Abstract
Granadilla (Passiflora ligularis) is a fruit species native to the Ecuadorian Andes. This study identifies agromorphological variables of interest that allow the selection of elite individuals from native populations in the provinces of Carchi, Imbabura, Pichincha, Cotopaxi, Tungurahua, Chimborazo, and Loja, these being the provinces where P. ligularis crops are grown. Fruits from 50 genotypes (10 fruits of each phenotype were characterized) collected from different farms were analyzed, evaluating 19 variables between fruit and seed. Fruit weight (55.5–112.5 g), polar and equatorial fruit diameter (5.60–7.52 cm, 5.61–7.18 cm), fleshy ring weight (4.9–12.3 g), seeded pulp weight (26.9–56.6 g), total seed weight (4.9–10.6 g), the number of seeds per fruit (93–307), seedless pulp weight (20.2–50.9 g), seed perimeter (1.84–2.08 cm), pH (4.28–5.28), and °Brix (10.25–16.10) differentiated the genotypes of P. ligularis. Spearman’s correlation analysis (p ≤ 0.05) among quantitative morphoagronomic variables showed that there were positive correlations among the distance of the polar diameter of the fruit (PD), the total seed weight (TSW) (r = 0.82), and the weight of the peel or epicarp (SHW) (r = 0.70). The weight of the fleshy ring (FRW) correlated with the weight of the fruit (W) (r = 0.80) and the weight of the pulp with seeds (WPWS) (r = 0.80). The weight of the pulp with seeds (WPWS) correlated with the weight of the fruit (W) (r = 0.94) and the weight of the seedless pulp (PW) (r = 0.96). The cluster analysis of the quantitative variables of the fruit classified the genotypes into four groups, without taking into account the collection area. The descriptors W, PD, ED, WPW, PW, TSW, NS, SP, pH, and °Brix allow differentiation of P. ligularis genotypes. Therefore, the superior phenotypic traits (size and weight) observed in the CAR14 and LY001 genotypes should be used as breeding options for crop improvement.
1. Introduction
The Andean region, geographically, is a mountain range, with a length of about 8500 km, from Chile to Venezuela, passing through countries of Argentina, Peru, Bolivia, Ecuador, and Colombia, with altitudes between 3000 and 4000 m above sea level, surrounding the coastal area of the Pacific Ocean [1]. Its width varies from 250 to 750 km and it occupies an area of about 2,870,000 km2 [2]. The tropical Andes represent 15% of the total world plant diversity, standing out for being a region with very high biodiversity [3,4], and many fruit species are native to these South American areas [5]. Izquierdo and Roca [6] characterized this “ecoregion” as one of the most fragile and misunderstood, where more than 60 million people live, half of them working in the fields and many of them with very low incomes.
Tropical fruits have been gaining importance in recent decades due to their high content of nutrients, minerals, vitamins, and sugars that contribute to the nutritional requirements of the human population [7]. According to Food and Agriculture Organization of the United Nations (FAO) [8], the production and marketing of fresh tropical fruits will increase in the next decade and developing countries will continue to produce 98% of this production. The FAO estimates a 9.6 percent increase in tropical fruit production in the coming years [9]. Tropical fruits occupy a unique niche in the global agricultural sector, contributing 62% of the world’s fresh fruit supply [10]. Currently, Asia is the largest producer in tropical fruit production with approximately 86% of global production added during the triennium 2015–2017 [11].
Tropical fruits, with their year-round production and supply, offer possibilities for expanding crops and increasing exports [12]. However, much remains to be carried out to ensure their proper cultivation and to understand their agroclimatic requirements. Thus, the development of Andean fruit crops is seen as an essential and healthy contribution to global food consumption [13]. Many of these “exotic” fruits are classified as important functional foods [4,14], not only in their countries of origin but also for populations in higher-latitude regions [15], resulting in an excellent export opportunity for many Andean countries [16].
Passifloraceae are a family of plants with a pantropical distribution, comprising 17 genera and about 660 species. In America they are represented by four genera and around 500 species, most of them from the genus Passiflora [17]. The largest number of species of the genus Passiflora is found in the Andean region and according to Hernández and Bernal [18], about 72% are located in areas with altitudes above 1500 m above sea level. South America has become a biological and commercial source of this genus of plants, achieving economic exploitation [19,20] of species such as Passiflora edulis Sims. var. flavicarpa (passion fruit), Passiflora ligularis Juss. (granadilla), P. tarminiana var. mollisima (curuba), and P. edulis Sims. (gulupa), due to their market plasticity either as fresh fruit or processed foods.
Granadilla (P. ligularis) is native to tropical America, extending from Mexico to Peru in South America; the name of the species (ligularis) is due to the glands, very elongated petiolate and liguliform ones, called ligules that cover the base of the leaf [21]. Due to its exquisite sweet and aromatic flavor, granadilla is a fruit widely accepted for fresh consumption; the sweet and pleasant juice is consumed with the seeds. Granadilla (P. ligularis) is classified within the group of “secondary tropical fruits”, which due to their production and importance in marketing could occupy a niche in the markets.
This species is the second most economically important of the Passiflora genus, after passion fruit (P. edulis), because it is marketed in national and international markets for its pleasant flavor and nutritional value (vitamin C, vitamin A, potassium, magnesium, calcium) [22,23]. The main producers globally are Colombia, Peru, and Ecuador, where it is cultivated by small farmers in specific locations, mainly in hillside areas and croplands no larger than 1.5 ha on average [24,25].
