Micropropagation and Acclimatization of Hypericum aucheri
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Surface Sterilization
2.2. Shoot Induction and Multiplication
2.3. Root Induction
2.4. Acclimatization
2.5. Statistical Analysis
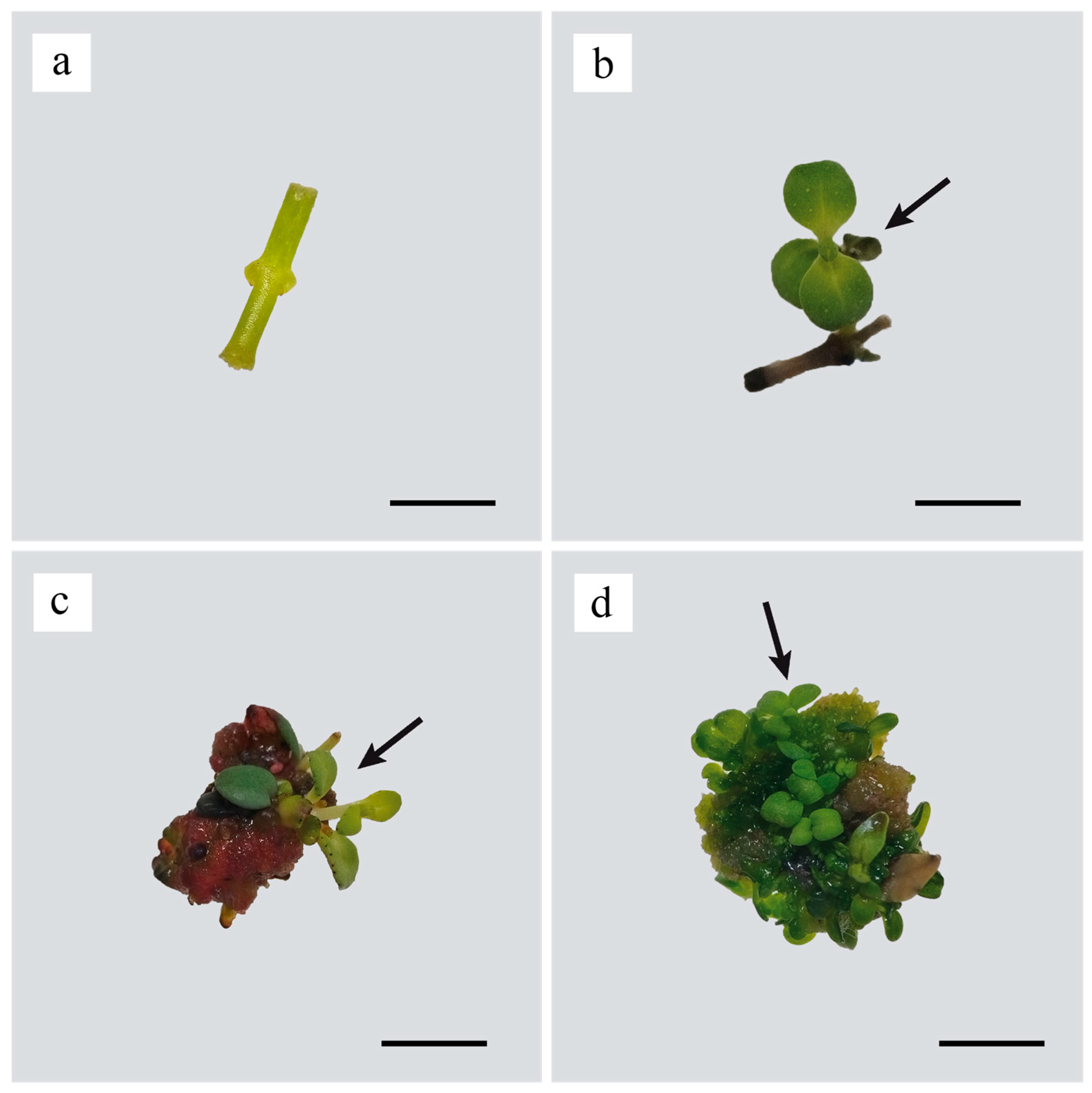
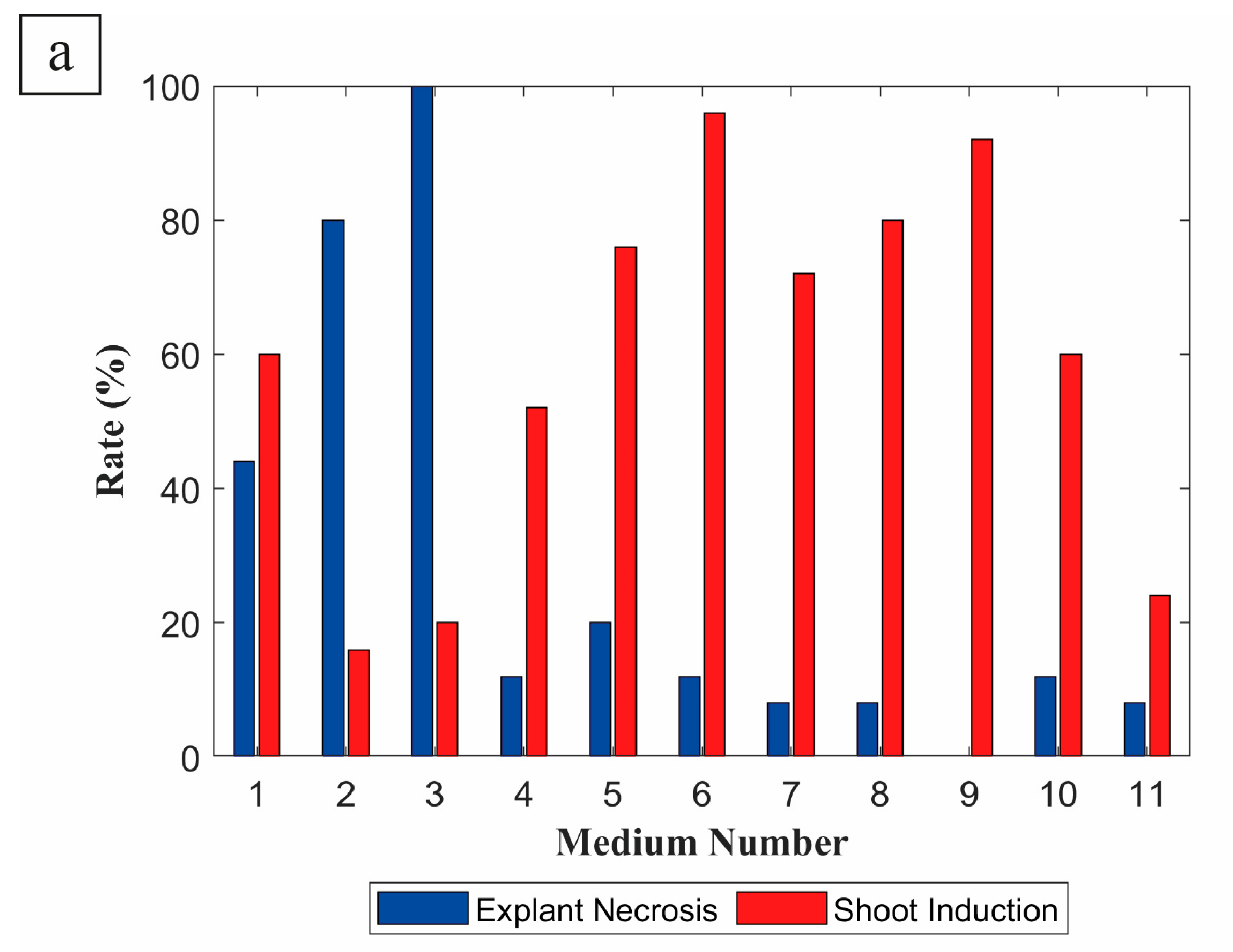
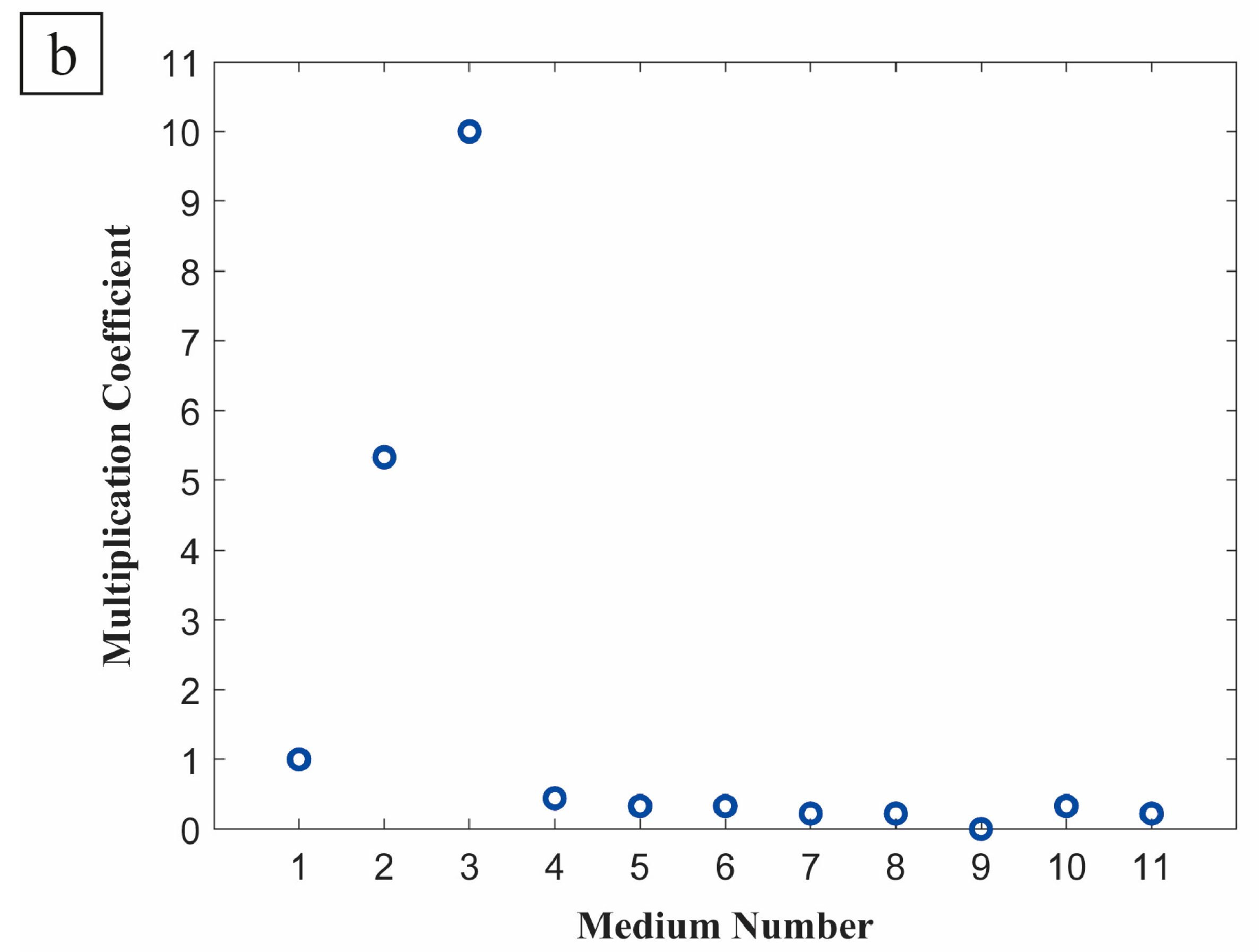
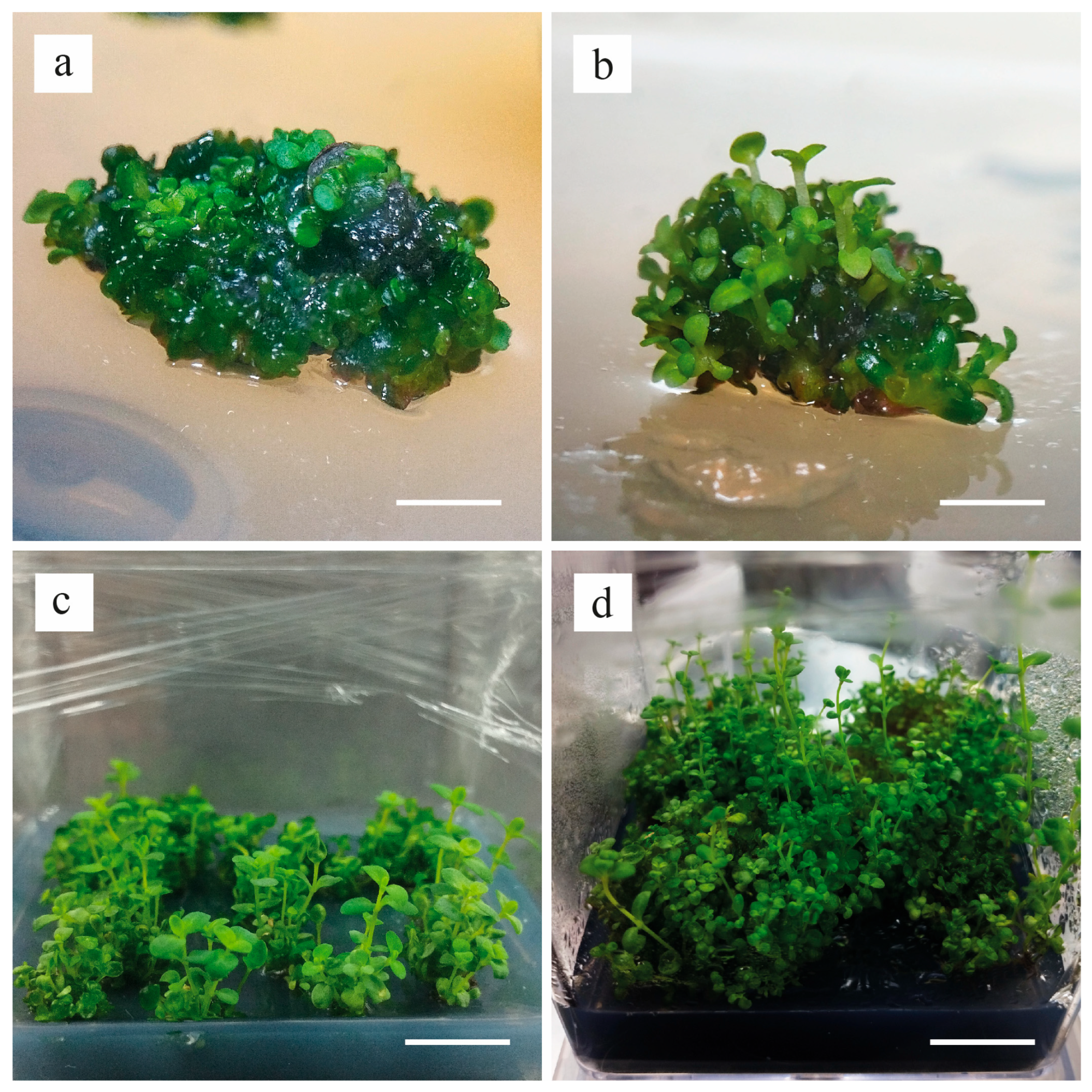
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Babacan, E.Y.; Bagci, E. Essential oil composition of Hypericum uniglandulosum Hausskn. ex Bornm. and Hypericum lydium Boiss. from Turkey. Int. J. Nat. Life Sci. 2017, 1, 12–16. [Google Scholar]
- POWO (Plants of the World Online). Hypericum L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002180-2 (accessed on 2 December 2024).
- Nürk, N.M.; Crockett, S.L. Morphological and phytochemical diversity among Hypericum species of the Mediterranean basin. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 14–28. [Google Scholar]
- Ersoy, E.; Özkan, E.E.; Boğa, M.; Mat, A. Evaluation of in vitro biological activities of three Hypericum species from Turkey. S. Afr. J. Bot. 2020, 130, 141–147. [Google Scholar] [CrossRef]
- Baytop, T. Therapy with Medicinal Plants in Turkey; Istanbul University Press: Istanbul, Turkey, 1999; pp. 66–167. [Google Scholar]
- Cirak, C.; Radusiene, J.; Jakstas, V.; Ivanauskas, L.; Seyis, F.; Yayla, F. Secondary metabolites of seven Hypericum species growing in Turkey. Pharm. Biol. 2016, 54, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Statti, G.; Conforti, F.; Menichini, F. New potential pharmaceutical applications of Hypericum species. Mini-Rev. Med. Chem. 2016, 16, 710–720. [Google Scholar] [CrossRef]
- Avato, P. A survey of the Hypericum genus: Secondary metabolites and bioactivity. Stud. Nat. Prod. Chem. 2005, 30, 603–634. [Google Scholar] [CrossRef]
- Bingol, U.; Cosge, B.; Gurbuz, B. Hypericum species in flora of Turkey. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 86–90. [Google Scholar]
- Senturk, H.; Kabay, S.; Ozden, H.; Bayramoglu, G.; Ustuner, M.C.; Ozturk, N.; Guven, G.; Kutlu, A.; Bilgi, G.; Ustuner, D.; et al. protective effect of Hypericum origanifolium in experimental renal ischemia/reperfusion injury in rats. Afr. J. Pharm. Pharmacol. 2013, 7, 2306. [Google Scholar] [CrossRef]
