Effects of Foliar Phosphorus Application at Harvest and Postharvest in Sweet Cherry (Prunus avium L.; cv. Regina) Produced in Southern Chile
Abstract
1. Introduction
2. Materials and Methods
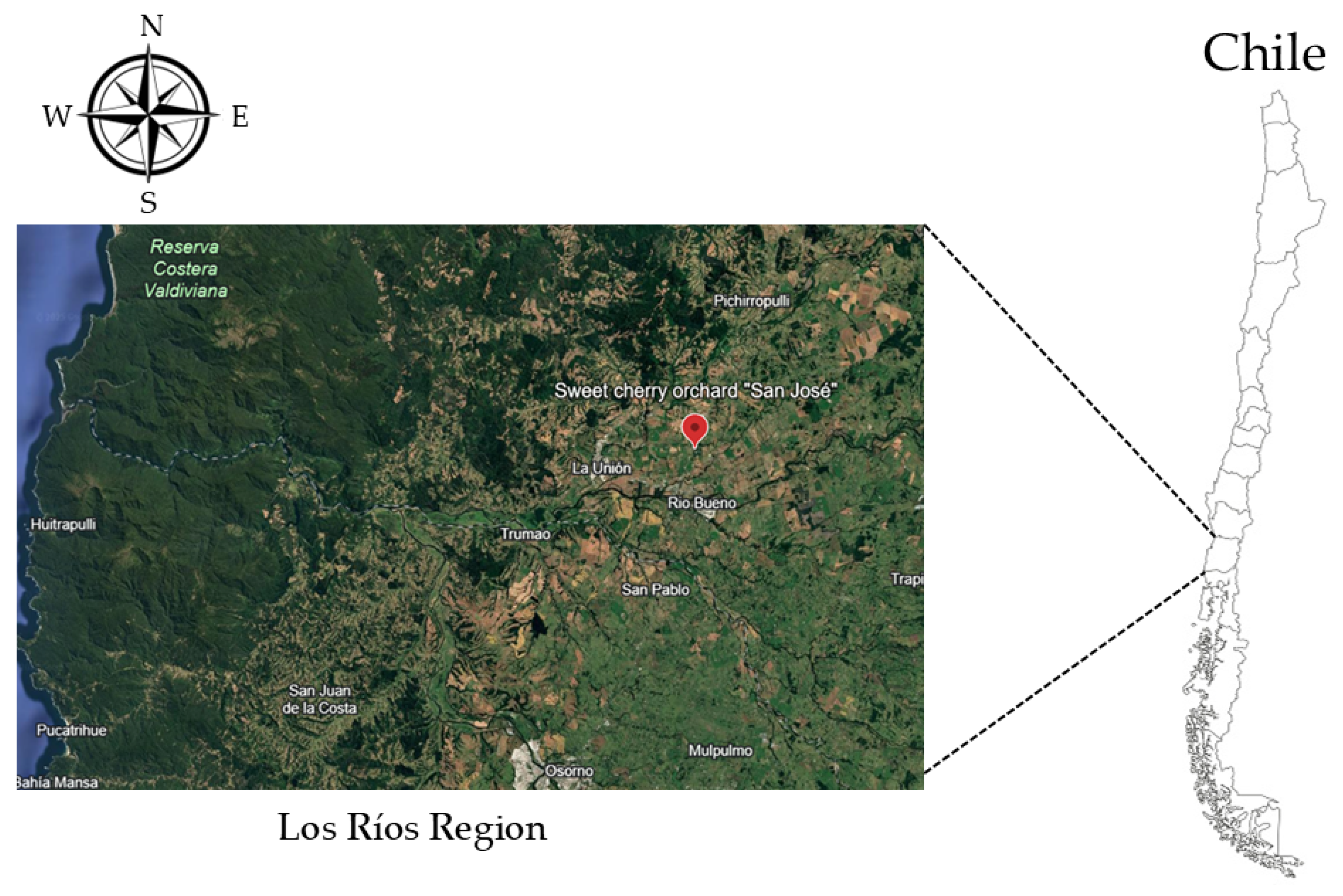
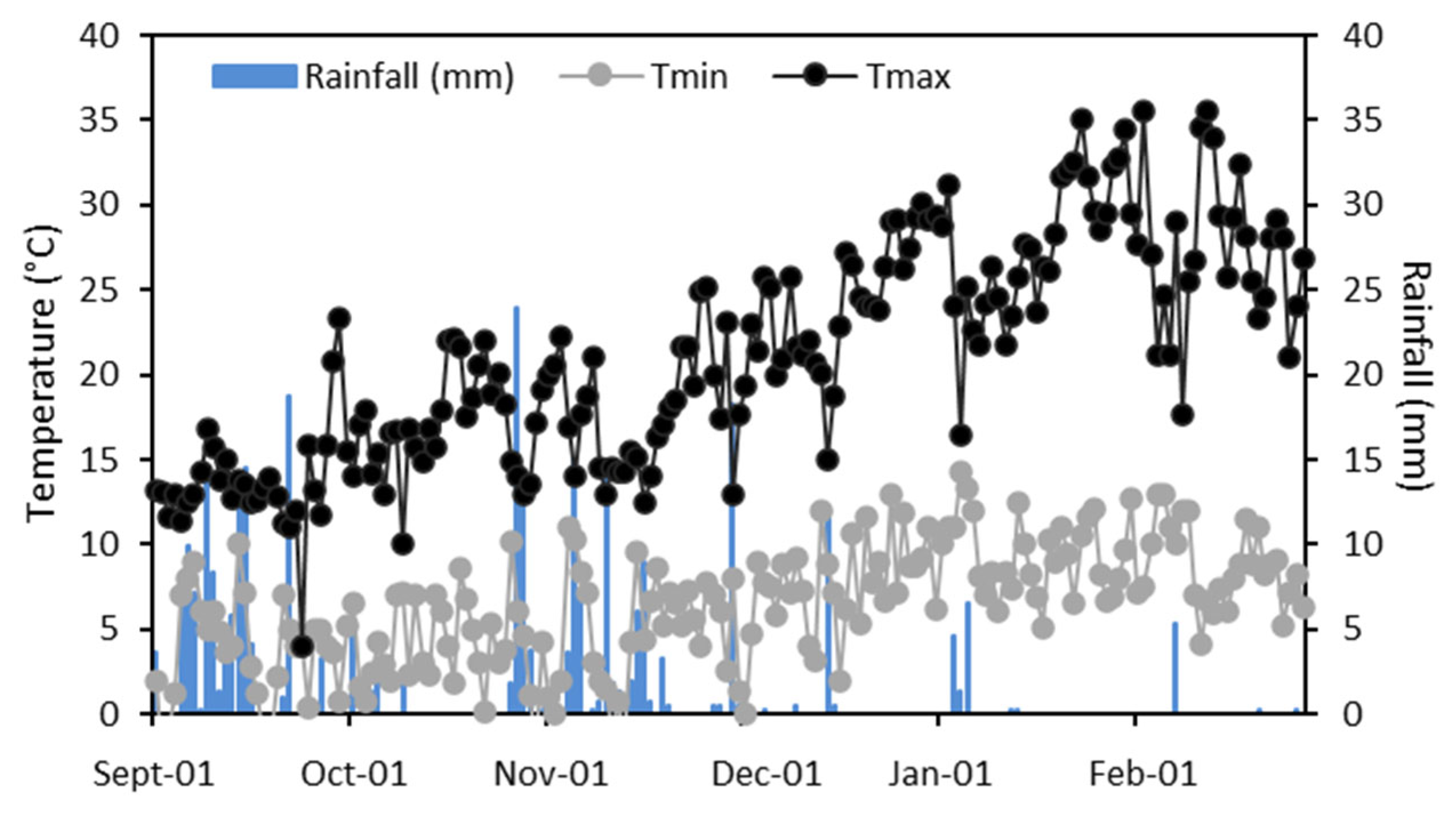
2.1. Experimental Site and Meteorological Measurements
2.2. Treatments
