Development of an Efficient Micropropagation Protocol for Philodendron erubescens ‘Pink Princess’ Using a Temporary Immersion System and Assessment of Genetic Fidelity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, In Vitro Establishment, and Chemicals
2.2. The Temporary Immersion Bioreactor (TIB) System Setup
2.3. Effect of Cytokinins on Shoot Induction of Philodendron ‘Pink Princess’
2.4. Effect of Auxins on Root Formation of Philodendron ‘Pink Princess’
2.5. Acclimatization of the Plantlets
2.6. Genetic Stability Assessment of the Plantlets
2.6.1. Genomic DNA Extraction
2.6.2. PCR Reaction and RAPD Analysis
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Effect of Cytokinins on Shoot Induction and Proliferation
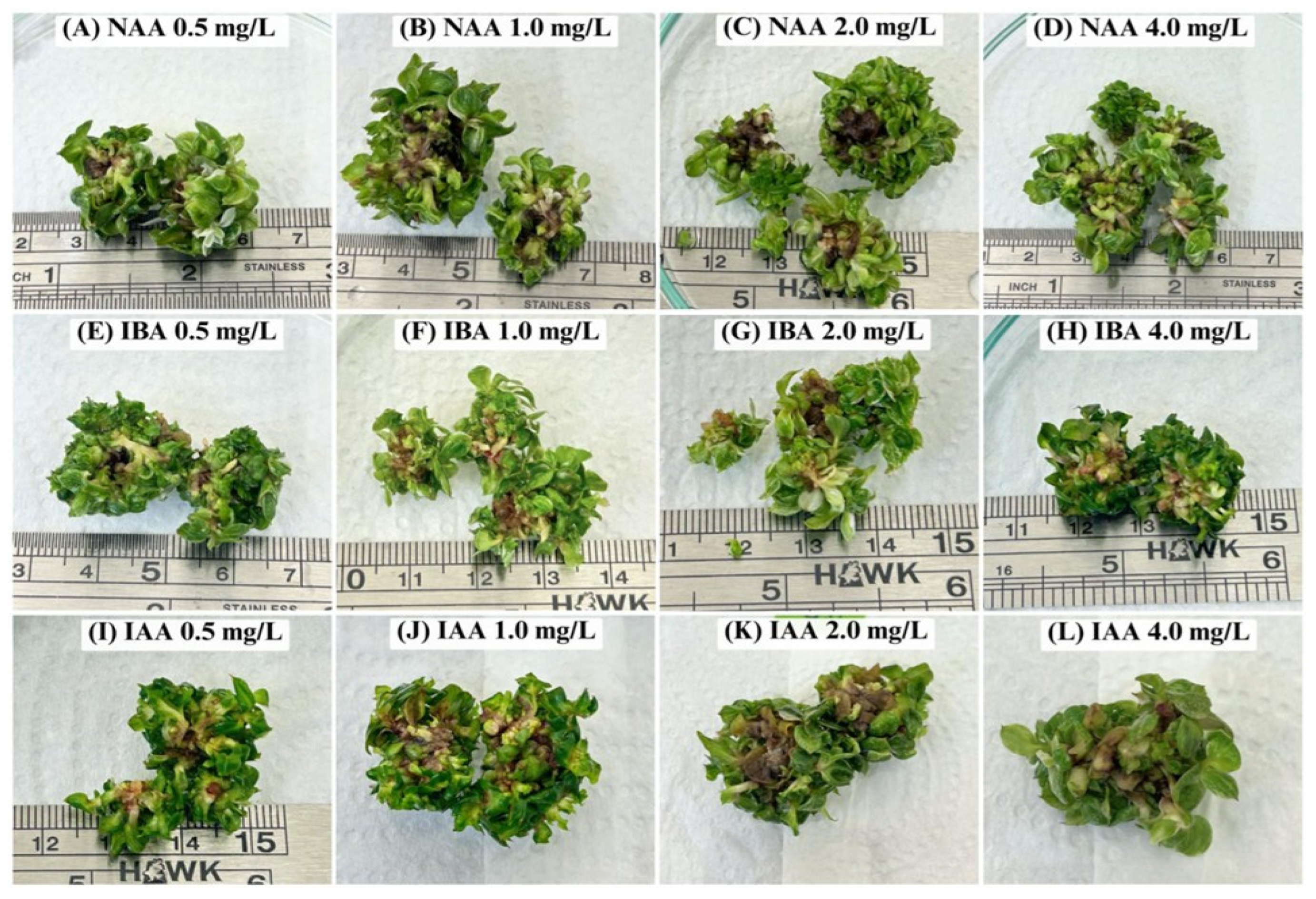
3.2. Effect of Auxin on Root Induction
3.3. Acclimatization of the Plantlets
3.4. Assessment of Genetic Stability of Micropropagated Plantlets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayo, S.J. A revision of Philodendron subgenus Meconostigma (Araceae). Kew Bull. 1991, 46, 601–681. [Google Scholar] [CrossRef]
- Croat, T.B. A revision of Philodendron subgenus Philodendron (Araceae) for Mexico and Central America. Ann. Mo. Bot. Gard. 1997, 84, 311–704. [Google Scholar] [CrossRef]
- Hovhannisyan, V.; Khachatryan, H. Ornamental plants in the United States: An econometric analysis of household-level demand system. Agribusiness 2017, 33, 226–241. [Google Scholar] [CrossRef]
- Chiewchan, N.; Saetiew, K.; Teerarak, M. The effect of BA on inducing shoots of Philodendron erubescens ‘Pink Princes’ in vitro. Int. J. Agric. Technol. 2023, 19, 2385–2398. [Google Scholar]
- Alawaadh, A.A.; Dewir, Y.H.; Alwihibi, M.S.; Aldubai, A.A.; El-Hendawy, S.; Naidoo, Y. Micropropagation of lacy tree Philodendron (Philodendron bipinnatifidum Schott ex Endl.). HortScience 2020, 55, 294–299. [Google Scholar] [CrossRef]
- Klanrit, P.; Kitwetcharoen, H.; Thanonkeo, P.; Thanonkeo, S. In Vitro propagation of Philodendron erubescens ‘Pink Princess’ and ex vitro acclimatization of the plantlets. Horticulturae 2023, 9, 688. [Google Scholar] [CrossRef]
- Yunita, R.; Nugraha, M.F.I. Effect of auxin type and concentration on the induction of Alternanthera Reineckii roots in vitro. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012073. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Han, B.H.; Park, B.M. In vitro micropropagation of Philodendron cannifolium. J. Plant Biotechnol. 2008, 35, 203–208. [Google Scholar] [CrossRef]
