Light–Nutrient Optimization Enhances Cherry Tomato Yield and Quality in Greenhouses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Disign
2.3. Plant Growth and Fruit Production
2.4. Fruits Quality of Nutritional, Flavor, Antioxidant Activity
2.5. Statistical Analysis
3. Results
3.1. Effects of Optimized Nutrient Solution and Supplementary Lighting on Growth of Cherry Tomatoes
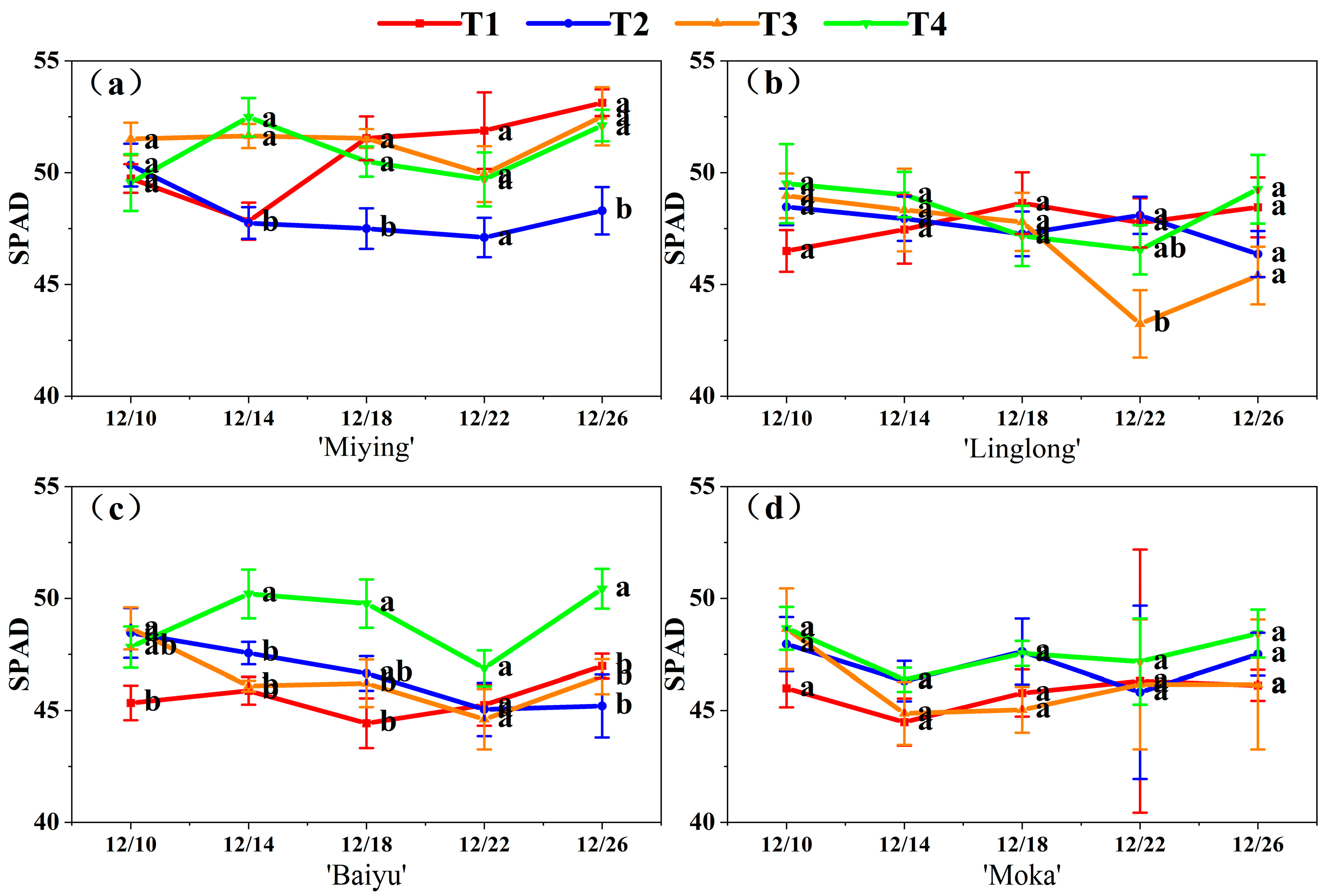
3.2. Effects of Optimized Nutrient Solution and Supplementary Lighting on SPAD Values of Cherry Tomato Leaves
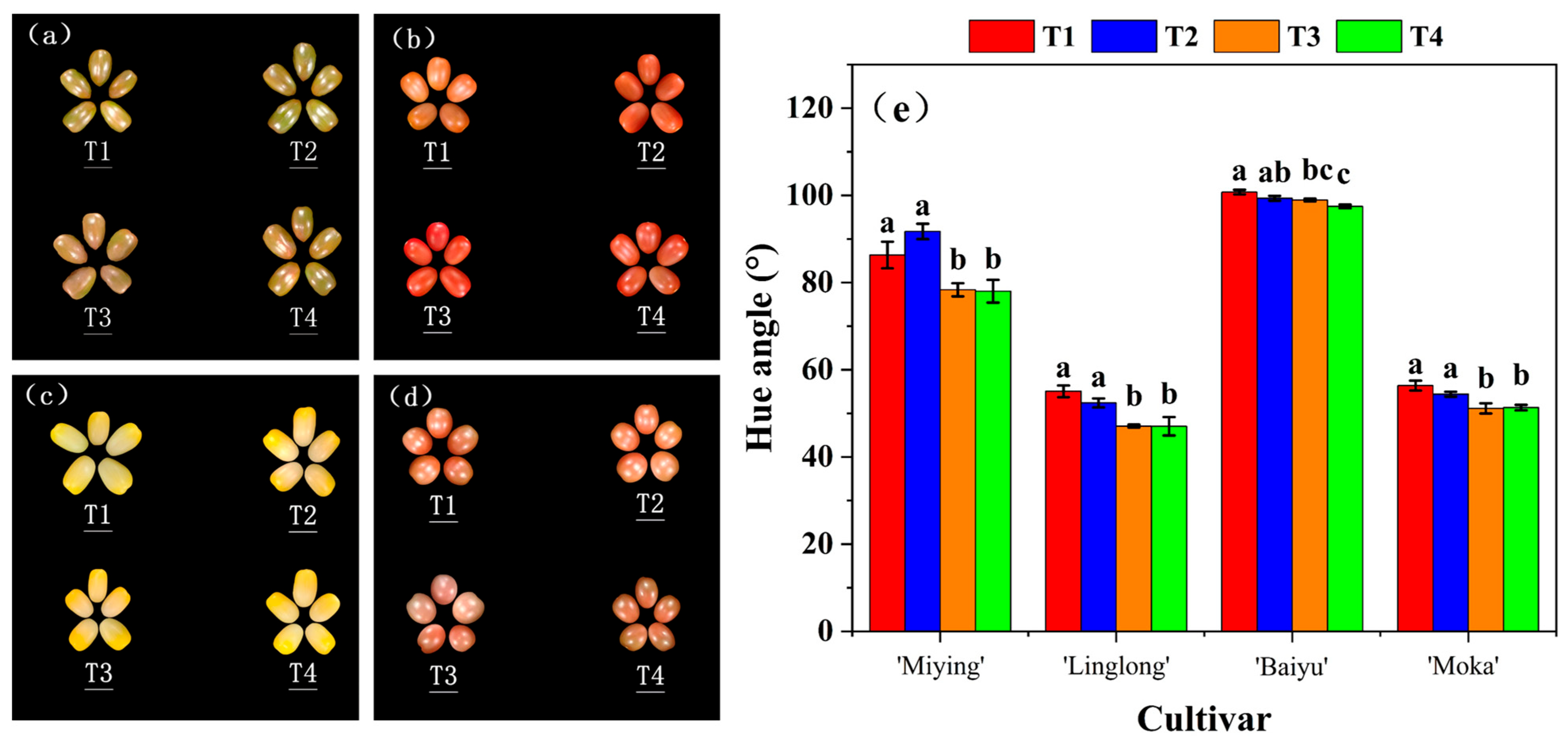
3.3. Effects of Optimized Nutrient Solution and Supplementary Lighting on the Appearance of Cherry Tomato Fruits
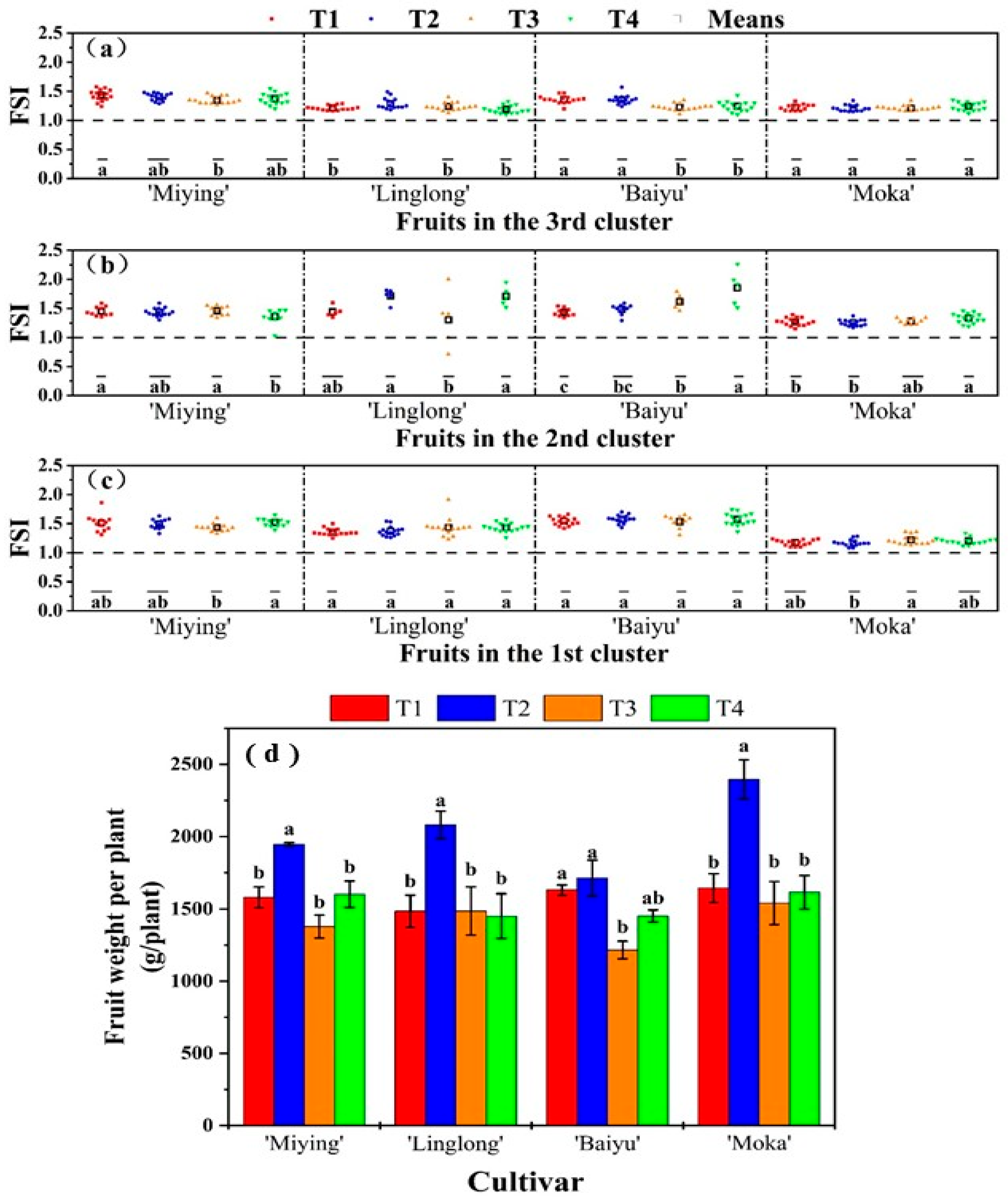
3.4. Effects of Optimized Nutrient Solution and Supplementary Lighting on the Shape and Weight of Cherry Tomato Fruits
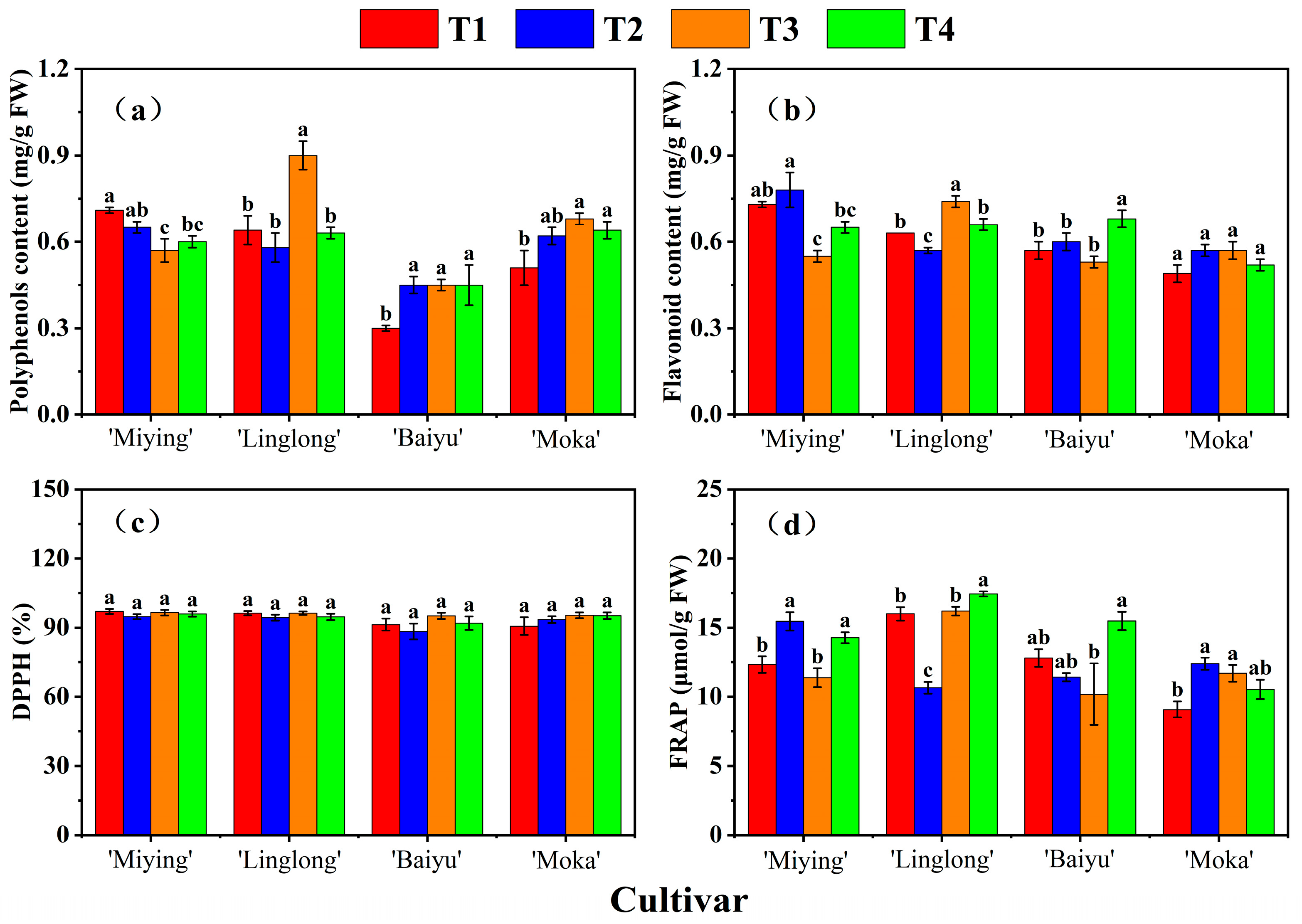
3.5. Effects of Optimized Nutrient Solution and Supplementary Lighting on Antioxidant Capacity of Cherry Tomatoes
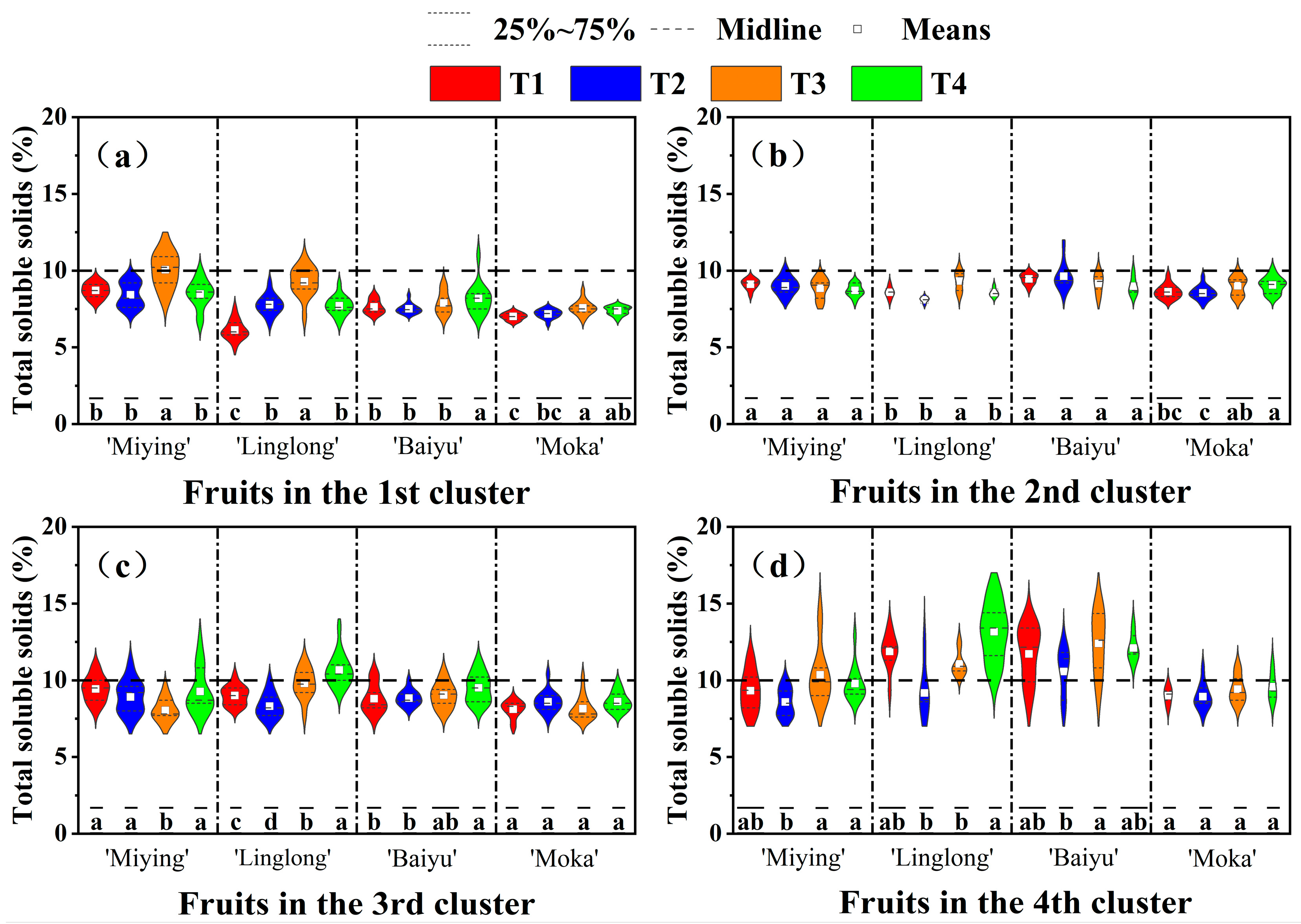
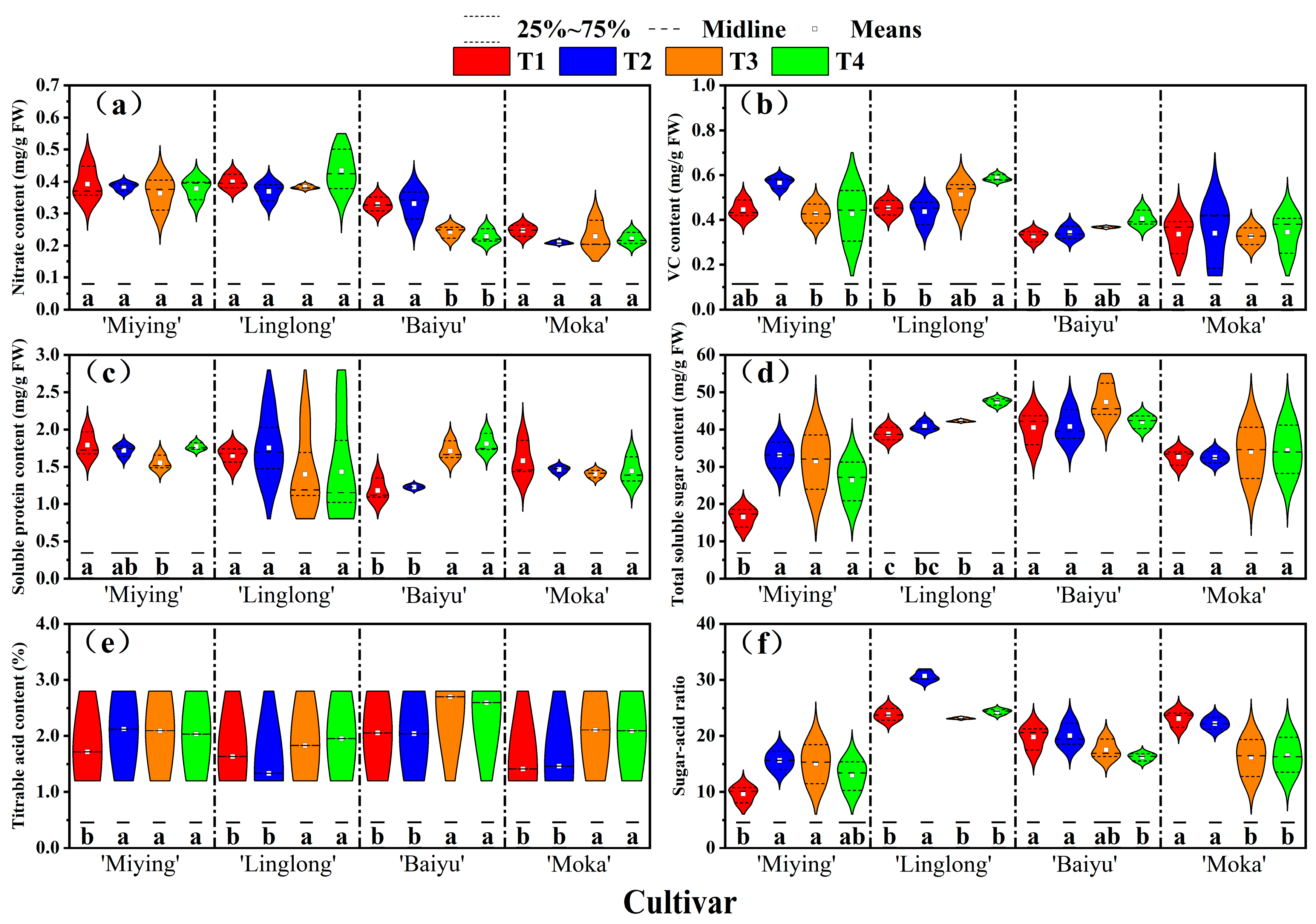
3.6. Effects of Optimized Nutrient Solution and Supplementary Lighting on Fruit Quality Attributes of Cherry Tomatoes
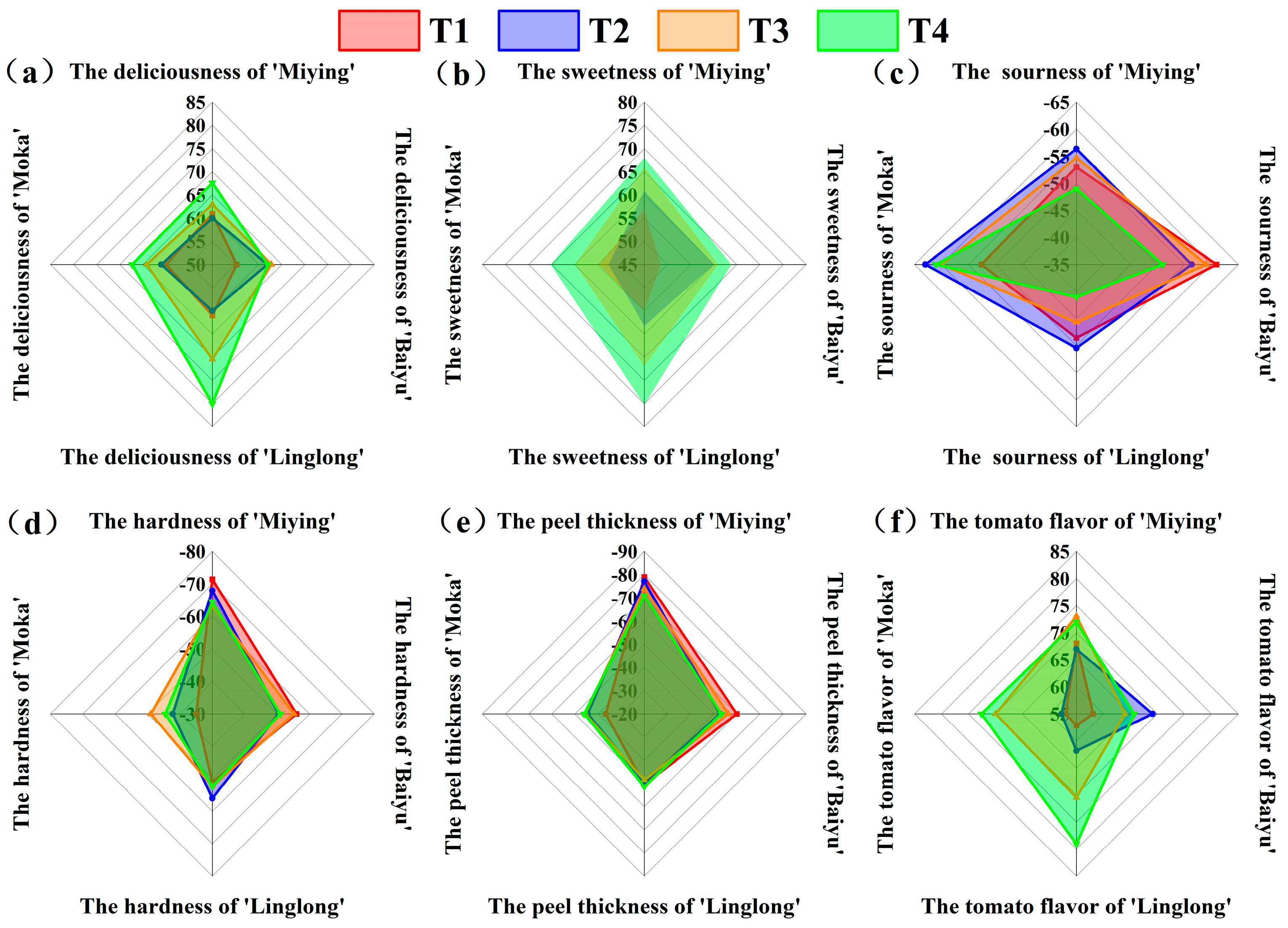
3.7. Comprehensive Analysis of the Effects of Optimized Nutrient Solution and Supplementary Lighting on the Fruit Production and Quality of Cherry Tomatoes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rapa, M.; Ciano, S.; Ruggieri, R.; Vinci, G. Bioactive compounds in cherry tomatoes (Solanum lycopersicum var. Cerasiforme): Cultivation techniques classiffcation by multivariate analysis. Food Chem. 2021, 355, e129630. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gao, L. Theory and technology for facility cultivation of high-quality tomato. China Veg. 2021, 2, 30–40, (In Chinese with English Abstract). [Google Scholar]
- Ou, X.; Liu, D.; Liu, A.; Liu, H.; Chen, R.; Zhang, Y. Effects of nutrient solution management modes on fruit production and quality of tomatoes grown in extremely root restriction. Sci. Hortic. 2023, 321, 112366. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H.; Wang, T.; Zhang, T.; Shi, C.; Jiang, W. Influence of the electrical conductivity of the nutrient solution in different phenological stages on the growth and yield of cherry tomato. Horticulturae 2022, 8, 378. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, H.; Sun, L.; Tian, Y.; Gao, L. Effects of different cultivation densities and K+, Na+ ion treatments on tomato yield and quality under substrate cultivation. China Veg. 2019, 368, 26–31, (In Chinese with English abstract). [Google Scholar]
- Almeselmani, M.; Pant, R.; Singh, B. Potassium level and physiological response and fruit quality in hydroponically grown tomato. Int. J. Veg. Sci. 2009, 16, 85–99. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Qin, M.; Xie, Z.; Chen, R.; Zhang, Y. Effects of supplemental lighting on potassium transport and fruit coloring of tomatoes grown in hydroponics. Int. J. Mol. Sci. 2021, 22, 2687. [Google Scholar] [CrossRef] [PubMed]