In Ecuador, passion fruit is cultivated in areas with a moderate cold climate, with average annual temperatures between 15 and 20 °C. It requires soils with a depth of at least 80 cm; that are well drained; with loam, sandy loam, or clayey loam textures; with a good organic matter content; and with a pH between 6 and 6.5. It is cultivated in areas located between 800 and 2600 m above sea level, in the provinces of Imbabura, Pichincha, Tungurahua, Azuay, Carchi, and Loja [26]. Each of these provinces has geographic, climatic, and agricultural characteristics that can significantly influence the characteristics of Passiflora ligularis (granadilla) fruit, such as size, flavor, yield, and quality. Some provinces, such as Imbabura, Pichincha, and Cotopaxi, have cooler, wetter climates, ideal for high-altitude crops, while provinces like Loja have warmer climates, which can influence fruit type and productivity. The water demand of granadilla varies between 1500 and 3200 mm, well distributed throughout the year [27].
According to the research carried out by Navarrete [28], the passion fruit in the province of Imbabura–Ecuador reaches an annual production of 4.5 to 6.0 t per hectare, considered low, since in Colombia yields between 10 and 14 t per hectare are reported [29], one of the main causes of low yields being inadequate nutritional management [30]. By 2024 the main important markets were Spain, Hong Kong, and Canada, where the price remained at 5.6 USD per kilogram; in this year, passion fruit exports reached 2000 t with a value of USD 7 million [31].
This study aims to characterize the fruit of 50 P. ligularis genotypes and their endocarps from accessions distributed in the Andean Region of Ecuador. Characterizing P. ligularis fruit will provide a deeper understanding of the morphological variation in this species, which will aid in the establishment of future breeding programs through crossbreeding to obtain superior genotypes with potential for international trade.
2. Materials and Methods
2.1. Characterization of the Area
This research was carried out where P. ligularis has a high distribution density, this being the provinces of Carchi, Imbabura, Pichincha, Cotopaxi, Tungurahua, Cotopaxi, Chimborazo, and Loja located in the mountain systems of the Andes of Ecuador (Figure 1). In each of the provinces, sampling sites were selected mainly where there are P. ligularis crops (because these crops are not distributed homogeneously throughout Ecuador, these crops are mainly influenced by climatic characteristics, height, and slope); these crop sites generally did not exceed two hectares. The height of the collection sites ranged between 1687 and 2986 m.a.l.s, with a slope between 5 and 30 degrees and the three-bolillo planting system being the most common. From each sample site, 5 adult plants were selected, one corresponding to the center of the cropland and others corresponding to each side of the cropland. From each plant, 10 fruits at maturity for consumption were collected, which were stored in airtight plastic bags with their respective labels (location, code, X, Y, Z coordinates, collection date). In addition, each plant was georeferenced using a GPS (Garmin Oregon 650 t, Olathe, KS, USA). The fruit re-harvest was carried out during the months of January–May 2024, because these are the months where the production is highest.
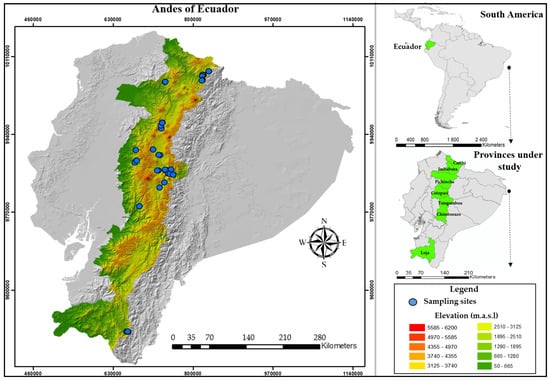
Figure 1.
Location of the study area in South America and Ecuador.
2.2. Morphological Analysis
The collected samples (50 genotypes, 10 fruits were collected from each genotype) were transferred to the Plant Production Laboratory of the Polytechnic School of Chimborazo (ESPOCH) in Riobamba, Ecuador, to evaluate fruit weight (W), polar fruit diameter (PD), equatorial fruit diameter (ED), peel width (SWE), peel or epicarp weight (SHW), fleshy ring weight (FRW) (Figure 2), seeded pulp weight (WPW), total seed weight (TSW), the number of seeds per fruit (NS), seedless pulp weight (PW), the mucilage weight of 10 seeds (MW), seed weight (WOS), equatorial seed diameter (EDS), polar seed diameter (PDS), seed width (SW), seed area (SA), seed perimeter (SP), pH, and soluble solid content (TSS, °Brix). The 19 variables were characterized for the 10 fruits of each genotype.

Figure 2.
Parts of the fruit of P. ligularis.
All fruit size measurements were completed using a digital caliper (electronic Vernier Digital® 0–150 mm, INSIZE, Shenzhen, China). Weights were measured using a precision digital balance (Radwag WTC 2000, Radwag Wagi Elektroniczne, Radom, Poland). TSS was measured using a handheld refractometer (Extech RF15, Extech Instruments, Nashua, NH, USA) from the juice obtained from the pulp. For seed measurements, due to their small size, they were characterized using a stereomicroscope (LEICA S-APO, LEICA, Wetzlar, Germany).
2.3. Statistical Analysis
Analyses of variance were performed for all fruit parameters for each province. The relationship between variables was determined by Spearman’s rank correlation analysis (Spearman’s analysis was used because it is a suitable tool for exploring whether there is a general association, regardless of whether it is linear or not) [32], using R software version 4.3.3 [33]. Principal component analysis (PCA) was based on the correlation matrix between the characteristics, which were graphed in a two-dimensional plane to group the genotypes with R Core Team. The distance matrix was constructed using the Euclidean distance for cluster analysis. The minimum Ward distance method was used as a clustering method, where each cluster was given by the smallest increase in the value of the total sum of the squares of the differences existing within each cluster of each observation for the cluster centroid. Additionally, a dendrogram was generated using the Euclidean distance method and hierarchical clustering.