- Ziv, M.; Ariel, T. Bud proliferation and plant regeneration in liquid-cultured Philodendron treated with ancymidol and paclobutrazol. J. Plant Growth Regul. 1991, 10, 53–57. [Google Scholar] [CrossRef]
- Bozkurt, T.; İnan, S.; Dündar, İ. Comparison of temporary immersion bioreactor (SETISTM) and classical solid culture in micropropagation of ‘Grand Naine’ (Musa spp.) banana cultivar. J. Agric. Sci. 2023, 15, 51–60. [Google Scholar] [CrossRef]
- Gupta, S.D.; Prasad, V.S.S. Matrix-supported liquid culture systems for efficient micropropagation of floricultural plants. Floric. Ornam. Plant Biotechnol. Adv. Trop. Issue 2006, 2, 488–495. [Google Scholar]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB-silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Ruffoni, B.; Savona, M. The temporary immersion system (T.I.S) for the improvement of micropropagation of ornamental plants. Acta Hortic. 2005, 683, 445–454. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Cruz-Cruz, C.A.; Cano-Ricárdez, A.; Bello-Bello, J.J. Assessment of different temporary immersion systems in the micropropagation of anthurium (Anthurium andreanum). 3 Biotech 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Phulwaria, M.; Harish; Gupta, A.K.; Shekhawat, N.; Jaiswal, U. Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tiss. Org. Cult. 2012, 111, 259–264. [Google Scholar] [CrossRef]
- Gupta, A.K.; Harish; Rai, M.K.; Phulwaria, M.; Agarwal, T.; Shekhawat, N. In vitro propagation, encapsulation, and genetic fidelity analysis of Terminalia arjuna: A cardioprotective medicinal tree. Appl. Biochem. Biotechnol. 2014, 173, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Alwahibi, M.S.; Alawaadh, A.A.; Dewir, Y.H.; Soliman, D.A.; Seliem, M.K. Assessment of genetic fidelity of lacy tree Philodendron (Philodendron bipinnatifidum Schott ex Endl.) micropropagated plants. Revis Bionatura 2022, 7, 10. [Google Scholar]
- Al-Aizari, A.A.; Dewir, Y.H.; Ghazy, A.-H.; Al-Doss, A.; Al-Obeed, R.S. Micropropagation and genetic fidelity of Fegra Fig (Ficus palmata Forssk.) and grafting compatibility of the regenerated plants with Ficus carica. Plants 2024, 13, 1278. [Google Scholar] [CrossRef]