2.3. Fruit Quality and Condition Analysis
2.4. Determination of Antioxidant-Related Parameters in Fruit
2.5. Postharvest Storage Conditions and Fruit Quality Assessment
2.6. Experimental Design and Statistical Analysis
3. Results
3.1. Fruit Physical Quality
3.2. Fruit Chemical Quality and Condition in P. avium Fruit
3.3. Antioxidant-Related Parameters in Sweet Cherry Fruit
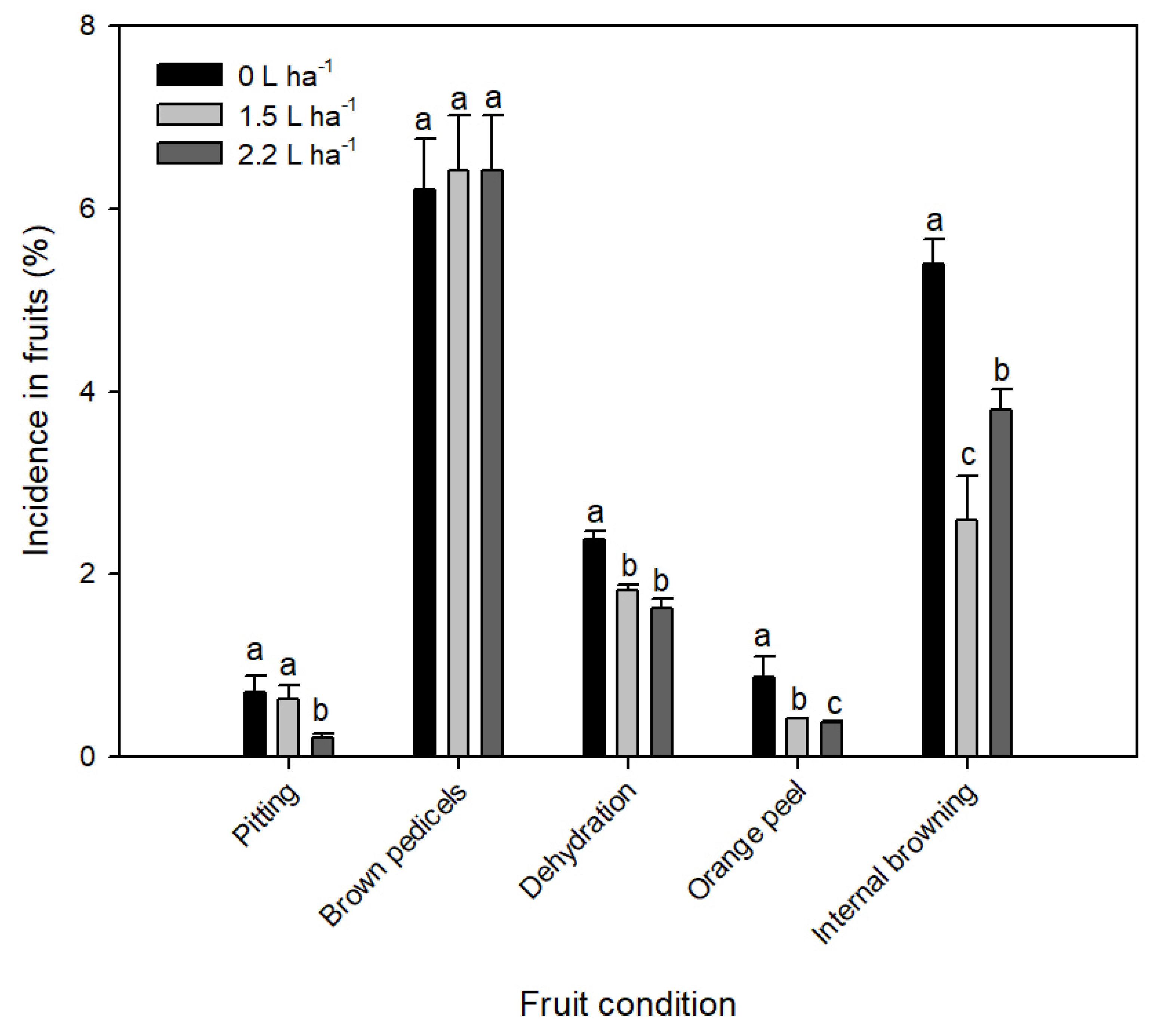
3.4. Fruit Quality and Conditions at Postharvest in Sweet Cherry cv. Regina
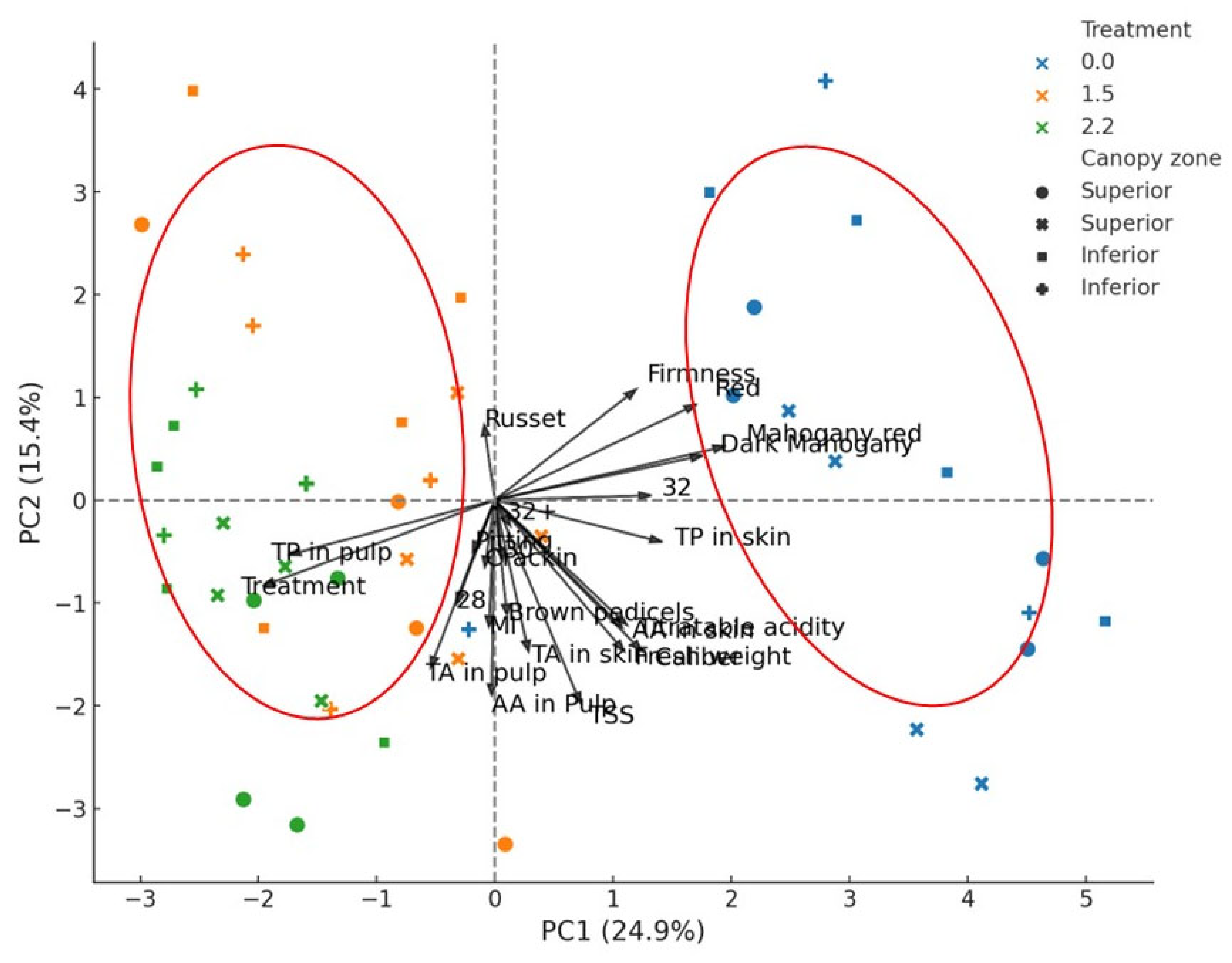
3.5. Principal Component Analysis (PCA) in Response to Foliar Phosphorus Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef]
- Salvadores, Y.; Bastías, R.M. Environmental Factors and Physiological Responses of Sweet Cherry Production under Protective Cover Systems: A Review. Chil. J. Agric. Res. 2023, 83, 484–498. [Google Scholar] [CrossRef]
- ODEPA. Boletín de Fruta, Marzo 2025. Oficina Estud. Políticas Agrar, 2025. Available online: https://opia.fia.cl/601/w3-article-128695.html (accessed on 15 May 2025).