3. Results and Discussion
Factors such as temperature, solar radiation, altitude, precipitation, wind, and atmospheric pressure are factors that influence Andean crops, as is the case of P. ligularis, which develop in complex and extreme environmental conditions, which is why many of the species have unique adaptations [34,35]. The tropics do not have temperature seasons as marked as those in temperate zones, so the wet and dry seasons define the seasons to which plants react physiologically [36]. P. ligularis plantations are found in inter-Andean valleys and on mountain slopes, where the topography is varied but suitable for cultivation. These valleys have a steep geography, which favors water drainage and prevents waterlogging. The microclimate also plays a crucial role, since many times the cultivation areas are protected from strong winds by the surrounding mountains (Figure 3).

Figure 3.
Andean rural landscape where P. ligularis is present: (A). Gruta de la Paz (Carchi province). (B). Monte Olivo (Imbabura province). (C). Yangana (Loja province).
3.1. Morphometric Characteristics
Granadilla (P. ligularis) is one of the most important fruits in Latin America, containing carbohydrates, vitamins, minerals, fiber, antioxidants, and phytochemicals; this makes it not only the economic base of various regions but also a source of health and well-being [37].
The characteristics of the fruits of 50 genotypes of P. ligularis collected in the mountain systems of the central Andes of Ecuador in 2024 were evaluated. Significant accession differences were identified for fruit weight, fleshy ring weight, pulp weight with seeds, total seed weight, the number of seeds per fruit, seedless pulp weight, seed perimeter, pH, and °Brix.
FW ranged from 66.5 g (Pichincha) to 112.4 g (Imbabura); the highest values were identified in the CAR14 fruit sample with 156.0 g corresponding to the province of Carchi, and the lowest weight was recorded in the PICH1 ascension (52.2 g) corresponding to the province of Pichincha. The highest values of our study are higher than those characterized Mora et al. [38], in characterization studies of fruits from two producing areas of Mexico, in which the weight did not exceed 75 g. De Borrero et al. [39] mentions, in his study carried out in the Municipality of San Eduardo (Boyacá), that the average weight identified was 102.6 g. On the contrary, Linares et al. [5] and Ocampo et al. [29] reported that FW ranges between 113 g and 143 g.
The epicarp was hard, smooth, and orange in color (Appendix A); the weight varied, in the provinces studied, between 30.4 and 49.1 g, with Pichincha being where the lowest value was identified and Tungurahua having the highest value (Table 1). The width of the epicarp ranged between 1.44 mm and 4.73 mm; the peel occupied approximately 44.53% of the total fruit; the weight of the fleshy ring ranged between 4.9 and 12.2 g, corresponding to the samples of Pichincha and Imbabura, respectively; and the fleshy ring had the function of covering the seeds and pulp [40]. According to Mora et al. [38], the percentage of peel of P. ligularis fruit corresponds to 38.38% of the total fruit. Linares et al. [5] mention that 60% of the fruit is edible and 40% corresponds to the peel and seed. For Miranda et al. [41], in their study on the integral management of the cultivation of granadilla (P. ligularis), cultivation, post-harvest, and marketing of passionflowers in Colombia determined that the average weight of the peel is 63.8 g. Carvajal et al. [42], in their study on the relationship between the popular uses of granadilla (P. ligularis) and its phytochemical composition, mention that the epicarp is used as nutritional feed for sheep because it provides 374.86 calories/kg and is 4.37% protein, taking into consideration that a significant intake of crude fiber (50.77%) is added simultaneously.

Table 1.
Morphological characteristics of P. ligularis fruits from growing areas of Ecuador.
The average polar diameter of the fruits varied from 5.60 to 7.52 cm. The most outstanding diameter was recorded in the genotype LY001 (8.61 cm), corresponding to the province of Loja; the smallest polar diameter was observed in the sample CAR06 (4.96 cm), corresponding to the province of Carchi (Table 1). For the equatorial diameter of the fruit, the values between the provinces ranged from 5.6 to 7.18 cm. The study by Miranda et al. [41], on P. ligularis in Colombia, mentions that the polar and equatorial diameters of the fruit range between 8 and 7 cm, respectively. Melgarejo et al. [43], in a study on the ecophysiological characterization of the crop in Colombia, determined that the average size of the polar and equatorial diameters is between 8 and 6.8 cm. De Borrero et al. [39] and García [40] mention that the polar and equatorial diameters of the fruit of P. ligularis in production areas range between 6 and 7 cm, respectively. These studies present higher values than those identified in the present study; this may be because in Colombia the intensive cultivation of granadilla with more than 1500 ha was established from the 1990s, which allows the country to be a pioneer in management and selection of superior genotypes to date. In the present study, values were higher than those reported in previous research conducted by Aguilar and Garza [44] and Mora et al. [38], where it was determined that the polar and equatorial diameters of P. ligularis range between 5.6 and 7.20 cm, respectively.