- Özkan, E.E.; Mat, A. An overview on Hypericum species of Turkey. J. Pharmacogn. Phytother. 2013, 5, 38–46. [Google Scholar] [CrossRef]
- Warnick, S.J., Jr.; Mehdi, L.; Kowalkowski, J. Wait—There’s evidence for that? Integrative medicine treatments for major depressive disorder. Int. J. Psychiatry Med. 2021, 56, 334–343. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Toma, V.A.; Sevastre, B.; Hanganu, D.; Vlase, L.; Benedec, D.; Oniga, I.; Baldea, I.; Olteanu, D.; Moldovan, R.; et al. Characterization and biological effects of Hypericum extracts on experimentally-induced anxiety, oxidative stress and inflammation in rats. J. Physiol. Pharmacol. 2018, 69, 789–800. [Google Scholar] [CrossRef]
- McFadden, S.L.; Hooker, B.L. Comparing perika St. John’s wort and sertraline for treatment of posttraumatic stress disorder in mice. J. Diet. Suppl. 2020, 17, 300–308. [Google Scholar] [CrossRef]
- Robson, N.K.B. Studies in the Genus Hypericum L. (Hypericaceae) 5(1). Sections 10. Olympia to 15/16. Crossophyllum. Phytotaxa 2010, 4, 5–126. [Google Scholar] [CrossRef]
- Robson, N.K.B. Studies in the Genus Hypericum L. (Hypericaceae) 9. Addenda, corrigenda, keys, lists and general discussion. Phytotaxa 2012, 72, 1–111. [Google Scholar] [CrossRef]
- Özbek, M.U.; Koç, M.; Hamzaoğlu, E. Contributions to the Hypericum L. section Oligostema (Boiss.) Stef. (Hypericaceae), and Hypericum turcicum sp. nov. as a New Species from Turkey. Turk. J. Bot. 2019, 43, 694–702. [Google Scholar] [CrossRef]
- Efe, R.; Sönmez, S.; Cürebal, İ.; Soykan, A. Ecological Conditions and Vegetation of Subalpine Zone of Kaz Mountain (Mount Ida, NW Turkey). In Climate Change Impacts on High-Altitude Ecosystems; Öztürk, M., Hakeem, K., Faridah-Hanum, I., Efe, R., Eds.; Springer: Cham, Switzerland, 2015; pp. 591–608. [Google Scholar] [CrossRef]
- Carta, A.; Probert, R.; Puglia, G.; Peruzzi, L.; Bedini, G. Local climate explains degree of seed dormancy in Hypericum elodes L. (Hypericaceae). Plant Biol. 2016, 18, 76–82. [Google Scholar] [CrossRef]
- Klein, J.D.; Cohen, S.; Hebbe, Y. Seasonal variation in rooting ability of myrtle (Myrtus communis L.) cuttings. Sci. Hortic. 2000, 83, 71–76. [Google Scholar] [CrossRef]
