Transcriptomic Analysis Reveals the Regulation Function of Calcium Ions Regarding Anthocyanin Biosynthesis in Lonicera japonica Under Cold Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatment
2.2. Pigment Content Measurement
2.3. Total RNA Extraction, cDNA Library Construction, and Sequencing
2.4. Gene Assembly and Expression Analysis
2.5. Functional Analysis
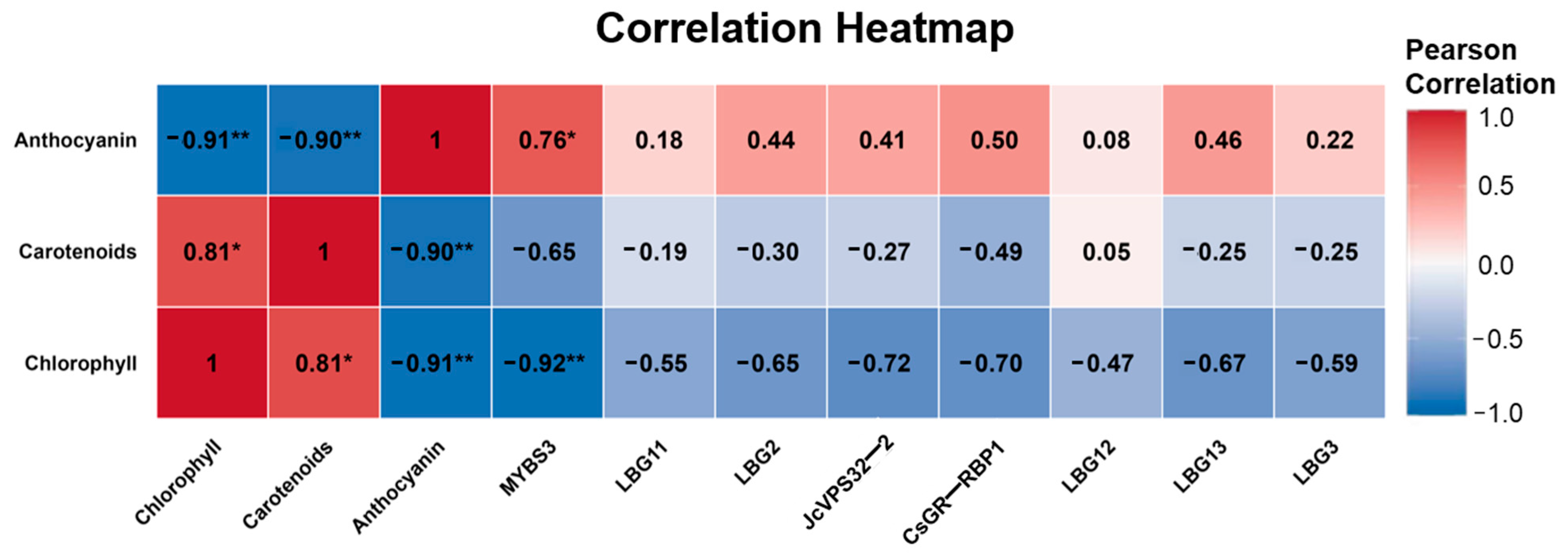