The juicy, transparent, sweet, and aromatic pulp, with a pleasant taste [40], is full of hard, blackish seeds, surrounded by a gelatinous, transparent, light-gray aril, with a very aromatic acidulous flavor; it contains vitamins A, C, and K, phosphorus, iron, and calcium [45]. The weight of the pulp in our study ranged between 26.8 and 56.5 g, occupying approximately 38.78% of the fruit weight; the weight of the seeds varied between 4.88 and 10.6 g, occupying 7.8% of the fruit weight; and the number of seeds identified in provinces ranged between 134.78 and 306 units. Mora et al. [38], in their study on the “Preliminary characterization of Chinese pomegranate fruits (P. ligularis) in Hueyapan and Teziutlán, Puebla-Mexico”, mention that the pulp occupies 35% of the fruit weight and the seeds occupy 25.33%, and the number of seeds is 248 per fruit. García [40], Castro et al. [37], and Rivera et al. [21] mention that the fruit of P. ligularis is composed of around 250 small edible seeds, dark brown or black, wrapped by a grayish, translucent, mucilaginous, and acidic ring that constitutes the edible part.
The seeds of P. ligularis are small, hard, blackish in color, and surrounded by a light gray gelatinous aril also called mucilage whose weight does not exceed 0.09 g. The equatorial diameter varied, in the provinces studied, between 0.37 and 0.45 cm, respectively (Table 1); the polar diameter of the endocarp ranged between 0.60 and 0.71 cm; and the weight ranged between 0.03 and 0.06 g, being representative of the samples from the province of Tungurahua. The perimeter of the endocarp varied between 1.84 and 2.06 cm (Table 1), the width ranged between 0.16 and 0.19 cm, with no significant difference between the provinces, and the area of the seed varied between 18.11 and 20.27 mm2. According to Cárdenas et al. [46], in their study on morphological and anatomical analysis of the seed coats of P. ligularis, they determined that the polar diameter ranged between 0.65 and 0.74 cm, the equatorial diameter was 0.39–0.44 cm, and the weight varied between 0.02 and 0.04 g. These data are similar to those identified in our research. López et al. [47] indicate that the seed could also be used in animal feed due to its protein and fat content.
TSS and °Brix in fruits are mainly composed of sugars dissolved in the cell juice. The main sugars in fruit juices are sucrose, glucose, and fructose, which account for around 75% of TSS [48]. The increase in soluble solids is a well-defined characteristic in the ripening of all fruits, which occurs as a response to the breakdown of larger polysaccharides such as starches stored in vacuoles and intercellular spaces during fruit growth [49,50]. In P. ligularis, °Brix varied between 10.25 and 16.10. De Borrero et al. [39] and Viera et al. [13] mention that °Brix degrees in P. ligularis range between 14.5 and 19.47 °Brix, these values being higher than those identified in our study. Espinosa et al. [51], in their study on the granadilla of Huila in Colombia, mention that the average °Brix degree is 13.3° and also mention that this value corresponds to the fruit that reaches commercial maturity. According to Verona [52], the flavor of the fruit depends on the maturity during its harvest, an important factor for the concentration of sugars to be higher and in this way for the total soluble solids to increase.
The flavor present in the pulp is related to the pH value [53]; in our study, the pH varied between 4.28 and 5.44, being higher than that in the records of Fonseca and Ospina [54], in their study on characterization of P. ligularis fruit in Colombia where the average pH ranged around 4.64. In addition, De Borrero et al. [39], in their study on the physical characterization and post-harvest behavior of P. ligularis, managed to determine that the average pH was 4.6.
3.2. Correlation Analysis
Spearman correlations (p ≤ 0.05) between variables are displayed in a correlation network graph (Figure 4). Phenotypic correlations indicate the linear dependence between two characteristics and depend on the interaction between the genotypes and the environment. Their magnitudes are determined by the degree of association between the two variables. It can be seen in the graph that the variables MW, SW, SA, WOS, ED, and NS are the variables that show the lowest correlations with the rest of the variables and are shown in the graph as quite distant from the rest. In turn, the other quantitative morphoagronomic variables show that there are moderately strong positive correlations between the polar diameter of the fruit (PD) and the total seed weight (TSW) (r = 0.82), polar diameter of the seed (PDS) (r = 0.58), pH (r = 0.55), seed width (SW) (0.59), and peel or epicarp weight (SHW) (r = 0.70). The weight of the fleshy ring (FRW) correlates with the weight of the fruit (W) (r = 0.80), weight of the pulp with seeds (WPWS) (r = 0.80), weight of the seedless pulp (PW) (r = 0.72), seed perimeter (PW) (r = 0.72), and seed perimeter (SP) (r = 0.56). The weight of the pulp with seeds (WPWS) correlates with the weight of the fruit (W) (r = 0.94), weight of the fleshy ring (FRW) (r = 0.80), weight of the seedless pulp (PW) (r = 0.96), and seed perimeter (SP) (r = 0.55). These correlations show the strong interaction that exists between all the variables analyzed in the present study and how these associations between the variables are decisive when characterizing the fruit of P. ligularis.
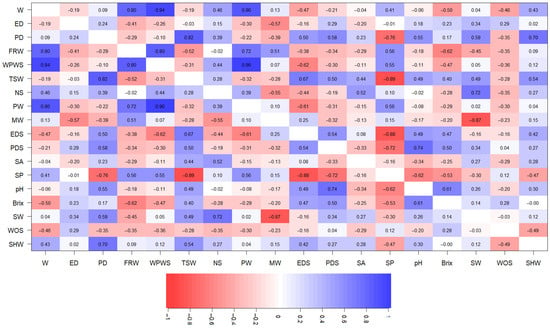
Figure 4.