- San-Martín-Hernández, C.; Gómez-Merino, F.C.; Rivera-Vargas, G.; Saucedo-Veloz, C.; Vaquera-Huerta, H.; Trejo-Téllez, L.I. Tomato fruit quality between clusters is differentially affected by nitrogen and potassium supply. Rev. Fitotec. Mex. Soc. Mex. Fitogenética 2022, 45, 183–192. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Z.; Sun, Y.; Cai, K.; Wang, W.; Zhao, M. Effect of nitrogen, phosphorus and potassium fertilizers on tomato cultivation in greenhouse. Soil Fertil. Sci. China 2016, 262, 65–71, (In Chinese with English Abstract). [Google Scholar]
- Tamai, D. The practical cultivation and technologist training in tomato low node order pinching and high density planting cultivation. Shisetsu Engei 2014, 165, 62–65. (In Japanese) [Google Scholar]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Effects of lower nitrogen concentration of nutrient solution combined with K supplementation and changing the concentration on growth, yield, and yellow-shoulder disorder for tomatoes grown in extremely low-volume substrate. Hortic. J. 2015, 84, 46–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Suzuki, K.; Liu, H.; Nukaya, A.; Kiriiwa, Y. Fruit yellow-shoulder disorder as related to mineral element uptake of tomatoes grown in high temperature. Sci. Hortic. 2018, 242, 25–29. [Google Scholar] [CrossRef]
- Liu, A.; Liu, D.; Yin, D.; Lian, H.; Zhang, Y.; Chen, R. Effects of extremely low drainage rate and nutrient solution concentration regulating on tomato fruit quality. China Veg. 2021, 10, 66–78, (In Chinese with English Abstract). [Google Scholar]
