.12 | 2.01 ± 0.04 bc | 140.30 ± 4.66 | 14.31 ± 0.31 |
| NaCl 1 × PH3 | 2.58 ± 0.14 | 2.22 ± 0.14 ab | 157.22 ± 11.38 | 14.11 ± 0.87 |
| NaCl 50 × Control | 1.28 ± 0.06 | 1.18 ± 0.05 d | 108.40 ± 4.46 | 10.87 ± 0.45 |
| NaCl 50 × PH1 | 1.48 ± 0.06 | 1.23 ± 0.06 d | 109.91 ± 3.65 | 11.19 ± 0.53 |
| NaCl 50 × PH2 | 1.28 ± 0.06 | 1.10 ± 0.07 d | 98.90 ± 3.75 | 11.08 ± 0.70 |
| NaCl 50 × PH3 | 1.36 ± 0.05 | 1.21 ± 0.05 d | 110.82 ± 5.19 | 10.95 ± 0.44 |
| Significance | ||||
| NaCl | *** | *** | *** | *** |
| B | * | *** | ** | ns |
| NaCl × B | ns | ** | ns | ns |
| Source of Variance | Mineral Elements (mg g−1 DW) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | P | S | K | Ca | Mg | Na | Cl | |
| NaCl level (mM) | ||||||||
| 1 | 25.31 ± 0.40 a | 4.62 ± 0.23 a | 12.07 ± 0.38 a | 41.90 ± 1.01 a | 34.14 ± 0.94 | 5.42 ± 0.17 a | 4.25 ± 0.32 b | 8.56 ± 0.41 b |
| 50 | 22.62 ± 0.25 b | 3.68 ± 0.16 b | 6.99 ± 0.30 b | 15.59 ± 0.71 b | 32.32 ± 0.90 | 3.35 ± 0.14 b | 27.86 ± 1.29 a | 72.06 ± 2.17 a |
| Biostimulant (B) | ||||||||
| Control | 23.56 ± 0.87 | 4.21 ± 0.48 | 9.48 ± 1.00 | 29.67 ± 5.00 | 35.00 ± 1.21 | 4.52 ± 0.42 | 17.87 ± 4.93 | 41.64 ± 14.98 a |
| PH1 | 23.89 ± 0.66 | 4.22 ± 0.26 | 10.11 ± 1.08 | 29.61 ± 5.65 | 32.36 ± 1.06 | 4.62 ± 0.36 | 14.78 ± 4.48 | 40.48 ± 12.02 ab |
| PH2 | 24.33 ± 0.59 | 3.97 ± 0.24 | 8.82 ± 0.97 | 27.06 ± 4.73 | 31.52 ± 1.44 | 4.22 ± 0.58 | 15.71 ± 4.40 | 44.15± 13.23 a |
| PH3 | 24.10 ± 0.65 | 4.20 ± 0.32 | 9.73 ± 1.22 | 28.64 ± 5.06 | 34.04 ± 1.42 | 4.17 ± 0.38 | 15.86 ± 4.77 | 35.61 ± 10.45 b |
| NaCl × B | ||||||||
| NaCl 1 × Control | 24.85 ± 1.41 | 4.87 ± 0.79 | 11.89 ± 0.61 | 42.23 ± 2.94 | 36.46 ± 2.21 | 5.54 ± 0.34 | 4.92 ± 0.71 | 9.87 ± 0.84 d |
| NaCl 1 × PH1 | 25.41 ± 0.68 | 4.69 ± 0.17 | 12.84 ± 0.61 | 44.42 ± 0.82 | 31.06 ± 1.44 | 5.47 ± 0.25 | 3.07 ± 0.29 | 8.72 ± 0.36 d |
| NaCl 1 × PH2 | 25.59 ± 0.52 | 4.36 ± 0.37 | 10.91 ± 1.09 | 39.16 ±1.75 | 33.14 ± 1.78 | 5.57 ± 0.55 | 4.19 ± 0.42 | 7.28 ± 1.35 d |
| NaCl 1 × PH3 | 25.41 ± 0.60 | 4.56 ± 0.49 | 12.64 ± 0.46 | 41.78 ± 1.86 | 35.89 ± 1.17 | 5.09 ± 0.19 | 4.83 ± 0.72 | 8.03 ± 0.32 d |
