Soluble Eugenol Formulation for Managing Ball Moss on Ornamental Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening Studies
2.2. Dose Response Studies
2.3. Selection of a Candidate Product
2.4. Field Studies
2.5. Treatment Feasibility Trials
3. Results
3.1. Screening Experiments
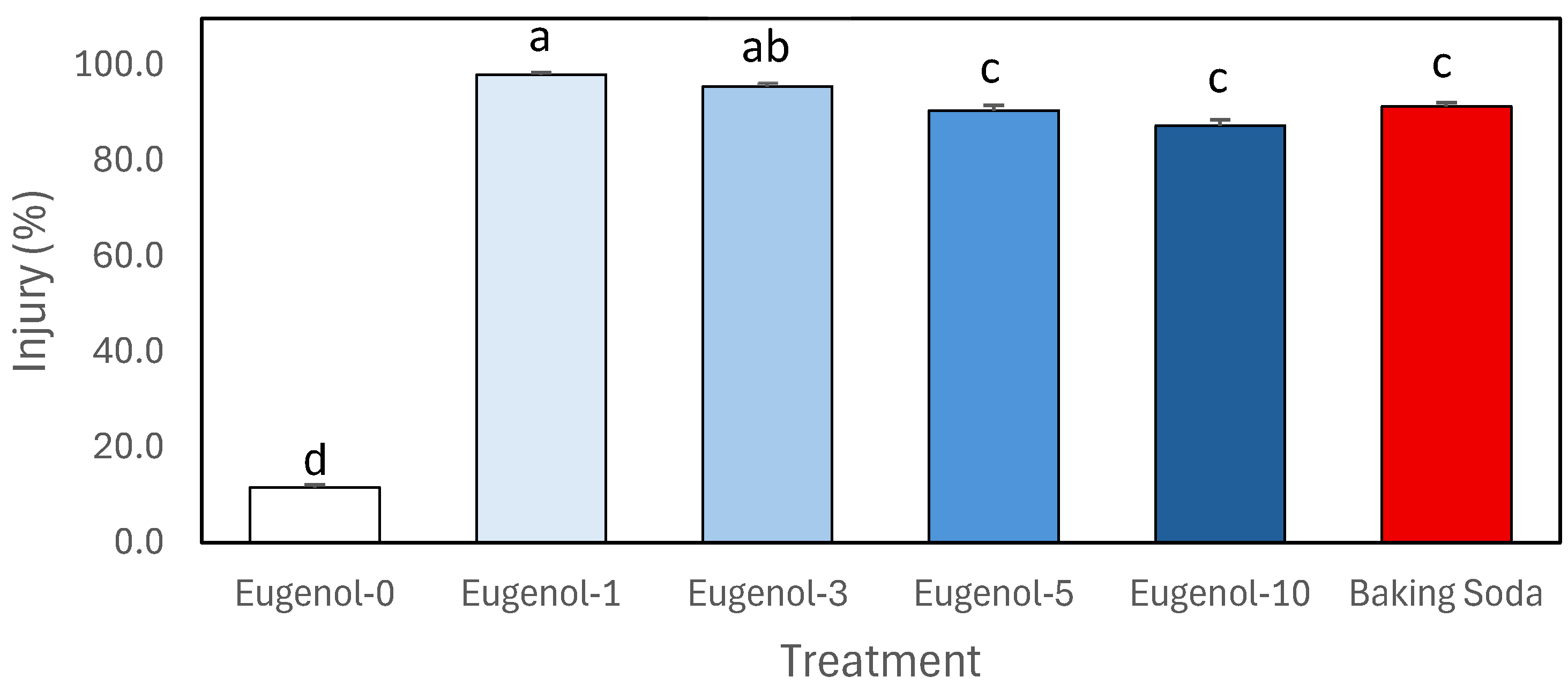
3.2. Dose Response Study
3.3. Outdoor Studies
3.4. Field Feasibility Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA, NRCS. The PLANTS Database: Tillandsia recurvata. 2021. Available online: https://plants.usda.gov/plant-profile/TIRE (accessed on 10 July 2025).