Correlation established between all the morphometric variables of P. ligularis. Fruit weight (W), fruit polar diameter (PD), fruit equatorial diameter (ED), epicarp weight (SHW), fleshy ring weight (FRW), seed pulp weight (WPWS), total seed weight (TSW), the number of seeds per fruit (NS), seedless pulp weight (PW), 10-seed mucilage weight (MW), seed weight (WOS), seed equatorial diameter (EDS), seed polar diameter (PDS), seed width (SW), seed area (SA), seed perimeter (SP), pH, and soluble solid content (TSS, °Brix). Values towards the number 1 indicate positive correlations, and those towards −1 indicate negative correlations. Dark-blue color indicates positive relationships between two variables, while red color indicates negative associations between variables.
3.3. Principal Component Analyses
The groups in a PCA are not related to each other except for the variables within each group [55]. The results of the PCA performed in this study identified that PC1 and PC2 components accounted for 43.1% of the total variation in the studied variables for P. ligularis (Figure 5).
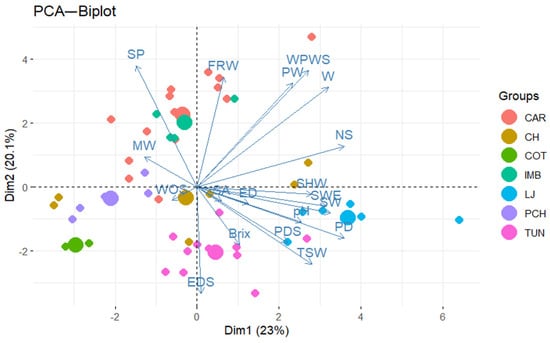
Figure 5.
Principal component analysis (PCA). P. ligularis genotypes collected in the Andes region of Ecuador in 2024.
In PC1, the variables with the highest factor loading were fruit polar diameter, the number of seeds in the fruit, and seed width. PC2 was mainly associated with the variables seed perimeter (SP), fleshy ring weight (FRW), seeded pulp weight (WPWS), equatorial seed diameter (EDS), and seedless pulp weight (PW) (Figure 5). These results suggest that fruit selection in P. ligularis should be based on fruit diameter, fruit weight, seedless pulp weight, and seed weight. Nakasone and Paull [56] recommended genetic improvement of Passiflora species based on fruit shape and size, juice content, acidity, and total soluble solids.
Cluster analysis of quantitative fruit and morphoagronomic variables grouped the genotypes into three groups (Figure 6). However, the groups were not established according to the collection area or collection site but rather by the main morphological characteristics of the genotypes evaluated. The first group was represented by 27 genotypes corresponding to the provinces of Tungurahua, Chimborazo, Cotopaxi, Carchi, and Pichincha, which presented the best characteristics with respect to the equatorial seed diameter (EDS) of 0.37–0.45 cm, mucilage weight of 10 seeds (MW) of 0.05–0.09 cm, and seed perimeter (SP) of 1.77–2.08 cm. The second group was represented mainly by the genotypes of the provinces of Imbabura and Carchi which presented representative characteristics in the weight of the fruit (W) of 108.3–112.5 g, equatorial diameter of the fruit (ED) of 6.1–6.5 g, and weight of the fleshy ring (FRW) of 9.6–3.1 g. The genotypes of the third group were made up of the samples from the province of Loja; this group was mainly made up of the most representative values corresponding to the thickness of the peel (SWE) of 4.73 mm, polar diameter of the fruit (PD) of 7.52 cm, total weight of the seeds (TSW) of 9.5 g, number of seeds per fruit (NS) of 307 units, and weight of the seedless pulp (PW) of 41.8 g.
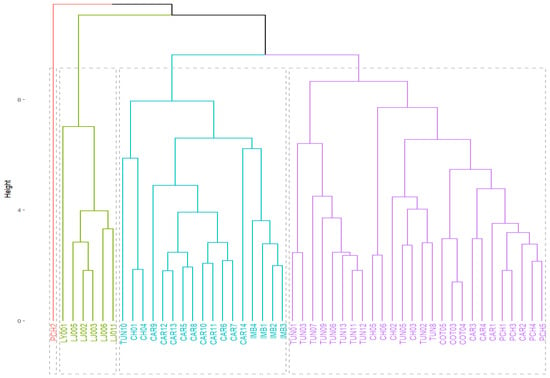
Figure 6.
The dendrogram shows three groups. (Each color groups genotypes with high similarity to each other based on the morphometric characteristics analyzed; purple: EDS–MW–SP; light turquoise: W–E–FRW; light green: SWE–PD–TSW–NS–PW).
Morphological characterization of P. ligularis fruit has revealed morphological variability that can be used in conservation and genetic improvement programs, as well as in the identification of elite materials, which can be used in alternative production programs and future genetic improvement programs. However, due to the limitations of this type of descriptor, these morphological characterization studies need to be complemented with biochemical and molecular data that allow for better germplasm discrimination. This study constitutes the first characterization of P. ligularis fruits in all of Ecuador carried out in 2024. Therefore, it is necessary to intensify research on P. ligularis crops over several years to obtain more robust results, considering aspects of the value chain and production from a long-term perspective.