- Liu, D.; Chen, J.; Hao, Y.; Yang, X.; Chen, R.; Zhang, Y. Effects of extreme root restriction on the nutritional and flavor quality, and sucrose metabolism of tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 813. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Li, J.; Luo, X.; Liu, M. Research progress on the effect of LED light quality on the growth and quality of vegetables. Hunan Agric. Sci. 2020, 12, 105–108, (In Chinese with English Abstract). [Google Scholar]
- Bertin, N.; Genard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Davis, P.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Tian, S.; Liang, Z.; Li, S.; Li, Y.; Wei, M.; Zhang, D. Effectemental lighting on water transport, photosynthetic carbon gain and water use efficiency in greenhouse tomato. Sci. Hortic. 2019, 256, 108630. [Google Scholar] [CrossRef]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime supplemental LED inter-lighting improves growth and yield of Single-Truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.; Mitchell, C. Supplemental lighting for greenhouse-grown tomatoes: Intracanopy LED towers vs. overhead HPS lamps. Acta Hortic. 2014, 1037, 855–862. [Google Scholar] [CrossRef]
- Heuvelink, E.; Bakker, M.; Hogendonk, L.; Janse, J.; Kaarsemaker, R.; Maaswinkel, R. Horticultural lighting in the Netherlands: New developments. Acta Hortic. 2006, 711, 25–33. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of supplemental lighting within the canopy at different developing stages on tomato yield and quality of single-truss tomato plants grown at high density. Environ. Control Biol. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; He, X.; Xia, S.; Zhang, Y.; Chen, R. Effects of supplemental blue light at different stages on tomato plant growth, fruit quality and nutrient uptake. North. Hortic. 2021, 11, 1–9, (In Chinese with English Abstract). [Google Scholar]