- Bogren, R.; Singh, R. Ball Moss Cropping up in Louisiana. 2015. Available online: https://www.lsuagcenter.com/portals/communications/news/news_archive/2015/february/headline_news/ball-moss-cropping-up-in-louisiana (accessed on 10 July 2025).
- Singh, R. Ballmoss. 2016. Available online: https://www.lsuagcenter.com/~/media/system/b/c/f/b/bcfbd6b48789d9c998694670db4a1b8d/pub%203421%20ballmoss_laplantpath_revpdf.pdf (accessed on 10 July 2025).
- Pérez-Noyola, F.J.; Flores, J.; Yáñez-Espinosa, L. Is ball moss (Tillandsia recurvata) a structural parasite of mesquite (Prosopis laevigata)? Anatomical and ecophysiological evidence. Trees 2021, 35, 135–144. [Google Scholar] [CrossRef]
- USDA, NRCS. The PLANTS Database: Tillandsia usneoides. 2021. Available online: https://plants.usda.gov/plant-profile/tius (accessed on 8 July 2025).
- Rogers, C.; Bush, E. Using Tillandsia recurvata (Ball Moss) as a Biological Indicator to Monitor Air Pollution and Retain Oil Pollution. Open J. Air Pollut. 2022, 11, 62–69. [Google Scholar] [CrossRef]
- Kirk-Ballard, H. What’s That Hanging in My Tree? 2020. Available online: https://www.lsuagcenter.com/profiles/rbogren/articles/page1583511527092 (accessed on 10 July 2025).
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wynn, S.G.; Fougère, B.J. Veterinary Herbal Medicine: A Systems-Based Approach. Vet. Herb. Med. 2009, 2007, 291–409. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cherian, S.; Ryu, S.B.; Cornish, K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant Biotechnol. J. 2019, 17, 2041–2061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahuja, N.A.; Batish, D.; Singh, H.P.; Kohli, R. Herbicidal activity of eugenol towards some grassy and broad-leaf weeds. J. Pest Sci. 2015, 88, 209–218. [Google Scholar] [CrossRef]
- Giannini, V.; Harris, J.R.; Todde, P.; McElroy, J.S. Concurrent weed growth suppression with essential oils and species-specific response to fractionated coconut oil. Ind. Crops Prod. 2022, 182, 114850. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Abdul-Raouf, U.M. Phytotoxic Interference of Culture Filtrates of Endophytic Bacteria Associated with Nerium oleander Leaf Against Seed Germination of the Invasive Noxious Weed Cenchrus echinatus. Curr. Microbiol. 2023, 80, 67. [Google Scholar] [CrossRef]
- O’Hara, F.M.; Liu, Z.; Davis, J.; Swale, D. Catalyzing systemic movement of inward rectifier potassium channel inhibitors for antifeedant activity against the cotton aphid, Aphis gossypii (Glover). Pest Manag. Sci. 2023, 79, 194–205. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, F.; McComic, S.; Cremades, A.; Norris, E.J.; Liu, Z.; Davis, J.A.; Bloomquist, J.; Swale, D. Characterization of N-arylamide insecticides to control populations of the green aphid, Myzus persicae. Pestic. Biochem. Physiol. 2025, 212, 106459. [Google Scholar] [CrossRef]
- Liu, Z. Terpene Glycosides and Their Combinations as Solubilizing Agents. U.S. Patent 8,551,507,B2, 8 October 2013. [Google Scholar]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stokłosa, A.; Matraszek, R.; Isman, M.B.; Upadhyaya, M.K. Phytotoxic Activity of Clove Oil, Its Constituents, and Its Modification by Light Intensity in Broccoli and Common Lambsquarters (Chenopodium album). Weed Sci. 2012, 60, 607–611. [Google Scholar] [CrossRef]