4. Conclusions
This study highlights the significant phenotypic variability (descriptors such as fruit weight and diameter, °Brix (BS), and pH) in P. ligularis genotypes grown in the Andean region of Ecuador, which opens up opportunities to improve the productivity and adaptability of this crop to environmental challenges. The main contribution of this research lies in the detailed agromorphological characterization of P. ligularis fruit genotypes from different regions of the Ecuadorian Andes, identifying correlations between various fruit and seed traits. Furthermore, the identification of the CAR14 and LY001 genotypes as superior in size and weight is crucial for the development of strategies to strengthen conservation and genetic improvement programs. These genotypes, in addition to offering agronomic benefits in terms of national and international market acceptance, represent an opportunity to improve food security and strengthen the economies of rural Ecuadorian communities, most of which live on less than 2 USD per day. Consequently, it is necessary to promote ongoing research focused on its adaptation to extreme conditions and its integration into public policies that promote the sustainability of the crop. Collaboration between researchers, producers, and local authorities will be essential to effectively leverage the results achieved in this research.
Author Contributions
Conceptualization, J.C.C., L.F.L.P. and V.C.-S.; formal analysis, J.C.C.; investigation, J.C.C., L.F.L.P. and V.C.-S.; resources, J.C.C. and L.F.L.P.; writing—original draft preparation, J.C.C. and L.F.L.P.; writing—review and editing, J.C.C. and V.C.-S.; supervision, L.F.L.P.; funding acquisition, J.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research in Ecuador was funded by Escuela Superior Politécnica de Chimborazo (ESPOCH).
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors would like to thank the Ministry of Environment, Water and Ecological Transition of Ecuador (MAATE) for facilitating the “Authorization for the Collection of Specimens of Biological Diversity Species No. 564”.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A


Figure A1.
Photographic record of characterized genotypes.
References
- Guerrero, A.; Gallucci, S.; Michalijos, P.; Visciarelli, S. Andean Countries: Theoretical contributions for an integrated approach from geographical and tourism perspectives. Huellas 2011, 15, 125. [Google Scholar]
- Orme, A.R. The Tectonic Framework of South America. In The Physical Geography of South America; Oxford Academic: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Peyre, G.; Balslev, H.; Font, X.; Tello, J. Fine-Scale Plant Richness Mapping of the Andean Páramo According to Macroclimate. Front. Ecol. Evol. 2019, 7, 377. [Google Scholar] [CrossRef]
- Campos, D.; Chirinos, R.; Gálvez, L.; Pedreschi, R. Chapter Eight—Bioactive Potential of Andean Fruits, Seeds, and Tubers. Adv. Food Nutr. Res. 2018, 84, 287–343. [Google Scholar] [CrossRef]
- Linares, J.; Castillo, B.; Londoño, M. Characterization of the mechanical properties of the sweet passion fruit (Passiflora ligularis Juss.). Agron. Colomb. 2013, 31, 208–214. [Google Scholar]
- Izquierdo, J.; Roca, W. Under-utilized andean food crops: Status and prospects of plant biotechnology for the conservation and sustainable agricultural use of genetic resources. Acta Hortic. 1998, 457, 157–172. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.I.L.; Villegas-Aguilar, M.D.C.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; Fernández-Ochoa, Á.; Alañón, M.E.; Segura-Carretero, A. Revalorization of Bioactive Compounds from Tropical Fruit By-Products and Industrial Applications by Means of Sustainable Approaches. Food Res. Int. 2020, 138, 109786. [Google Scholar] [CrossRef]
- FAO. Medium-Term Prospects for Agricultural Commodities: Projections to 2010; FAO: Rome, Italy, 2004; Available online: http://www.fao.org/3/a-y5143s.pdf (accessed on 7 March 2025).
- FAO. Major Tropical Fruits: Market Review 2020; FAO: Rome, Italy, 2021; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9e02f4c9-5d4a-4aa1-a4db-368900b22e56/content (accessed on 7 March 2025).
- Kusumaningrum, D.; Lee, S.H.; Lee, W.H.; Mo, C.; Cho, B. A Review of technologies to prolong the shelf life of fresh tropical fruits in southeast Asia. J. Biosyst. Eng. 2015, 40, 345–358. [Google Scholar] [CrossRef]
- Altendorf, S. Minor Tropical Fruits (Mainstreaming a Niche Market). Food Outlook 2018, 67–75. Available online: https://acortar.link/BSDr07 (accessed on 7 March 2025).
- Blancke, R. Tropical Fruits and Other Edible Plants of the World: An Illustrated Guide; Cornell University Press: Ithaca, NY, USA, 2016; Available online: https://acortar.link/xxqWo3 (accessed on 7 March 2025).
- Viera, W.; Shinohara, T.; Samaniego, I.; Terada, N.; Sanada, A.; Ron, L.; Koshio, K. Pulp Mineral Content of Passion Fruit Germplasm Grown in Ecuador and Its Relationship with Fruit Quality Traits. Plants 2022, 11, 697. [Google Scholar] [CrossRef]
- Moreno, E.; Ortiz, B.L.; Restrepo, L.P. Total phenolic content and antioxidant activity of pulp extracts of six tropical fruits. Rev. Colomb. Quím. 2014, 43, 41–48. [Google Scholar] [CrossRef]
- Ramadan, M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): An overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- Moreno-Miranda, C.; Moreno-Miranda, R.; Pilamala Rosales, A.A.; Molina-Sánchez, J.I. The fruit and vegetable sector in Ecuador: Main socio-productive characteristics of the agri-food network of Cape gooseberry (Physalis peruviana). Cienc. Agric. 2019, 16, 31–55. [Google Scholar] [CrossRef]
- Escobar, L.K. Novedades en Passiflora (Passifloraceae) de Colombia. Mutisia 1988, 71, 1–8. [Google Scholar]
- Hernández, A.; Bernal, R. Lista de especies de Passifloraceae de Colombia. Biota Colomb. 2000, 2. Available online: http://www.redalyc.org/articulo.oa?id=49110302 (accessed on 7 March 2025).