- Heywood, V.H.; Iriondo, J.M. Plant conservation: Old problems, new perspectives. Biol. Conserv. 2003, 113, 321–335. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Volume 1. The Background, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Pence, V.C. The possibilities and challenges of in vitro methods for plant conservation. Kew Bull. 2010, 65, 539–547. [Google Scholar] [CrossRef]
- Gürel, A.; Hayta, Ş.; Nartop, P.; Bayraktar, M.; Fedakar, S.O. Bitki Hücre, Doku ve Organ Kültürü Uygulamaları; Ege Üniversitesi Basımevi: İzmir, Turkey, 2013; pp. 47–66. [Google Scholar]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2021, 63, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Malik, C. Applications of biotechnology innovations in pharmaceutics and nutraceutics in multitherapeutic medicinal and special plants. In Pharmaceutics and Nutraceutics; Singh, K., Jahdon, M.L., Singh, D., Eds.; Aavishkar Publishers: Jaipur, India, 2007; Volume II, pp. 243–265. [Google Scholar]
- Jamsheed, S.; Rasool, S.; Koul, S.; Azooz, M.M.; Ahmad, P. Crop Improvement through Plant Tissue Culture. In Crop Improvement: New Approaches and Modern Techniques; Hakeem, K.R., Ahmad, P., Ozturk, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 123–148. [Google Scholar] [CrossRef]
- Khan, I.; Khan, M.A.; Shehzad, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Alyemeni, M.N.; Ahmad, P. Micropropagation and production of health-promoting lignans in Linum usitatissimum. Plants 2020, 9, 728. [Google Scholar] [CrossRef]
- Pretto, F.R.; Santarém, E.R. Callus formation and plant regeneration from Hypericum perforatum leaves. Plant Cell Tissue Organ Cult. 2000, 62, 107–113. [Google Scholar] [CrossRef]
- Ayan, A.K.; Cirak, C. In vitro multiplication of Hypericum heterophyllum, an endemic Turkish species. Am. J. Plant Physiol. 2006, 1, 76–81. [Google Scholar]
- Palmer, C.D.; Keller, W.A. Plant regeneration from petal explants of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2011, 105, 129–134. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandyopadhyay, S.; Raychaudhuri, S.S. In vitro regeneration of Hypericum perforatum L. using thidiazuron and analysis of genetic stability of regenerants. Indian J. Biotechnol. 2012, 11, 92–98. [Google Scholar]
- Mir, M.Y.; Kamili, A.N.; Hassan, Q.P.; Rafi, S.; Parray, J.A.; Jan, S. In vitro regeneration and free radical scavenging assay of Hypericum perforatum L. Natl. Acad. Sci. Lett. 2019, 42, 161–167. [Google Scholar] [CrossRef]