- Torres-Pagán, N.; Muñoz, M.; Barbero, S.; Mamone, R.; Peiró, R.; Carrubba, A.; Sánchez-Moreiras, A.M.; Gómez de Barreda, D.; Verdeguer, M. Herbicidal Potential of the Natural Compounds Carvacrol, Thymol, Eugenol, p-Cymene, Citral and Pelargonic Acid in Field Conditions: Indications for Better Performance. Agronomy 2024, 14, 537. [Google Scholar] [CrossRef]
- Somala, N.; Laosinwattana, C.; Teerarak, M. Formulation process, physical stability and herbicidal activities of Cymbopogon nardus essential oil-based nanoemulsion. Sci. Rep. 2022, 12, 10280. [Google Scholar] [CrossRef] [PubMed]
- Almarie, A. Bioherbicidal Potential of Eucalyptus and Clove Oil and their Combinations on Four Weedy Species. Iraqi. J. Sci. 2021, 62, 1494–1502. [Google Scholar] [CrossRef]
- Evans, G.J.; Bellinder, R.R.; Goffinet, M.C. Herbicidal Effects of Vinegar and a Clove Oil Product on Redroot Pigweed (Amaranthus retroflexus) and Velvetleaf (Abutilón theophrasti). Weed Technol. 2009, 23, 292–299. [Google Scholar] [CrossRef]
- Koleva, I.Z.; Kamenova, K.; Petrov, P.D.; Tzachev, C.T. Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying. Pharmaceuticals 2025, 18, 944. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Koh, G.Y.; Dong, X.; Hollingsworth, J.; Zhang, J.; Russo, P.S.; Yang, P.; Stout, R.W. Cytotoxic and antiangiogenic paclitaxel solubilized and permeation-enhanced by natural product nanoparticles. Anticancer Drugs 2015, 26, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Ginting, E.V.; Retnaningrum, E.; Widiasih, D.A. Antibacterial activity of clove (Syzygium aromaticum) and cinnamon (Cinnamomum burmannii) essential oil against extended-spectrum β-lactamase-producing bacteria. Vet. World 2021, 14, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osanloo, M.; Sedaghat, M.M.; Esmaeili, F.; Amani, A. Larvicidal Activity of Essential Oil of Syzygium aromaticum (Clove) in Comparison with Its Major Constituent, Eugenol, against Anopheles stephensi. J. Arthropod Borne Dis. 2018, 12, 361–369. [Google Scholar] [PubMed]
- Jyotsna, B.; Patil, S.; Prakash, Y.S.; Rathnagiri, P.; Kavi Kishor, P.B.; Jalaja, N. Essential oils from plant resources as potent insecticides and repellents: Current status and future perspectives. Biocatal. Agric. Biotechnol. 2024, 61, 103395. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Bainard, L.D.; Isman, M.B.; Upadhyaya, M.K. Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci. 2006, 54, 833–837. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Skoczowski, A. Early physiological response of broccoli leaf to foliar application of clove oil and its main constituents. Ind. Crops Prod. 2015, 74, 523–529. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, J.; Stout, R.W.; Russo, P.S.; Liu, Z. Solubilization of Paclitaxel with Natural Compound Rubusoside toward Improving Oral Bioavailability in a Rodent Model. Pharmaceutics 2024, 16, 1104. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Choi, S.H.; Ahn, J.Y.; Sohn, Y.G.; Kim, C.-G.; Lee, J.J. Herbicidal action of clove oil on cucumber seedlings. Weed Biol. Manag. 2011, 11, 235–240. [Google Scholar] [CrossRef]