- Huerta-Mendoza, V.; Catalán-Paine, R.; Romero, I.; González-Villagra, J.; Tighe-Neira, R.; Bota, J.; Jorquera-Fontena, E. Effect of Late Preharvest Deficit Irrigation on Physiological and Agronomical Responses in ‘Regina’/Gisela 6 Sweet Cherry (Prunus avium L.) Cultivar. Plants 2025, 14, 517. [Google Scholar] [CrossRef]
- ASOEX. Temporada 2023–2024: Exportaciones de Cerezas Chilenas Cierran Con Nuevo Récord En China. Available online: https://www.asoex.cl/component/content/article/25-noticias/1415-temporada-2023-2024-exportaciones-de-cerezas-chilenas-cierran-con-nuevo-record-en-china.html (accessed on 16 October 2024).
- Chiang, A.; Schnettler, B.; Mora, M.; Aguilera, M. Perceived quality of and satisfaction from sweet cherries (Prunus avium L.) in China: Confirming relationships through structural equations. Cienc. Investig. Agrar. 2018, 45, 210–219. [Google Scholar] [CrossRef]
- Jansen, S.; Foster, W.; Anriquez, G. Quality and the window of opportunity: The importance of attributes and timing for cherry import process in the Chinese market. J. Agribus. Dev. Emerg. Econ. 2025, 15, 1–12. [Google Scholar] [CrossRef]
- Mora, M.L.; Cartes, P.; Demanet, R.; Cornforth, I.S. Effects of Lime and Gypsum on Pasture Growth and Composition on an Acid Andisol in Chile, South America. Commun. Soil Sci. Plant Anal. 2002, 33, 2069–2081. [Google Scholar] [CrossRef]
- Hirzel, J. (Ed.) Diagnóstico Nutricional y Principios de Fertilización en Frutales y Vides, 2nd ed.; Trama Impresores: Chillan, Chile, 2014. [Google Scholar]
- Rojas, G.; Fernandez, E.; Whitney, C.; Luedeling, E.; Cuneo, I.F. Adapting Sweet Cherry Orchards to Extreme Weather Events—Decision Analysis in Support of Farmers’ Investments in Central Chile. Agric. Syst. 2021, 187, 103031. [Google Scholar] [CrossRef]
- Palma, M.; Sepúlveda, Á.; Yuri, J.A. Effect of Plastic Roof and High Tunnel on Microclimate, Physiology, Vegetative Growth and Fruit Characteristics of “Santina” Sweet Cherry. Sci. Hortic. 2023, 317, 112037. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High Tunnel Cultivation of Sweet Cherry (Prunus avium L.): Physiological and Production Variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- González-Villagra, J.; Tighe-Neira, R.; González, T.; González, A.; Reyes-Díaz, M.; Inostroza-Blancheteau, C. Photosynthetic and antioxidants-related properties in blueberry under low- and high-density covering material. Agronomy 2023, 11, 1856–1866. [Google Scholar] [CrossRef]
- González-Villagra, J.; Chicahual, C.; Jorquera-Fontena, E.; Falquetto-Gomes, P.; Nunes-Nesi, A.; Reyes-Díaz, M. Salicylic Acid Improves Yield, Fruit Quality, and Post-Harvest Storage in Sweet Cherry (Prunus avium L.) cv. Lapins Subjected to Late-Deficit Irrigation. Horticulturae 2024, 10, 707. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Dong, J.; Jiang, L.; Hong, H. Overview of fruit cracking in sweet cherry (Prunus avium L.): Causes, testing methods, mitigation strategies, and research perspectives. Front. Sustain. Food Syst. 2025, 9, 1534778. [Google Scholar] [CrossRef]