- Baruah, A.; Sarma, D.; Saud, J.; Singh, R.S. In vitro regeneration of Hypericum patulum Thunb.—A medicinal plant. Indian J. Exp. Biol. 2001, 39, 947–949. [Google Scholar] [PubMed]
- Karakas, O.; Toker, Z.; Tilkat, E.; Ozen, H.C.; Onay, A. Effects of different concentrations of benzylaminopurine on shoot regeneration and hypericin content in Hypericum triquetrifolium Turra. Nat. Prod. Res. 2009, 23, 1459–1465. [Google Scholar] [CrossRef]
- Shilpashree, H.P.; Rai, R. In vitro plant regeneration and accumulation of flavonoids in Hypericum mysorense. Int. J. Integr. Biol. 2009, 8, 43–49. [Google Scholar]
- Oluk, E.; Orhan, S. Thidiazuron induced micropropagation of Hypericum triquetrifolium Turra. Afr. J. Biotechnol. 2009, 8, 3506–3510. [Google Scholar]
- Akbas, F.; Isikalan, C.; Namli, S.; Karakus, P.; Basaran, D. Direct plant regeneration from in vitro-derived leaf explants of Hypericum spectabile, a medicinal plant. J. Med. Plants Res. 2011, 5, 2175–2181. [Google Scholar]
- Cirak, C.; Radušienė, J.; Kurtarc, E.S.; Marksa, M.; Ivanauskas, L. In vitro plant regeneration and jasmonic acid induced bioactive chemical accumulations in two Hypericum species from Turkey. S. Afr. J. Bot. 2020, 128, 312–318. [Google Scholar] [CrossRef]
- Yamaner, O.; Erdağ, B. Callus induction and adventitious shoot regeneration of Hypericum adenotrichum Spach. Eskişehir Tek. Üniversitesi Bilim Ve Teknol. Derg.-C Yaşam Bilim. Ve Biyoteknoloji 2020, 9, 98–108. [Google Scholar] [CrossRef]
- Turker, H.; Unal, B.T. Development of a micropropagation protocol for endangered Hypericum bilgehan-bilgilii Başkose & Savran (Hypericaceae), local endemic to Turkey. Pak. J. Bot. 2022, 54, 1089–1095. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Blinova, K.F.; Akhtardzhiev, K.; Rumenin, V. Flavonoids of Hypericum aucheri. Chem. Nat. Compd. 1979, 15, 760–761. [Google Scholar] [CrossRef]
- Kitanov, G.M. Biflavone, Flavonol, and Xanthone Glycosides from Hypericum aucheri. Chem. Nat. Compd. 1988, 24, 390–391. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical Scavenging and Antioxidant Activities of Methanolic Extracts from Hypericum Species Growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef]