- Thanonkeo, S.; Kitwetcharoen, H.; Thanonkeo, P.; Klanrit, P. Temporary immersion bioreactor (TIB) system for large-scale micropropagation of Musa sp. cv Kluai Numwa Pakchong 50. Horticulturae 2024, 10, 1030. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Emery, R.J.N.; Kisiala, A. The roles of cytokinins in plants and their response to environmental stimuli. Plants 2020, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Khamrit, R.; Jongrungklang, N. Determining optimal mutation induction of Philodendron billietiae using gamma radiation and in vitro tissue culture techniques. Horticulturae 2024, 10, 1164. [Google Scholar] [CrossRef]
- Maikaeo, L.; Puripunyavanich, M.; Limtiyayotin, M.; Orpong, P.; Kongpeng, C. Micropropagation and gamma irradiation mutagenesis in Philodendron billietiae. Thai J. Agric. Sci. 2024, 57, 11–19. [Google Scholar]
- Kang, I.; Sivanesan, I. Micropropagation of Philodendron ‘White Knight’ via shoot regeneration from petiole explants. Plants 2025, 14, 1714. [Google Scholar] [CrossRef]
- Klaocheed, S.; Jehsu, W.; Choojun, W.; Thammasiri, K.; Prasertsongskun, S.; Rittirat, S. Induction of direct shoot organogenesis from shoot tip explants of an ornamental aquatic plant, Cryptocoryne wendtii. Walailak J. Sci. Technol. 2018, 17, 293–302. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Wiesman, Z.; Riov, J.; Epstein, E. Comparison of movement and metabolism of indole-3-acetic acid and indole-3-butyric acid in mung bean cuttings. Physiol. Plant. 1988, 74, 556–560. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, E.J.; Kim, E.A.; Jang, I.T.; Lee, S.; Nam, S.Y. Effects of different concentrations of exogenous auxins (IAA, IBA, and NAA) on growth and rooting ability of Philodendron hederaceum var. oxycardium (Schott) Croat stem cuttings. J. People Plants Environ. 2024, 27, 279–289. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Org. Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Hassan, H.M.S.; Ali, M.A.M.; Soliman, D.A. Effect of low-cost gelling agents and some growth regulators on micropropagation of Philodendron selloum. J. Plant Prod. 2016, 7, 169–176. [Google Scholar] [CrossRef][Green Version]
- Akramian, M.; Khaleghi, A.R.; Salehi-Arjmand, H. Optimization of plant growth regulators for in vitro mass propagation of Philodendron cv. Birkin through shoot tip culture. Greenh. Plant Prod. J. 2024, 1, 55–62. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Hausman, J. Changes in peroxidase activity, auxin level and ethylene production during root formation by poplar shoots raised in vitro. Plant Growth Regul. 1993, 13, 263–268. [Google Scholar] [CrossRef]