- Bustamante, M.; Muñoz, A.; Romero, I.; Osorio, P.; Mánquez, S.; Arriola, R.; Reyes-Díaz, M.; Ribera-Fonseca, A. Impact of Potassium Pre-Harvest Applications on Fruit Quality and Condition of Sweet Cherry (Prunus avium L.) Cultivated under Plastic Covers in Southern Chile Orchards. Plants 2021, 10, 2778. [Google Scholar] [CrossRef]
- González-Villagra, J.; Palacios-Peralta, C.; Muñoz-Alarcón, A.; Reyes-Díaz, M.; Osorio, P.; Ribera-Fonseca, A. Influence of Fruit Load Regulation on Harvest and Postharvest Fruit Quality and Antioxidant-Related Parameters in Sweet Cherry (Prunus avium L.) cv. Regina Cultivated under Plastic Covers in Southern Chile. Plants 2024, 13, 2257. [Google Scholar] [CrossRef]
- Muñoz-Alarcón, A.; Palacios-Peralta, C.; González-Villagra, J.; Carrasco-Catricura, N.; Osorio, P.; Ribera-Fonseca, A. Impact of Reflective Ground Film on Fruit Quality, Condition, and Post-Harvest of Sweet Cherry (Prunus avium L.) cv. Regina Cultivated Under Plastic Cover in Southern Chile. Agronomy 2025, 15, 520. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Chen, Q.; Nie, T.; Li, Y.; Li, H.; Sun, Y.; Wu, Y.; Zhang, Y.; Wang, M. Optimized Phosphorus Application Under Water Stress Enhances Photosynthesis, Physiological Traits, and Yield in Soybean During Flowering Stage. Agronomy 2025, 15, 444. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ryu, K.S. Effect of foliar applied phosphatic fertilizer on absorption pathways, yield and quality of sweet persimmon. Sci. Hortic. 2009, 122, 626–632. [Google Scholar] [CrossRef]
- Dang, L.V.; Ngoc, N.P.; Hung, N.N. Effects of foliar fertilization on nutrient uptake, yield, and fruit quality of pomelo (Citrus grandis Osbeck) grown in the mekong delta soils. Int. J. Agron. 2022, 2022, 7903796. [Google Scholar] [CrossRef]
- Tasmin, R.; Calderwood, L.; Tooley, B.; Wang, L.; Zhang, Y.-J. Are Foliar Fertilizers Beneficial to Growth and Yield of Wild Lowbush Blueberries? Agronomy 2022, 12, 470. [Google Scholar] [CrossRef]
- Sarricolea, P.; Herrera-Ossandon, M.; Meseguer-Ruiz, O. Climatic regionalisation of continental Chile. J. Maps 2017, 13, 66–73. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-Based Biostimulant as Sustainable Alternative to Synthetic Growth Regulators in Two Sweet Cherry Cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, A.; Riponi, C. Radical Scavenging Activities of Peels and Pulps from cv. Golden Delicious Apples as Related to Their Phenolic Composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Strack, D.; Wray, V. Anthocyanins. In Methods in Plant Biology. Plant Phenolics; Harborne, J.B., Ed.; Academic Press: London, UK; Harcourt Brace Jovanovich: Orlando, FL, USA, 1989; Volume 1. [Google Scholar]