- He, R.; Wei, J.; Zhang, J.; Tan, X.; Li, Y.; Gao, M.; Liu, H. Supplemental blue light frequencies improve ripening and nutritional qualities of tomato fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Liang, J.; Xie, Z.; Qin, M.; Yang, X.; Chen, R.; Zhang, Y. Comprehensive evaluation of 6 cherry tomato varieties under the substrate bag cultivation mode in multi-span greenhouse in south China. Guangdong Agric. Sci. 2023, 50, 143–145, (In Chinese with English Abstract). [Google Scholar]
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Khan, I.; Azam, A.; Mahmood, A. The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acid and vitamin C contents of tomato (Lycopersicon esculentum). Environ. Monit. Assess. 2013, 185, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Almeida, J.; Otaiza, S.N.; Duffy, T.; da Silva, J.V.C.; de Godoy, F.; Asís, R.; Bermúdez, L.; Fernie, A.R.; Carrari, F.; et al. Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol. Biol. 2012, 81, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Inffuence of nutrient solution concentration and composition on the growth, uptake patterns of nutrient elements and fruit coloring disorder for tomatoes grown in extremely low-volume substrate. Hortic. J. 2015, 84, 37–45. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, J.; Liu, H.; Chen, R.; Yang, X.; Song, S.; Zhang, Y. Influence of combined supplemental lighting and nutrient solution concentration on fruit production and quality of cherry tomato. Horticulturae 2024, 10, 990. [Google Scholar] [CrossRef]