- Epstein, E.; Ludwig-Müller, J. Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Epstein, E.; Sagee, O.; Zelcer, A. Uptake and metabolism of indole-3-butyric acid and indole-3-acetic acid by petunia cell suspension culture. Plant Growth Regul. 1993, 13, 31–40. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, N.; Anis, M. The role of cytokinins on in vitro shoot production in Salix tetraspera Roxb.: A tree of ecological importance. Trees 2011, 25, 577–584. [Google Scholar] [CrossRef]
- Abdalla, N.; Ragab, M.; El-Miniawy, S.; Arafa, N.; Taha, H. A new aspect for in vitro propagation of Jerusalem artichoke and molecular assessment using RAPD, ISSR and SCoT marker techniques. Egypt. J. Bot. 2021, 61, 203–218. [Google Scholar] [CrossRef]
- Gonbad, R.A.; Moghaddam, S.S.; Sinniah, U.R.; Aziz, M.A.; Safarpour, M. Determination of potting media for effective acclimatization in micropropagated plants of tea clone Iran 100. Int. J. For. Soil Erosion. 2013, 3, 40–44. [Google Scholar]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Effects of supporting materials in in vitro acclimatization stage on ex vitro growth of wasabi plants. Sci. Hortic. 2020, 261, 109042. [Google Scholar] [CrossRef]
- Erol, M.H.; Dönmez, D.; Biçen, B.; Şimşek, Ö.; Kaçar, Y.A. Modern approaches to in vitro clonal banana production: Next-generation tissue culture systems. Horticulturae 2023, 9, 1154. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S. Evaluation of temporary immersion bioreactors for in vitro micropropagation of banana (Musa spp.) and genetic fidelity assessment using flow cytometry and simple-sequence repeat markers. S. Afr. J. Bot. 2023, 157, 553–565. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Lal, D.; Singh, N. Mass multiplication of Celastrus paniculatus Willd: An important medicinal plant under in vitro conditions via nodal segments. Int. J. Biodivers. Conserv. 2010, 2, 140–145. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Premvaranon, P.; Vearasilp, S.; Thanapornpoonpong, S.N.; Karladee, D.; Gorinstein, S. In vitro studies to produce double haploid in Indica hybrid rice. Biologia 2011, 66, 1074–1081. [Google Scholar] [CrossRef]
- Gantait, S.; Mandal, N.; Bhattacharyya, S.; Das, P.K. In vitro mass multiplication with pure genetic identity in Anthurium andreanum Lind. Plant Tissue Cult. Biotechnol. 2009, 18, 113–122. [Google Scholar] [CrossRef]
- EL-Banna, A.N.; Khatab, I.A. Assessing genetic diversity of some potato (Solanum tuberosum L.) cultivars by protein and RAPD markers. Egypt. J. Genet. Cytol. 2013, 42, 89–101. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd EL-Aziz, M.; Abd ELwahed, M.S.; Shaaban, E. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Venkataramareddy, S.R.; Neelwarne, B. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotechnol. 2007, 10, 106–113. [Google Scholar] [CrossRef]