- Youryon, P.; Supapvanich, S.; Kongtrakool, P.; Wongs-Aree, C. Calcium chloride and calcium gluconate peduncle infiltrations alleviate the internal browning of Queen pineapple in refrigerated storage. Hortic. Environ. Biotechnol. 2018, 59, 205–213. [Google Scholar] [CrossRef]
- Palacios-Peralta, C.; Reyes-Díaz, M.; González-Villagra, J.; Ribera-Fonseca, A. The potential roles of the N and P supplies on the internal browing incidence in sweet cherries in the Southern Chile. Horticulturae 2022, 8, 1209. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in mineral nutrition transport and signal transduction in rosaceae fruit quality and postharvest storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef]
- Schreiner, R.P. Foliar Sprays Containing Phosphorus (P) Have Minimal Impact on ‘Pinot noir’ Growth and P Status, Mycorrhizal Colonization, and Fruit Quality. HortSci. Horts. 2010, 45, 815–821. [Google Scholar] [CrossRef]
- Khalid, S.; Malik, A.U.; Ullah, M.I.; Khalid, M.S.; Naseer, M. Influence of fertilizers and plant growth regulators application on physicochemical attributes of ‘kinnow’ mandarin fruit. Int. J. Fruit Sci. 2021, 21, 758–767. [Google Scholar] [CrossRef]
- Nartvaranant, P. Fruit growth, fruit carbohydrate concentration and fruit nutrient concentration in dropped pummelo (Citrus grandis (L.) Osbeck) fruit. Int. J. Fruit Sci. 2019, 19, 91–103. [Google Scholar] [CrossRef]
- Li, H.; He, K.; Zhang, Z.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef]
- Stampar, F.; Bizjak, J.; Veberic, R.; Jakopic, J. Foliar application of phosphorus improves apple fruit color during ripening. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 1195–1200. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Tang, W.; Chen, H.; Gong, R. Insights into the Coloring Mechanism of Dark-Red and Yellow Fruit in Sweet Cherry through Transcriptome and Metabolome Analysis. Agronomy 2023, 13, 2397. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.; Goncalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Villavicencio, J.D.; Zoffoli, J.P.; Plotto, A.; Contreras, C. Aroma Compounds Are Responsible for an Herbaceous Off-Flavor in the Sweet Cherry (Prunus avium L.) cv. Regina during Fruit Development. Agronomy 2021, 11, 2020. [Google Scholar] [CrossRef]
- Villavicencio, J.D.; Tobar, J.; O’Brien, J.A.; Contreras, C. Identification, characterization, and expression of lipoxygenase genes in sweet cherry (Prunus avium L.) cv. Regina and their relationship with the development of an herbaceous off-flavor during fruit ripening. Plant Physiol. Biochem. 2024, 206, 108271. [Google Scholar] [CrossRef]



| Treatment | Control | 1.5 L ha−1 | 2.2 L ha−1 | p-Value | |
|---|---|---|---|---|---|
| Fresh weight (g) | Upper | 14.8 ± 0.6 a | 13.6 ± 0.3 a | 14.2 ± 0.2 a | 0.23 |
| Lower | 14.0 ± 0.7 a | 13.1 ± 0.3 a | 13.8 ± 0.4 a | 0.52 | |
| Caliber (mm) | Upper | 32.0 ± 0.1 a | 31.7 ± 0.1 a | 31.4 ± 0.0 a | 0.15 |