- Chen, X. Effects of Different Light Emitting Diode Sources on Physiological Characteristics and Quality in Chinese Chive. Master’s Thesis, Shandong Agricultural University, Taian, China, 2012. (In Chinese with English Abstract). [Google Scholar]
- Serio, F.; Leo, J.J.; Parente, A.; Santamaria, P. Potassium nutrition increases the lycopene content of tomato fruit. J. Hortic. Sci. Biotechnol. 2015, 82, 941–945. [Google Scholar] [CrossRef]
- Liu, H.; Son, J.; Niu, G.; Li, Q. Editorial: Growth and quality formation regulated by light in horticulture plants. Front. Plant Sci. 2024, 15, 1414970. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Masuda, E.; Tamashiro, T.; Maharjan, G.; Maruo, T. Comparison of supplemental LED top- and interlighting for year-round production of cherry tomato. Agronomy 2022, 12, 1878. [Google Scholar] [CrossRef]
- Aponov, M.; Verheul, M.; Dobrev, P.; Paponov, I. Additive effects of light and branching on fruit size and chemical fruit quality of greenhouse tomatoes. Front. Plant Sci. 2023, 14, 1221163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wu, H.; Zhang, X.; Liu, J.; Li, Y.; Song, Y.; Wang, J.; Zheng, Y. Integrating omics reveals insights into tomato abaxial/adaxial leafy supplemental lighting. Front. Plant Sci. 2023, 14, 1118895. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, H.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Effects of potassium application on flavor compounds of cherry tomato fruits. J. Plant Nutr. 2009, 32, 1451–1468. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Song Zhang, Y.; Su, W.; Liu, H. Effects of photoperiod interacted with nutrient solution concentration on nutritional quality and antioxidant and mineral content in lettuce. Agronomy 2020, 10, 920. [Google Scholar] [CrossRef]
- Kim, Y.X.; Son, S.; Lee, S.; Jung, E.; Lee, Y.; Sung, J.; Lee, C. Combined effects of nutrients×water×light on metabolite composition in tomato fruits (Solanum lycopersicum L.). Plants 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]


| Cultivar | Treatments | Plant Height | Stem Diameter | Leaf Number | Leaf Area |
|---|---|---|---|---|---|
| (C) | (cm) | (mm) | Per Plant | (cm2/Plant) | |
| ‘Miying’ | T1 | 127.57 ± 3.11 a | 13.64 ± 1.00 b | 25.83 ± 1.45 a | 468.25 ± 25.80 b |
| T2 | 128.71 ± 3.44 a | 13.72 ± 0.39 b | 25.20 ± 1.31 a | 506.27 ± 31.13 ab | |
| T3 | 127.74 ± 2.43 a | 14.60 ± 0.41 ab | 27.56 ± 1.45 a | 576.99 ± 18.83 ab | |
| T4 | 128.76 ± 1.99 a | 15.70 ± 0.44 a | 28.17 ± 1.08 a | 585.69 ± 20.91 a | |
| ‘Linglong’ | T1 | 148.17 ± 1.63 ab | 14.63 ± 0.39 b | 25.63 ± 0.50 b | 509.34 ± 21.13 a |
| T2 | 144.20 ± 1.36 b | 15.62 ± 0.79 ab | 25.67 ± 0.71 b | 498.66 ± 47.38 a | |
| T3 | 150.61 ± 2.83 ab | 14.70 ± 0.44 b | 29.40 ± 1.17 a | 484.18 ± 43.46 a | |
| T4 | 153.88 ± 2.19 a | 16.53 ± 0.29 a | 29.60 ± 1.02 a | 413.47 ± 32.48 a | |
| ‘Baiyu’ | T1 | 139.13 ± 1.56 b | 14.61 ± 0.32 b | 23.83 ± 0.51 a | 711.53 ± 58.60 a |
| T2 | 144.77 ± 0.99 ab | 14.78 ± 0.52 ab | 25.57 ± 0.65 a | 773.96 ± 36.17 a | |
| T3 | 138.28 ± 2.27 b | 14.77 ± 0.34 ab | 23.86 ± 0.74 a | 656.47 ± 59.28 a | |