- Krasteva, I.; Nedelcheva, A.; Pavlova, D.; Zdraveva, P.; Nikolov, S.; Mitov, K. Influence of Serpentine Soils on the Flavonoid Content of Hypericum Populations Growing in Bulgaria. Afr. J. Pharm. Pharmacol. 2013, 7, 1762–1765. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Ilieva, Y.; Zheleva-Dimitrova, D.; Kokanova-Nedialkova, Z.; Momekov, G. Three New Prenyloxy Chromanones from Aerial Parts of Hypericum aucheri. Fitoterapia 2019, 139, 104421. [Google Scholar] [CrossRef] [PubMed]
- Marinov, T.; Kokanova-Nedialkova, Z.; Nedialkov, P. UHPLC-HRMS-Based Profiling and Simultaneous Quantification of the Hydrophilic Phenolic Compounds from the Aerial Parts of Hypericum aucheri Jaub. & Spach (Hypericaceae). Pharmacia 2024, 71, 1–11. [Google Scholar] [CrossRef]
- Dimitrov, M.; Nikolova, I.; Benbasat, N.; Kitanov, G.; Danchev, N. Acute Toxicity, Antidepressive and MAO Inhibitory Activity of Mangiferin Isolated from Hypericum aucheri. Biotechnol. Biotechnol. Equip. 2011, 25, 2668–2671. [Google Scholar] [CrossRef]
- Marinov, T.; Kondeva-Burdina, M.; Kokanova-Nedialkova, Z.; Nedialkov, P.T. Phenolic Constituents from Hypericum aucheri Jaub. & Spach—Isolation, Identification, and Preliminary Evaluation for hMAO-A/B and Neuroprotective Activity. Chemistry 2024, 6, 1535–1551. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Paek, K.Y.; Yu, K.J.; Park, S.I.; Sung, N.; Park, C. Micropropagation of Rehmannia glutinosa as medicinal plant by shoot tip and root segment culture. Acta Hortic. 1995, 390, 113–120. [Google Scholar] [CrossRef]
- Borthakur, M.; Singh, R.S. Direct plantlet regeneration from male inflorescence of medicinal yam (Dioscorea floribunda Mart. & Gal.). Vitr. Cell. Dev. Biol.-Plant 2002, 38, 183–185. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Brown, D.C.; Saxena, P.K.; Moo-Young, M. Improvement of ginseng by in vitro culture: Challenges and opportunities. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, MA, USA, 2011; Volume 2, pp. 317–329. [Google Scholar]
- Önlü, Ş.; Yaman, C.; Kurtul, E.; Önlü, H.; Bahadir-Acikara, Ö.; Tusevski, O.; Simic, S.G.; Özcan, S. Production of elicitor-induced phytochemicals in callus and shoot cultures of Hypericum heterophyllum. S. Afr. J. Bot. 2025, 177, 295–304. [Google Scholar] [CrossRef]