| NaCl 50 × Control | 22.26 ± 0.64 | 3.54 ± 0.41 | 7.07 ± 0.62 | 17.11 ± 1.66 | 33.54 ± 0.75 | 3.51 ± 0.18 | 30.82 ± 1.05 | 83.99 ± 0.30 a |
| NaCl 50 × PH1 | 22.37 ± 0.14 | 3.74 ± 0.37 | 7.38 ± 0.24 | 14.81 ± 1.39 | 33.65 ± 1.45 | 3.77 ± 0.25 | 26.48 ± 1.44 | 72.24 ± 1.37 b |
| NaCl 50 × PH2 | 23.37 ± 0.53 | 3.58 ± 0.15 | 6.73 ± 0.51 | 14.95 ± 1.88 | 29.90 ± 2.19 | 2.87 ± 0.28 | 27.24 ± 1.31 | 71.81 ± 4.10 bc |
| NaCl 50 × PH3 | 22.79 ± 0.66 | 3.84 ± 0.39 | 6.81 ± 1.02 | 15.50 ± 0.96 | 32.18 ± 2.40 | 3.25 ± 0.27 | 26.90 ± 4.95 | 63.18 ± 1.45 c |
| Significance | ||||||||
| NaCl | *** | ** | *** | *** | ns | *** | *** | *** |
| B | ns | ns | ns | ns | ns | ns | ns | *** |
| NaCl × B | ns | ns | ns | ns | ns | ns | ns | *** |
| Source of Variance | Dry Matter (%) | Firmness (N mm−1) | Total Soluble Solids (°Brix) | Titratable Acidity (g of Citric Acid 100 g−1 FW) | Citric Acid (mg g−1 DW) | Malic Acid (mg g−1 DW) | Oxalic Acid (mg g−1 DW) |
|---|---|---|---|---|---|---|---|
| NaCl level (mM) | |||||||
| 1 | 9.40 ± 0.11 b | 1.41 ± 0.02 b | 7.88 ± 0.09 b | 0.62 ± 0.02 b | 29.18 ± 0.63 | 4.44 ± 0.15 a | 0.41 ± 0.03 a |
| 50 | 10.97 ± 0.11 a | 1.61 ± 0.03 a | 9.38 ± 0.22 a | 0.79 ± 0.02 a | 29.56 ± 0.68 | 3.68 ± 0.07 b | 0.30 ± 0.01 b |
| Biostimulant (B) | |||||||
| Control | 10.22 ± 0.26 | 1.32 ± 0.03 b | 8.78 ± 0.22 b | 0.67 ± 0.02 | 29.98 ± 0.79 | 4.17 ± 0.23 | 0.43 ± 0.05 a |
| PH1 | 10.11 ± 0.36 | 1.38 ± 0.04 b | 9.33 ± 0.51 a | 0.71 ± 0.03 | 27.88 ± 0.92 | 3.97 ± 0.21 | 0.34 ± 0.03 b |
| PH2 | 10.24 ± 0.26 | 1.66 ± 0.04 a | 8.14 ± 0.15 c | 0.72 ± 0.03 | 30.32 ± 1.03 | 4.15 ± 0.30 | 0.33 ± 0.02 b |
| PH3 | 10.17 ± 0.44 | 1.68 ± 0.04 a | 8.29 ± 0.33 c | 0.70 ± 0.07 | 29.30 ± 0.82 | 3.97 ± 0.09 | 0.31 ± 0.01 b |
| NaCl × B | |||||||
| NaCl 1 × Control | 9.63 ± 0.12 | 1.33 ± 0.04 def | 8.23 ± 0.06 de | 0.62 ± 0.01 cd | 28.83 ± 0.72 | 4.65 ± 0.26 | 0.54 ± 0.04 a |
| NaCl 1 × PH1 | 9.32 ± 0.30 | 1.25± 0.04 f | 8.05 ± 0.19 de | 0.66 ± 0.02 c | 28.92 ± 0.92 | 4.44 ± 0.18 | 0.41 ± 0.03 b |