| Treatment | Description | Active Ingredients (ai) | ai Concentration in Product | Solvent | ai Concentration in Spray Solution | Role |
|---|---|---|---|---|---|---|
| Copper fungicide | 3000-O Copper Fungicide | Copper hydroxide | 46.1% | Water | 0.68% | New agent |
| Herbicide | Fertilome | Sethoxydim | 18% | Water | 1.07% | New agent |
| Eugenol in ethanol | Eugenol 99% pure | 10% | 10% | Ethanol (90%) | 10% | New agent |
| Thymol in ethanol | Thymol 99% pure | 10% | 10% | Ethanol (90%) | 10% | New agent |
| Eugenol-SL | Eugenol soluble liquid (ESL) | 5% | 5% | Water | 5% | New agent |
| Thymol-SL | Thymol soluble liquid | 5% | 5% | Water | 5% | New agent |
| Oleander-L | Oleander leaf extract | Oleandrin | Unknown | Ethanol (90%) | Unknown | New agent |
| Oleander-T | Oleander twig extract | Oleandrin | Unknown | Ethanol (90%) | Unknown | New agent |
| Baking Soda | Baking Soda | sodium bicarbonate | 100% | Water | 50% | Positive control |
| Chemical | Injury (%) ± Standard Error of the Mean (n = 6) |
|---|---|
| Baking Soda | 100.00 ± 0.00 a |
| Copper fungicide | 7.2 ± 0.86 d |
| Herbicide | 5.2 ± 0.80 d |
| Eugenol in ethanol | 85.3 ± 1.48 bc |
| Thymol in ethanol | 83.7 ± 1.64 bc |
| Eugenol-SL | 93.7 ± 0.73 b |
| Thymol-SL | 91.0 ± 0.75 bc |
| Oleander-L ethanol | 90.8 ± 0.64 bc |
| Oleander-T ethanol | 87.8 ± 0.80 c |
| Ethanol 90% | 80.5 ± 0.99 c |
| Water | 6.8 ± 1.61 d |
| Season | Treatment | Injury (%) ± Standard Error of the Mean |
|---|---|---|
| Spring (n = 6) | 3% ESL | 53.7 ± 1.35 b |
| Baking Soda | 100.0 ± 0.00 a | |
| Water | 6.8 ± 2.68 c | |
| Summer (n = 6) | 3% ESL | 93.2 ± 1.75 b |
| Baking Soda | 100.0 ± 0.00 a | |
| Water | 5.5 ± 1.22 c | |
| Fall (n = 9) | 3% ESL | 98.8 ± 0.49 a |
| Baking Soda | 100 ± 0.00 a | |
| Water | 4.0 ± 1.66 b | |
| Winter (n = 8) | 3% ESL | 84.5 ± 3.59 a |
| Baking Soda | 100.0 ± 0.00 a | |
| Water | 6.7 ± 2.38 b |
| Live Oak | Branch Number | Treatment | Injury (%) ± Standard Error of the Mean (n = 12) |
|---|---|---|---|
| Tree 1—hand spray | One | 3% ESL | 99.1 ± 0.40 a |
| Untreated | 10.7 ± 5.00 b | ||
| Two | 3% ESL | 47.6 ± 11.73 a | |
| Untreated | 11.1 ± 3.72 b | ||
| Tree 2—broadcast | One | 3% ESL | 3.8 ± 0.55 a |
| Untreated | 4.2 ± 0.54 a | ||
| Two | 3% ESL | 3.3 ± 0.41 a | |
| Untreated | 2.9 ± 0.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slade, B.; Elftmann, K.; Kirk-Ballard, H.; Liu, Z. Soluble Eugenol Formulation for Managing Ball Moss on Ornamental Trees. Horticulturae 2025, 11, 1090. https://doi.org/10.3390/horticulturae11091090
Slade B, Elftmann K, Kirk-Ballard H, Liu Z. Soluble Eugenol Formulation for Managing Ball Moss on Ornamental Trees. Horticulturae. 2025; 11(9):1090. https://doi.org/10.3390/horticulturae11091090
Chicago/Turabian StyleSlade, Brianna, Kali Elftmann, Heather Kirk-Ballard, and Zhijun Liu. 2025. "Soluble Eugenol Formulation for Managing Ball Moss on Ornamental Trees" Horticulturae 11, no. 9: 1090. https://doi.org/10.3390/horticulturae11091090
APA StyleSlade, B., Elftmann, K., Kirk-Ballard, H., & Liu, Z. (2025). Soluble Eugenol Formulation for Managing Ball Moss on Ornamental Trees. Horticulturae, 11(9), 1090. https://doi.org/10.3390/horticulturae11091090