| Lower | 31.6 ± 0.2 a | 30.7 ± 0.1 a | 31.2 ± 0.1 a | 0.24 | |
| Firmness (g mm−1) | Upper | 323.8 ± 9.3 a | 321.6 ± 8.1 a | 306.0 ± 8.0 a | 0.18 |
| Lower | 340.0 ± 4.7 a | 331.2 ± 6.0 a | 293.2 ± 9.1 b | 0.01 |
| Canopy | Foliar P | Fruit Size Distribution (%) | |||
|---|---|---|---|---|---|
| Zone | Treatment | 28 mm | 30 mm | 32 mm | >32 mm |
| Upper | Control | 9.28 ± 0.56 b | 21.78 ± 1.02 a | 23.22 ± 0.92 b | 43.40 ± 2.0 b |
| 1.5 L ha−1 | 12.86 ± 0.70 a | 23.92 ± 1.02 a | 37.86 ± 0.72 a | 37.86 ± 1.84 b | |
| 2.2 L ha−1 | 8.40 ± 0.48 b | 20.18 ± 0.96 a | 16.60 ± 0.94 c | 48.90 ± 2.04 a | |
| p-value | 0.0029 | 0.18 | 0.0018 | 0.0016 | |
| Lower | Control | 5.54 ± 0.64 b | 23.76 ± 0.92 b | 22.50 ± 0.90 a | 46.62 ± 1.74 a |
| 1.5 L ha−1 | 11.60 ± 0.72 a | 24.10 ± 1.04 b | 16.26 ± 0.72 b | 43.40 ± 2.44 b | |
| 2.2 L ha−1 | 13.22 ± 0.66 a | 31.08 ± 1.18 a | 15.72 ± 0.54 b | 31.96 ± 1.70 c | |
| p-value | 0.011 | 0.023 | 0.014 | 0.028 | |
| Canopy | Foliar P | Fruit Color Distribution (%) | ||
|---|---|---|---|---|
| Zone | Treatment | Red | Mahogany Red | Dark Mahogany |
| Upper | Control | 20.18 ± 0.82 a | 43.92 ± 1.48 a | 35.90 ± 1.30 b |
| 1.5 L ha−1 | 18.40 ± 0.66 a | 41.26 ± 1.24 a | 40.36 ± 1.34 a | |
| 2.2 L ha−1 | 18.04 ± 0.64 a | 38.76 ± 1.42 a | 43.22 ± 1.62 a | |
| p-value | 0.104 | 0.254 | 0.0012 | |
| Lower | Control | 24.82 ± 0.88 a | 47.50 ± 1.56 a | 27.68 ± 1.06 c |
| 1.5 L ha−1 | 16.26 ± 0.60 b | 37.32 ± 1.26 b | 46.42 ± 1.78 a | |
| 2.2 L ha−1 | 22.86 ± 0.58 a | 41.78 ± 1.32 a | 35.54 ± 1.18 b | |
| p-value | 0.002 | 0.001 | 0.0029 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Muñoz-Alarcón, A.; Pirce, F.; Müller, E.; Ribera-Fonseca, A. Effects of Foliar Phosphorus Application at Harvest and Postharvest in Sweet Cherry (Prunus avium L.; cv. Regina) Produced in Southern Chile. Horticulturae 2025, 11, 1052. https://doi.org/10.3390/horticulturae11091052
González-Villagra J, Muñoz-Alarcón A, Pirce F, Müller E, Ribera-Fonseca A. Effects of Foliar Phosphorus Application at Harvest and Postharvest in Sweet Cherry (Prunus avium L.; cv. Regina) Produced in Southern Chile. Horticulturae. 2025; 11(9):1052. https://doi.org/10.3390/horticulturae11091052
Chicago/Turabian StyleGonzález-Villagra, Jorge, Ariel Muñoz-Alarcón, Fanny Pirce, Eric Müller, and Alejandra Ribera-Fonseca. 2025. "Effects of Foliar Phosphorus Application at Harvest and Postharvest in Sweet Cherry (Prunus avium L.; cv. Regina) Produced in Southern Chile" Horticulturae 11, no. 9: 1052. https://doi.org/10.3390/horticulturae11091052
APA StyleGonzález-Villagra, J., Muñoz-Alarcón, A., Pirce, F., Müller, E., & Ribera-Fonseca, A. (2025). Effects of Foliar Phosphorus Application at Harvest and Postharvest in Sweet Cherry (Prunus avium L.; cv. Regina) Produced in Southern Chile. Horticulturae, 11(9), 1052. https://doi.org/10.3390/horticulturae11091052