| NaCl 1 × PH2 | 9.59 ± 0.17 | 1.44 ± 0.03 cde | 7.80 ± 0.06 de | 0.66 ± 0.02 c | 31.13 ± 1.73 | 4.61 ± 0.53 | 0.36 ± 0.02 bc |
| NaCl 1 × PH3 | 9.08 ± 0.16 | 1.60 ± 0.04 bc | 7.45 ± 0.09 e | 0.54 ± 0.03 d | 27.86 ± 1.26 | 4.07 ± 0.17 | 0.31 ± 0.02 bc |
| NaCl 50 × Control | 10.81 ± 0.24 | 1.31 ± 0.04 ef | 9.33 ± 0.11 b | 0.72 ± 0.01 bc | 31.12 ± 1.24 | 3.68 ± 0.16 | 0.31 ± 0.04 bc |
| NaCl 50 × PH1 | 10.89 ± 0.31 | 1.51 ± 0.05 cd | 10.60 ± 0.29 a | 0.77 ± 0.03 b | 26.84 ± 1.54 | 3.51 ± 0.18 | 0.27 ± 0.01 c |
| NaCl 50 × PH2 | 10.89 ± 0.03 | 1.89 ± 0.04 a | 8.48 ± 0.15 cd | 0.78 ± 0.02 ab | 29.51 ± 1.25 | 3.68 ± 0.06 | 0.30 ± 0.01 bc |
| NaCl 50 × PH3 | 11.26 ± 0.25 | 1.75 ± 0.06 ab | 9.13 ± 0.21 bc | 0.87 ± 0.02 a | 30.74 ± 0.38 | 3.86 ± 0.06 | 0.31 ± 0.02 bc |
| Significance | |||||||
| NaCl | *** | *** | *** | *** | ns | *** | *** |
| B | ns | *** | *** | ns | ns | ns | *** |
| NaCl × B | ns | *** | *** | *** | ns | ns | ** |
| Source of Variance | FRAP (mM Fe2+ 100 g−1 FW) | DPPH (mM Trolox 100 g−1 FW) | Total Phenols (mM GAE 100 g−1 FW) | Flavonoids (mM QE 100 g−1 FW) | Proline (mM Pro 100 g−1 FW) |
|---|---|---|---|---|---|
| NaCl level (mM) | |||||
| 1 | 1.09 ± 0.06 b | 1.70 ± 0.06 b | 416.01 ± 15.53 a | 40.83 ± 1.68 | 672.87 ± 52.76 b |
| 50 | 2.27 ± 0.08 a | 2.42 ± 0.05 a | 366.00 ± 7.48 b | 41.79 ± 1.25 | 2127.33 ± 43.40 a |
| Biostimulant (B) | |||||
| Control | 1.23 ± 0.14 c | 1.76 ± 0.11 c | 327.48 ± 9.11 c | 38.31 ± 2.41 b | 1257.93 ± 64.04 b |
| PH1 | 1.67 ± 0.17 b | 2.00 ±0.12 b | 391.60 ± 13.83 b | 39.30 ± 133 b | 1315.24 ± 61.37 b |
| PH2 | 1.76 ± 0.21 b | 2.17 ± 0.11 ab | 390.89 ± 11.71 b | 37.91 ± 1.01 b | 1491.76 ± 72.62 a |
| PH3 | 2.06 ± 0.15 a | 2.34 ± 0.09 a | 460.35 ± 19.75 a | 50.17 ± 1.60 a | 1535.47 ± 74.33 a |
| NaCl × B | |||||
| NaCl 1 × Control | 0.75 ± 0.07 | 1.41 ± 0.08 | 299.40 ± 9.77 e | 30.29 ± 1.61 e | 494.89 ± 96.83 |
| NaCl 1 × PH1 | 1.11 ± 0.09 | 1.58 ± 0.08 | 417.96 ± 14.47 bc | 40.13 ± 1.56 bcd | 624.04 ± 83.85 |
| NaCl 1 × PH2 | 1.04 ± 0.08 | 1.84 ± 0.09 | 429.97 ± 6.52 b | 39.58 ± 0.69 cd | 790.00 ± 118.59 |
| NaCl 1 × PH3 | 1.46 ± 0.06 | 1.99 ± 0.06 | 500.06 ± 23.62 a | 51.92 ± 2.89 a | 782.55 ± 118.59 |