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A Review Update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef]
- Zhang, J.; Koike, R.; Yamamoto, A.; Ukiya, M.; Fukatsu, M.; Banno, N.; Akihisa, T. Glycosidic Inhibitors of Melanogenesis from Leaves of Passiflora edulis. Chem. Biodivers. 2013, 10, 1851–1865. [Google Scholar] [CrossRef]
- Rivera, B.; Miranda, D.; Ávila, L.A.; Nieto, A.M. Comprehensive Management of Passion Fruit Cultivation (Passiflora ligularis Juss); Litoas: Manizales, Colombia, 2008; 130p, Available online: https://www.researchgate.net/publication/315614738 (accessed on 7 March 2025).
- Yockteng, R.; Coppens, G.; Souza, T. Pasiflora L.; Springer: Berlin/Heidelberg, Germany, 2011; 129p. [Google Scholar]
- Arias, J.; Ocampo, J.; Urrea, R. Pollination systems in sweet granadilla (Passiflora ligularis Juss.) as a basis for genetic and conservation studies. Acta Agron. 2016, 65, 197203. [Google Scholar] [CrossRef]
- Parra, M. Competitiveness Agreement for the Passionflower Production Chain in Colombia; Ministry of Agriculture and Rural Development: Bogotá, Colombia, 2013; 173p. Available online: https://sioc.minagricultura.gov.co/Pasifloras/Normatividad/004%20-%20D.C.%20-%20Acuerdo%20de%20Competitividad%20Cadena%20Pasifloras.pdf (accessed on 7 March 2025).
- Gutiérrez, C.; Klein, A. Floral Larceny by the stingless bee Trigona almalhea on grandadila (Passiflora ligularis Juss). J. Pollinat. Ecol. 2018, 22, 75–81. [Google Scholar] [CrossRef]
- Villavicencio, A.; Vásquez, W. Guía Técnica de Cultivos; Instituto Nacional de Investigaciones Agropecuarias: Quito, Ecuador, 2008; 444p, Available online: https://repositorio.iniap.gob.ec/jspui/handle/41000/851 (accessed on 7 March 2025).
- Melgarejo, L. (Ed.) Passion Fruit (Passiflora ligularis Juss): Ecophysiological Characterization of the Crop; Universidad Nacional de Colombia: Bogotá, Colombia, 2015; 304p. [Google Scholar]
- Navarrete, J. Estudio de la Producción y Comercialización de la Granadilla (Passiflora ligularis) en la Provincia de Imbabura. Bachelor’s Thesis, Universidad Técnica del Norte, Ibarra, Ecuador, 2017. Available online: https://repositorio.utn.edu.ec/handle/123456789/6953 (accessed on 7 March 2025).
- Ocampo, J.; Arias, J.C.; Urrea, R. Collect and identification of elite genotypes of sweet granadilla (Passiflora ligularis Juss.) in Colombia. Rev. Colomb. Cienc. Hortícolas 2015, 9, 9–23. [Google Scholar] [CrossRef]
- Moura, R.; Sousa, A.; Santos, E.; Silva, G.; Medeiros, T.; Moreira, E. Nutritional status of yellow passion fruit submitted to nitrogen sources by fertigation. Comun. Sci. 2017, 8, 562–569. [Google Scholar] [CrossRef]
- Coba, G. Granadilla from Intag, Ecuador, Is Exported to Europe. Primicias. 2024. Available online: https://www.primicias.ec/noticias/economia/granadilla-intag-ecuador-exportacion-europa/ (accessed on 7 March 2025).
- Borda, M.; Tuesca, R.; Navarro, E. Quantitative Methods, 4th ed.; Universidad del Norte Press: Barranquilla, Colombia, 2013; Available online: https://editorial.uninorte.edu.co/gpd-metodos-cuantitativos-4ta-edicion-revisda-y-aumentada.html (accessed on 7 March 2025).
- R Core Team. Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.R-project.org (accessed on 20 March 2025).
- Fischer, G.; Melgarejo, L.M. The Ecophysiology of Cape Gooseberry (Physalis peruviana L.)—An Andean Fruit Crop. A Review. Rev. Colomb. Cienc. Hort. 2020, 14, 76–89. [Google Scholar] [CrossRef]
- Restrepo-Díaz, H.; Sánchez-Reinoso, A.D. Chapter 5—Ecophysiology of Fruit Crops: A Glance at Its Impact on Fruit Crop Productivity. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–66. [Google Scholar] [CrossRef]
- Fischer, G.; Parra-Coronado, A. Influence of Environmental Factors on the Feijoa (Acca sellowiana [Berg] Burret) Crop. A Review. Agron. Colomb. 2020, 38, 388–397. [Google Scholar] [CrossRef]
- Castro, M.L.; Cifuentes, M.C.B.; Lancheros, J.E.C.; Giraldo, H.F.Y. Passion fruit, nature’s potential contribution to food security. Andin. Res. 2006, 8. Available online: https://www.redalyc.org/articulo.oa?id=239017506007 (accessed on 20 March 2025).
- Mora, O.F.; Reyes, J.R.T.; Ruiz, R.Q.; Huerta, A.G. Preliminary characterization of Chinese pomegranate fruits (Passiflora ligularis Juss.) in Hueyapan and Teziutlán, Puebla. Cienc. Ergo Sum 2008, 15, 54–60. [Google Scholar]
- De Borrero, F.V.; Gutiérrez, C.; Pulido, A. Passion fruit, its physical characteristics and post-harvest behavior. Eng. Res. 1992, 28, 14–23. [Google Scholar] [CrossRef]
- García, M. Passion Fruit Handling, Harvesting, and Postharvest Manual; Corpoica: Bogotá, Colombia, 2008; Available online: http://hdl.handle.net/20.500.12324/14434 (accessed on 20 March 2025).