- Cabo, S.; Ferreira, L.; Carvalho, A.; Martins-Lopes, P.; Martín, A.; Lima-Brito, J.E. Potential of start codon targeted (SCoT) markers for DNA fingerprinting of newly synthesized tritordeums and their respective parents. J. Appl. Genet. 2014, 55, 307–312. [Google Scholar] [CrossRef]
- Fang-Yong, C.; Ji-Hong, L. Germplasm genetic diversity of Myrica rubra in Zhejiang province studied using inter-primer binding site and start codon-targeted polymorphism markers. Sci. Hortic. 2014, 170, 169–175. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle-an endemic and endangered medicinal plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef] [PubMed]



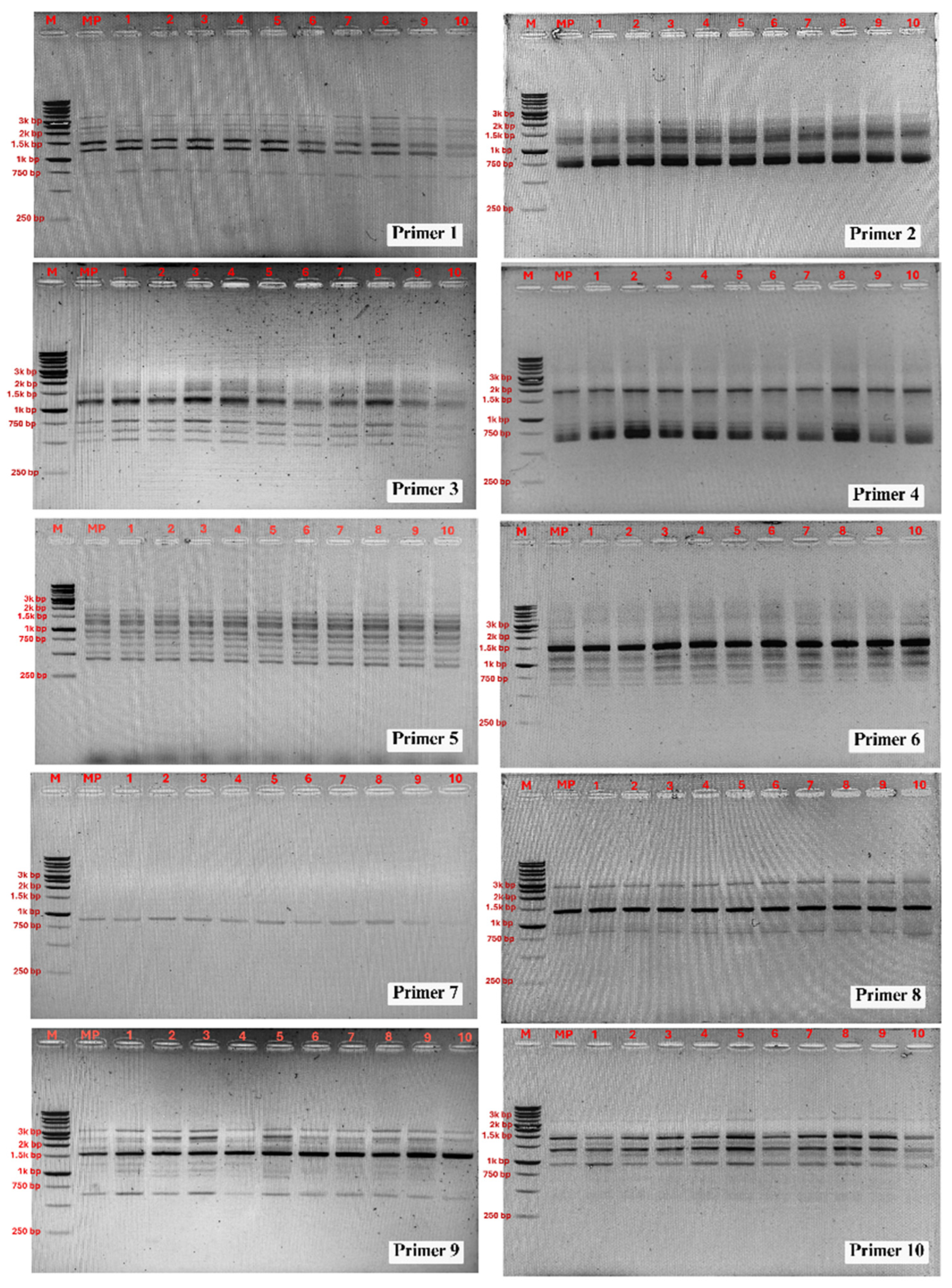
| Primer Code | Primer Sequence (5′→3′) | Number of Scorable Bands | Size of Amplicon (bp) |
|---|---|---|---|
| RAPD 1 | CAGGCCTTTC | 7 | 750–3500 |
| RAPD 2 | AGTCAGCCAC | 8 | 750–2500 |
| RAPD 3 | AGGGGTCTTG | 7 | 500–2000 |
| RAPD 4 | GGGTAACGCC | 6 | 650–2000 |
| RAPD 5 | TCGGCGATAG | 10 | 480–3200 |
| RAPD 6 | CAGCACCCAC | 10 | 500–3000 |
| RAPD 7 | TTCCGAACCC | 1 | 900 |
| RAPD 8 | AGCCAGCGAA | 4 | 750–3500 |
| RAPD 9 | GACCGCTTGT | 10 | 650–3200 |
| RAPD 10 | GTGCAACGTG | 10 | 400–3500 |
| Total | 73 |
| Treatment | Concentration (mg/L) | Number of Shoots (Shoots/Explant) | Shoot Length (cm) |
|---|---|---|---|
| Control (MS without hormone) | 0 | 19.70 ± 2.08 abc | 2.05 ± 0.20 cde |
| BAP | 0.5 | 20.40 ± 1.41 abc | 2.08 ± 0.16 cde |
| 1.0 | 20.90 ± 1.75 abc | 2.38 ± 0.23 ab | |
| 2.0 | 21.87 ± 2.08 a | 2.43 ± 0.17 a | |
| 4.0 | 20.40 ± 1.91 abc | 2.16 ± 0.13 bcde | |
| 8.0 | 20.40 ± 2.28 abc | 2.08 ± 0.11 cde | |
| Kn | 0.5 | 16.07 ± 2.31 de | 2.22 ± 0.20 abcd |
| 1.0 | 16.67 ± 2.26 de | 2.29 ± 0.21 abc | |
| 2.0 | 15.93 ± 2.77 de | 2.10 ± 0.14 cde | |
| 4.0 | 15.80 ± 3.30 de | 2.14 ± 0.19 bcde | |
| 8.0 | 14.00 ± 2.65 e | 2.03 ± 0.14cde | |
| TDZ | 0.5 | 18.70 ± 2.68 bcd | 2.16 ± 0.15 bcde |
| 1.0 | 18.40 ± 2.11 bcd | 1.90 ± 0.25 e | |
| 1.5 | 16.50 ± 1.82 de | 2.02 ± 0.15 de | |
| 2.0 | 17.87 ± 2.02 cd | 1.98 ± 0.13 de |
| Treatment | Concentration (mg/L) | Number of Roots (Roots/Explant) | Root Length (cm) |
|---|---|---|---|
| Control (MS without hormone) | 0 | 0.00 ± 0.00 e | 0.00 ± 0.00 f |
| NAA | 0.5 | 0.00 ± 0.00 f | 0.00 ± 0.00 f |
| 1.0 | 1.00 ± 0.00 e | 0.70 ± 0.21 cd | |
| 2.0 | 1.40 ± 0.55 de | 0.84 ± 0.28 bc | |