| NaCl 50 × Control | 1.78 ± 0.06 | 2.10 ± 0.10 | 348.54 ± 8.41 de | 45.19 ± 1.65 abc | 2020.96 ± 83.85 |
| NaCl 50 × PH1 | 2.17 ± 0.16 | 2.42 ± 0.09 | 368.53 ± 19.94 bcd | 37.98 ± 2.53 cde | 2006.44 ± 89.64 |
| NaCl 50 × PH2 | 2.48 ± 0.08 | 2.50 ± 0.09 | 356.70 ± 11.14 cde | 36.45 ± 1.68 de | 2193.51 ± 83.85 |
| NaCl 50 × PH3 | 2.59 ± 0.08 | 2.65 ± 0.05 | 404.76 ± 8.83 bcd | 48.12 ± 0.23 ab | 2288.39 ± 89.64 |
| Significance | |||||
| NaCl | *** | *** | *** | ns | *** |
| B | *** | *** | *** | *** | * |
| NaCl × B | ns | ns | *** | *** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Chami, A.; Ceccarelli, A.V.; Monterisi, S.; Colla, G.; El-Nakhel, C.; Rouphael, Y.; Pii, Y.; Cardarelli, M. Protein Hydrolysates Modulate Quality Traits of Tomato Fruit Under Salt Stress by Regulating the Expression Patterns of Genes Related to Sugar Metabolism. Horticulturae 2025, 11, 1108. https://doi.org/10.3390/horticulturae11091108
El Chami A, Ceccarelli AV, Monterisi S, Colla G, El-Nakhel C, Rouphael Y, Pii Y, Cardarelli M. Protein Hydrolysates Modulate Quality Traits of Tomato Fruit Under Salt Stress by Regulating the Expression Patterns of Genes Related to Sugar Metabolism. Horticulturae. 2025; 11(9):1108. https://doi.org/10.3390/horticulturae11091108
Chicago/Turabian StyleEl Chami, Antonio, Angela Valentina Ceccarelli, Sonia Monterisi, Giuseppe Colla, Christophe El-Nakhel, Youssef Rouphael, Youry Pii, and Mariateresa Cardarelli. 2025. "Protein Hydrolysates Modulate Quality Traits of Tomato Fruit Under Salt Stress by Regulating the Expression Patterns of Genes Related to Sugar Metabolism" Horticulturae 11, no. 9: 1108. https://doi.org/10.3390/horticulturae11091108
APA StyleEl Chami, A., Ceccarelli, A. V., Monterisi, S., Colla, G., El-Nakhel, C., Rouphael, Y., Pii, Y., & Cardarelli, M. (2025). Protein Hydrolysates Modulate Quality Traits of Tomato Fruit Under Salt Stress by Regulating the Expression Patterns of Genes Related to Sugar Metabolism. Horticulturae, 11(9), 1108. https://doi.org/10.3390/horticulturae11091108