- Saliwan, S.; Saensouk, S.; Saensouk, P. In vitro propagation of Kaempferia koratensis Picheans., an endemic plant of Thailand. Koch Cha Sarn J. Sci. 2022, 44, 1–13. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Vivanco, J.M. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2002, 60, 289–293. [Google Scholar] [CrossRef]
- Ayan, A.K.; Çirak, C.; Kevseroğlu, K.; Sökmen, A. Effects of explant types and different concentrations of sucrose and phytohormones on plant regeneration and hypericin content in Hypericum perforatum L. Turk. J. Agric. For. 2005, 29, 197–204. [Google Scholar]
- Ahmed, H.A.A.; Uranbey, S.; Salaj, T.; Mistrikova, V. Cell Suspension Cultures and High Frequency Shoot Regeneration of Some Hypericum species. J. Agri. Sci. 2025, 31, 319–331. [Google Scholar] [CrossRef]
- Namli, S.; Akbas, F.; Isikalan, C.; Tilkat, E.A.; Basaran, D. The Effect of Different Plant Hormones (PGRs) on Multiple Shoots f’Hypericum retusum Aucher. Plant Omics 2010, 3, 12–17. [Google Scholar]
- Swain, D.; Lenka, S.; Hota, T.; Rout, G.R. Micro-propagation of Hypericum gaitii Haines, an endangered medicinal plant: Assessment of genetic fidelity. Nucleus 2016, 59, 7–13. [Google Scholar] [CrossRef]
- Cirak, C.; Ayan, A.K.; Kevseroğlu, K. Direct and indirect regeneration of plants from internodal and leaf explants of Hypericum bupleuroides Gris. J. Plant Biol. 2007, 50, 24–28. [Google Scholar] [CrossRef]
- Asan, H.S.; Ozen, H.C.; Onay, A.; Nurettin, A. Effect of different BAP concentrations on proliferation and hypericin content of Hypericum retusum Aucher. Biol. Sci. Res. J. 2014, 7, 10–14. [Google Scholar]
- Varghese, R.J.; Bayyapureddy, A.; Pushparaj, S.P.; Seeni, S. A high-efficiency in vitro regeneration protocol and clonal uniformity analysis in Hypericum hookerianum Wight & Arn. Braz. J. Bot. 2016, 39, 377–386. [Google Scholar] [CrossRef]
- Bernardi, A.P.M.; Maurmann, N.; Rech, S.B.; Von Poser, G. Benzopyrans in Hypericum polyanthemum Klotzsch ex Reichardt cultured in vitro. Acta Physiol. Plant. 2007, 29, 165–170. [Google Scholar] [CrossRef]
- Coste, A.; Halmagyi, A.; Butiuc-Keul, A.L.; Deliu, C.; Coldea, G.; Hurdu, B. In vitro propagation and cryopreservation of Romanian endemic and rare Hypericum species. Plant Cell Tissue Organ Cult. 2012, 110, 213–226. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Savio, L.E.B.; Astarita, L.V.; Santarém, E.R. Secondary metabolism in micropropagated Hypericum perforatum L. grown in non-aerated liquid medium. Plant Cell Tissue Organ Cult. 2012, 108, 465–472. [Google Scholar] [CrossRef]