| 4.0 | 1.00 ± 0.00 e | 0.53 ± 0.18 de | |
| IBA | 0.5 | 1.81 ± 0.36 cd | 0.75 ± 0.34 cd |
| 1.0 | 2.53 ± 0.55 b | 0.93 ± 0.25 bc | |
| 2.0 | 2.52 ± 0.51 b | 0.96 ± 0.25 abc | |
| 4.0 | 2.96 ± 0.49 a | 1.21 ± 0.48 a | |
| IAA | 0.5 | 3.03 ± 0.36 a | 1.05 ± 0.30 ab |
| 1.0 | 2.47 ± 0.63 b | 0.78 ± 0.41 bcd | |
| 2.0 | 2.19 ± 0.55 bc | 0.68 ± 0.44 cd | |
| 4.0 | 2.17 ± 0.71 bc | 0.35 ± 0.15 e |
| Planting Substrate | Survival (%) | Growth Parameters | |||
|---|---|---|---|---|---|
| Number of Leaves (Leaves/Plantlet) | Number of Roots (Roots/Plantlet) | Root Length (cm) | Plant Height (cm) | ||
| 30 days | |||||
| PPV (2:1:1) | 100 ± 0.00a | 7.90 ± 0.99 a | 6.40 ± 0.70 a | 2.20 ± 0.57 a | 1.55 ± 0.20 a |
| PV (2:1) | 90 ± 31.62a | 7.22 ± 0.67 a | 5.11 ± 0.78 b | 2.08 ± 0.58 a | 1.34 ± 0.16 b |
| PP (2:1) | 90 ± 31.62a | 6.00 ± 1.73 b | 3.89 ± 0.78 c | 1.13 ± 0.33 b | 1.20 ± 0.20 b |
| 45 days | |||||
| PPV (2:1:1) | 100 ± 0.00a | 9.20 ± 1.55 a | 8.40 ± 2.41 a | 2.74 ± 0.74 a | 1.85 ± 0.33 a |
| PV (2:1) | 90 ± 31.62a | 8.11 ± 1.45 ab | 5.67 ± 1.22 b | 2.57 ± 0.96 a | 1.53 ± 0.17 b |
| PP (2:1) | 90 ± 31.62a | 7.33 ± 1.94 b | 4.89 ± 0.93 b | 1.22 ± 0.66 b | 1.37 ± 0.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vy, B.K.; Klanrit, P.; Thanonkeo, S.; Thanonkeo, P. Development of an Efficient Micropropagation Protocol for Philodendron erubescens ‘Pink Princess’ Using a Temporary Immersion System and Assessment of Genetic Fidelity. Horticulturae 2025, 11, 1085. https://doi.org/10.3390/horticulturae11091085
Vy BK, Klanrit P, Thanonkeo S, Thanonkeo P. Development of an Efficient Micropropagation Protocol for Philodendron erubescens ‘Pink Princess’ Using a Temporary Immersion System and Assessment of Genetic Fidelity. Horticulturae. 2025; 11(9):1085. https://doi.org/10.3390/horticulturae11091085
Chicago/Turabian StyleVy, Bui Khanh, Preekamol Klanrit, Sudarat Thanonkeo, and Pornthap Thanonkeo. 2025. "Development of an Efficient Micropropagation Protocol for Philodendron erubescens ‘Pink Princess’ Using a Temporary Immersion System and Assessment of Genetic Fidelity" Horticulturae 11, no. 9: 1085. https://doi.org/10.3390/horticulturae11091085
APA StyleVy, B. K., Klanrit, P., Thanonkeo, S., & Thanonkeo, P. (2025). Development of an Efficient Micropropagation Protocol for Philodendron erubescens ‘Pink Princess’ Using a Temporary Immersion System and Assessment of Genetic Fidelity. Horticulturae, 11(9), 1085. https://doi.org/10.3390/horticulturae11091085