2.6. Correlation Analysis Between TF Expression and Pigment Contents
2.7. Quantitative Real-Time (qRT) PCR Analysis
2.8. Statistical Analysis
3. Results
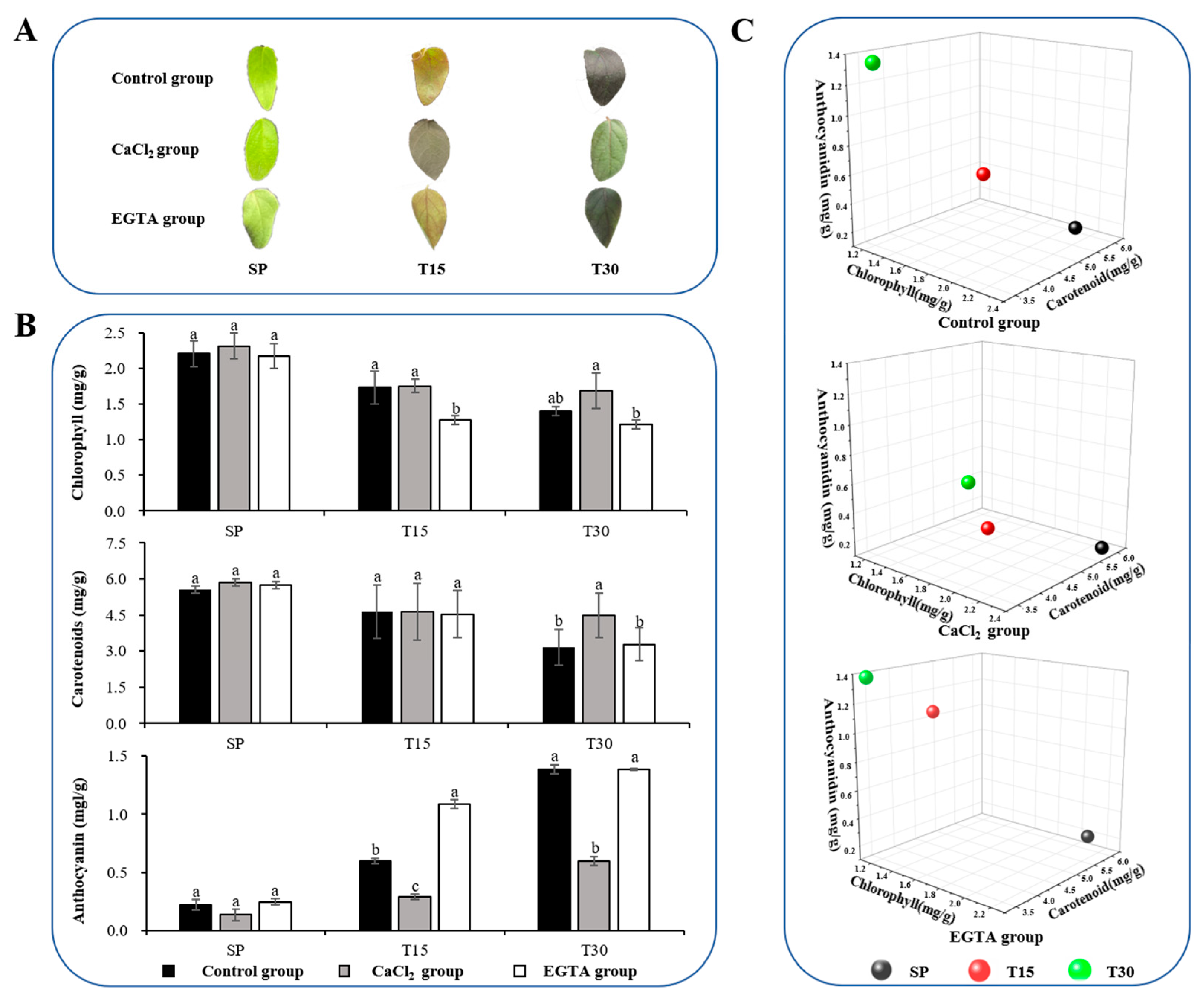
3.1. Morphological and Physiological Responses of L. japonica Leaves Under Cold Stress
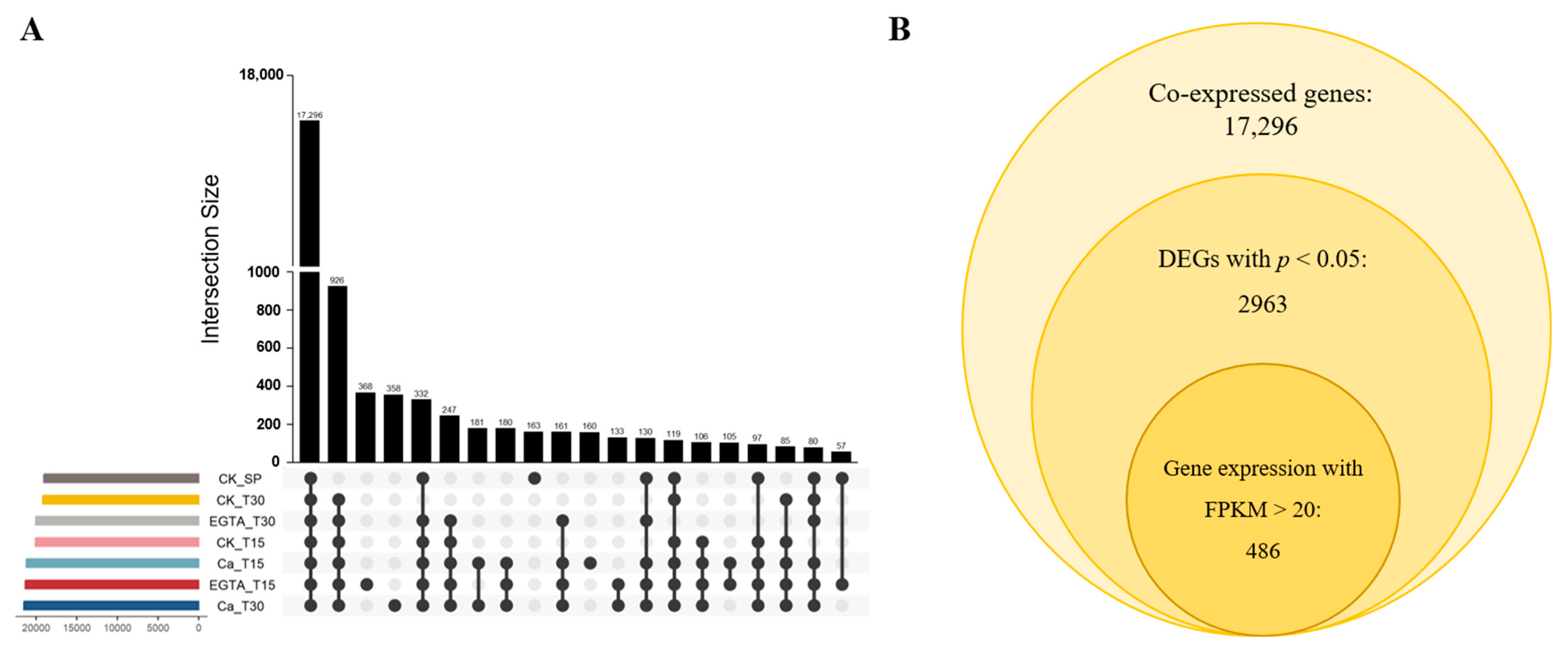
3.2. Transcriptomic Analysis of Leaves Under Cold Stress