- Oluk, E.A.; Orhan, S.; Çakir, A.; Gönüz, A. High efficiency indirect shoot regeneration and hypericin content in embryogenic callus of Hypericum triquetrifolium Turra. Afr. J. Biotechnol. 2010, 9, 2229–2233. [Google Scholar]
- Shilpashree, H.P.; Rai, V.R. Effect of plant growth regulators on callus formation, plant regeneration and hypericin production in Hypericum mysorense Hyne. Int. J. Plant Dev. Biol. 2010, 4, 31–36. [Google Scholar]
- Abdollahpoor, M.; Kalantari, S.; Azizi, M.; Saadat, Y.A. In vitro shoot proliferation of Hypericum perforatum L. through indirect and direct plant regeneration. J. Med. Plants By-Prod. 2017, 6, 81–89. [Google Scholar] [CrossRef]
- Cui, X.H.; Chakrabarty, D.; Lee, E.J.; Paek, K.Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Hypericins: Biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef] [PubMed]
- Shiao, T.L.; Doran, P.M. Root hairiness: Effect on fluid flow and oxygen transfer in hairy root cultures. J. Biotechnol. 2000, 83, 199–210. [Google Scholar] [CrossRef]
- Cristea, T.O.; Falticeanu, M.; Prisecaru, M. Studies regarding the morphogenetic reaction of Hypericum perforatum L. explants to in vitro medium conditions. Studii şi Comunicări Compl. Muz. Şt. Nat. „Ion Borcea” Bacău. 2006, 21, 164–167. [Google Scholar]
- Peng, L.C.; Qu, S.P.; Su, Y.; Zhang, Y.P.; Wang, L.H. Study on rapid propagation of Hylocereus undatus by two steps. Southwest China J. Agric. Sci. 2014, 27, 2529–2533. [Google Scholar]
- Máximo, W.P.F.; Santos, B.R.; Martins, J.P.R.; Beijo, L.A.; Barbosa, S. Multiplication and in vitro rooting of Handroanthus impetiginosus (Mart. Ex DC.) Mattos. Cienc. Florest. 2020, 30, 658–668. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, J.C. Development of an improved micropropagation protocol for red-fleshed pitaya ‘Da Hong’ with and without activated charcoal and plant growth regulator combinations. Horticulturae 2022, 8, 104. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Piri, K.; Abdoli, A.; Tohidfar, M. Effect of activated charcoal on in vitro propagation of Lythrum salicaria L. J. Med. Plants By-Prod. 2024, 13, 293–302. [Google Scholar]