- Miranda, D.; Fischer, G.; Carranza, C.; Magnitskiy, S.; Casierra, F.; Piedrahíta, W.; Flórez, L.E. Cultivation, Post-Harvest, and Marketing of Passion Fruit in Colombia: Passion Fruit, Granadilla, Gulupa, and Curuba; Colombian Society of Horticultural Sciences: Bogotá, Colombia, 2009. [Google Scholar]
- Carvajal-de Pabón, L.M.; Turbay, S.; Álvarez, L.M.; Rodríguez, A.; Alvarez, J.M.; Bonilla, K.; Parra, M. Relationship between the folk uses of the granadilla plant (Passiflora ligularis Juss) and its phytochemical composition. Biotecnol. Sector Agropecu. Agroind. 2014, 12, 185–196. [Google Scholar]
- Melgarejo, L.M.; Hernández, M.S.; Miranda Lasprilla, D.; Fischer, G.; Rodríguez-Castillo, N.A.; Rodríguez-León, A.K.; Moreno Echeverry, D.L. Granadilla (Passiflora ligularis Juss): Caracterización Ecofisiológica del Cultivo; Melgajero, L., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2015; ISBN 9789587753974. Available online: https://repositorio.unal.edu.co/handle/unal/84150?show=full (accessed on 20 March 2025).
- Aguilar, Z.A.; Garza, D. La Granada China (Passiflora ligularis Juss.); Una Alternativa de Cultivo para el Estado de Hidalgo; Folleto técnico No. 1. INIFAP; C.E. Pachuca: Pachuca, Mexico, 2000. [Google Scholar]
- Ocampo, J. Study of the Genetic Diversity of Genus Passiflora L. and Its Distribution in Colombia. Ph.D. Thesis, Centre International d’Etudes Supérieures en Sciences Agronomiques (SupAgro), Montpellier, France, 2007. [Google Scholar] [CrossRef]
- Cárdenas-Hernández, J.; Miranda, D.; Magnitskiy, S.; Carranza, C. Morphological and Anatomical Analyses of the Seed Coats. Agron. Colomb. 2011, 29, 377–385. Available online: http://www.redalyc.org/articulo.oa?id=180322995005 (accessed on 20 March 2025).
- López, L.F.; González, D.; Gómez, J.A.; Albarracín, C. Agenda Prospectiva de Investigación y Desarrollo Tecnológico Para la Cadena Productiva de Plantas Aromáticas, Medicinales, Condimentarias y Afines con Énfasis en Ingredientes Naturales Para la Industria Cosmética en Colombia; Ministerio de Agricultura y Desarrollo Rural, Instituto Alexander von Humboldt, Universidad Nacional de Colombia y Cámara de Comercio de Bogotá: Bogotá, Colombia, 2009; 182p. [Google Scholar]
- Rodríguez, M. Industrialización de la granadilla (Passiflora ligularis Juss). In Cultivo, Poscosecha y Comercialización de las Pasifloráceas en Colombia: Maracuyá, Granadilla, Gulupa y Curuba; Sociedad Colombiana de Ciencias Hortícolas: Bogotá, Colombia, 2009; pp. 283–302. Available online: https://www.researchgate.net/publication/259346111 (accessed on 20 March 2025).
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest—An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals; CAB International: Wallingford, UK, 2007; p. 227. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1761721 (accessed on 20 March 2025).
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; Taylor & Francis Group: New York, NY, USA, 2010; p. 217. [Google Scholar] [CrossRef]
- Espinosa, D.S.; Melgarejo, L.M.; Hernández, M.S.; Melo, S.E.; Fernández-Trujillo, J.P. Caracterización fisiológica y bioquímica de la granadilla dulce (Passiflora ligularis Juss) en diferentes ubicaciones. Acta Hortic. 2018, 1194, 1459–1464. [Google Scholar] [CrossRef]
- Verona-Ruiz, A.; Urcia-Cerna, J.; Paucar-Menacho, L.M. Pitahaya (Hylocereus spp.): Cultivation, physicochemical characteristics, nutritional composition, and bioactive compounds. Sci. Agropecu. 2020, 11, 439–453. [Google Scholar] [CrossRef]
- Mercado-Silva, E.M. Pitaya—Hylocereus undatus (Haw). In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 339–349. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128031384000459 (accessed on 20 March 2025).
- Fonseca, D.I.; Ospina, N.M. Relación Semilla/Fruto en dos Passifloras: Granadilla (Passiflora ligularis Juss.) y Gulupa (Passiflora edulis Sims.). Bachelor’s Thesis, Facultad de Agronomía, Universidad Nacional de Colombia, Bogotá, Colombia, 2006. [Google Scholar]
- Iezzoni, A.F.; Pritts, M.P. Applications of principal component analysis to horticultural research. HortScience 1991, 26, 334–338. [Google Scholar] [CrossRef]
- Nakasone, H.Y.; Paull, R.E. Tropical Fruits; CAB International: Wallingford, UK, 1998. Available online: https://www.cabidigitallibrary.org/cms/asset/3483f1d8-b085-4142-8275-e57ac2bff085/9781800623798.0000.preview.pdf (accessed on 20 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).