| T4 | 149.05 ± 2.25 a | 16.17 ± 0.50 a | 25.26 ± 0.71 a | 775.62 ± 27.58 a | |
| ‘Moka’ | T1 | 142.24 ± 2.46 bc | 16.42 ± 0.28 ab | 24.20 ± 1.00 b | 536.74 ± 48.03 a |
| T2 | 140.50 ± 3.14 bc | 15.50 ± 0.64 b | 24.33 ± 0.90 b | 537.42 ± 47.77 a | |
| T3 | 149.81 ± 2.64 ab | 18.24 ± 1.33 a | 27.71 ± 0.68 a | 536.95 ± 34.70 a | |
| T4 | 153.22 ± 2.37 a | 16.97 ± 0.46 ab | 27.73 ± 0.81 a | 566.27 ± 25.03 a | |
| ANOVA significance | C | ** | ** | ** | ** |
| N | ** | ** | ** | NS | |
| L | NS | NS | NS | NS | |
| C × N | * | NS | * | NS | |
| C × L | NS | * | NS | NS | |
| N × L | NS | NS | NS | NS | |
| C × N × L | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xie, Z.; Zhao, T.; Li, H.; Chen, R.; Song, S.; Zhang, Y. Light–Nutrient Optimization Enhances Cherry Tomato Yield and Quality in Greenhouses. Horticulturae 2025, 11, 874. https://doi.org/10.3390/horticulturae11080874
Li J, Xie Z, Zhao T, Li H, Chen R, Song S, Zhang Y. Light–Nutrient Optimization Enhances Cherry Tomato Yield and Quality in Greenhouses. Horticulturae. 2025; 11(8):874. https://doi.org/10.3390/horticulturae11080874
Chicago/Turabian StyleLi, Jianglong, Zhenbin Xie, Tiejun Zhao, Hongjun Li, Riyuan Chen, Shiwei Song, and Yiting Zhang. 2025. "Light–Nutrient Optimization Enhances Cherry Tomato Yield and Quality in Greenhouses" Horticulturae 11, no. 8: 874. https://doi.org/10.3390/horticulturae11080874
APA StyleLi, J., Xie, Z., Zhao, T., Li, H., Chen, R., Song, S., & Zhang, Y. (2025). Light–Nutrient Optimization Enhances Cherry Tomato Yield and Quality in Greenhouses. Horticulturae, 11(8), 874. https://doi.org/10.3390/horticulturae11080874