- Hazarika, B.N.; Teixeira da Silva, J.A.; Talukdar, A. Effective acclimatization of in vitro cultured plants: Methods, physiology and genetics. In Floriculture, Ornamental and Plant Biotechnology; Teixeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2006; Volume 2, pp. 427–438. [Google Scholar]
- Onlu, S. In Vitro Plant Regeneration and Secondary Metabolite Production in Hypericum Species. Ph.D. Thesis, Department of Field Crops, University of Ankara, Ankara, Turkey, 2019; 50p. [Google Scholar]

| Medium No | PGRs (mg L−1) | ||
|---|---|---|---|
| BAP | KIN | 2,4-D | |
| 1 | 0 | 0 | 0 |
| 2 | 2.0 | 0 | 0 |
| 3 | 0 | 2.0 | 0 |
| 4 | 0 | 1.0 | 0.5 |
| 5 | 0 | 1.0 | 1.0 |
| 6 | 0 | 2.0 | 0.5 |
| 7 | 0 | 2.0 | 1.0 |
| 8 | 1.0 | 0 | 0.5 |
| 9 | 1.0 | 0 | 1.0 |
| 10 | 2.0 | 0 | 0.5 |
| 11 | 2.0 | 0 | 1.0 |
| Replicate No | Number of Shoots per Explant |
Shoot Length (cm) | Number of Nodes per Shoot | Number of Leaves per Shoot |
|---|---|---|---|---|
| 1 | 90.00 ± 9.60 a | 1.49 ± 0.07 b | 2.83 ± 0.18 a | 3.06 ± 0.16 b |
| 2 | 87.40 ± 11.11 a | 1.27 ± 0.05 a | 1.96 ± 0.13 b | 2.30 ± 0.10 a |
| 3 | 92.40 ± 16.34 a | 1.39 ± 0.05 ab | 2.03 ± 0.16 a | 3.03 ± 0.16 b |
| Means | 89.93 ± 6.80 | 1.38 ± 0.03 | 2.27 ± 0.10 | 2.80 ± 0.09 |
| Root Induction Medium | Rooting Rate (%) | Number of Roots per Shoot | Root Length (cm) | Root Morphology |
|---|---|---|---|---|
| 1 mg L−1 IAA | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | - |
| 1 mg L−1 NAA 1 mg L−1 PVP | 76.00 ± 9.79 b | 2.04 ± 0.29 b | 0.55 ± 0.05 b | Hairy |
| 1 mg L−1 NAA 1 mg L−1 AC | 100.00 ± 0.00 c | 2.28 ± 0.20 b | 4.16 ± 0.31 c | Normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambaz, E.; Çördük, N. Micropropagation and Acclimatization of Hypericum aucheri. Horticulturae 2025, 11, 1069. https://doi.org/10.3390/horticulturae11091069
Cambaz E, Çördük N. Micropropagation and Acclimatization of Hypericum aucheri. Horticulturae. 2025; 11(9):1069. https://doi.org/10.3390/horticulturae11091069
Chicago/Turabian StyleCambaz, Ebru, and Nurşen Çördük. 2025. "Micropropagation and Acclimatization of Hypericum aucheri" Horticulturae 11, no. 9: 1069. https://doi.org/10.3390/horticulturae11091069
APA StyleCambaz, E., & Çördük, N. (2025). Micropropagation and Acclimatization of Hypericum aucheri. Horticulturae, 11(9), 1069. https://doi.org/10.3390/horticulturae11091069