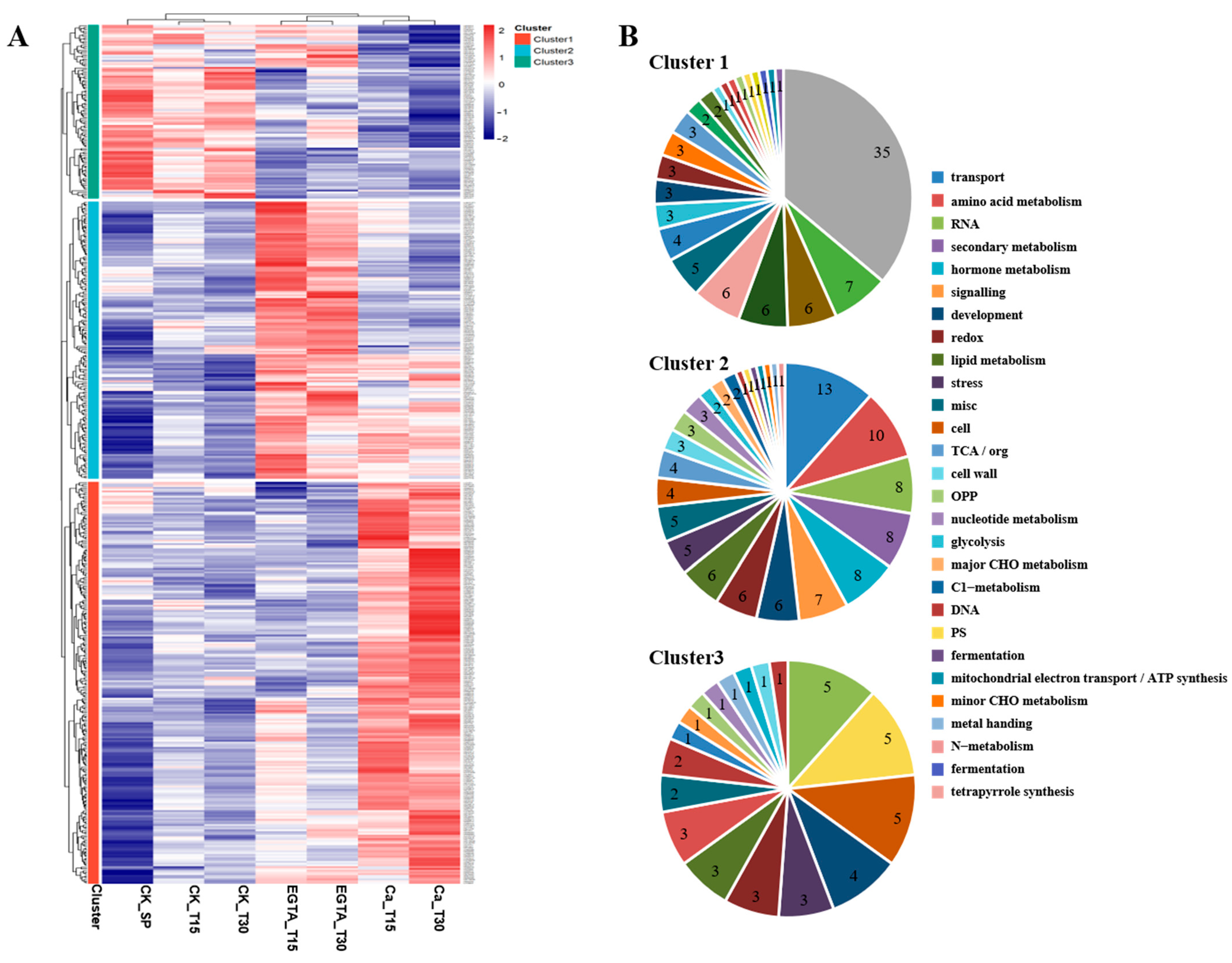
3.3. Clustering Analysis of DEGs Expression Patterns
3.4. Function Annotation and Enrichment
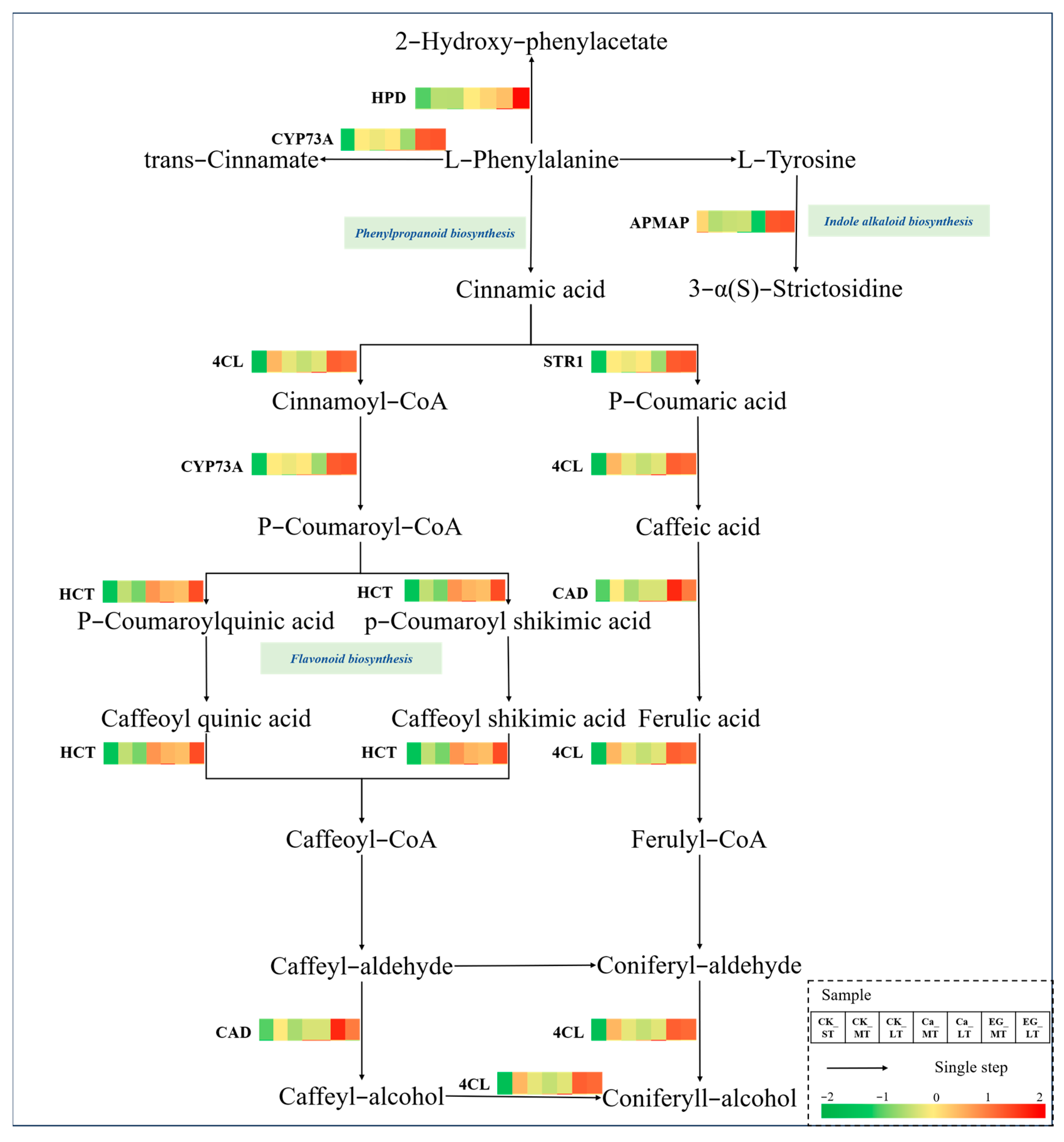
3.5. Analysis of Amino Acid Metabolism Pathways in L. japonica Under Cold Stress
3.6. Response of Secondary Metabolism to Cold Stress
3.7. Expression Analysis of Genes Related to RNA, Hormones, and Transport
3.8. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TF | transcription factor |
| SP | start point |
| BR | brassinosteroid |
| BRH1 | brassinosteroid-responsive RING-H2 |
| BAK1 | BRI1-associated kinase 1 |
| BES1/BZR1 | BRI1 EMS SUPPRESSOR 1/BRASSINAZOLE RESISTANT 1 |
| CAST | calcium-binding protein CAST |
| DEGs | differentially expressed genes |
References
- Miller, K.E.; Gorchov, D.L. The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia 2004, 139, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Hon, K.L.E.; Leung, P.C.; Sam, S.W.; Fung, K.P.; Lee, M.Y.H.; Lau, H.Y.A. Traditional Chinese medicine for atopic eczema: PentaHerbs formula suppresses inflammatory mediators release from mast cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar] [CrossRef]
- Liu, T.; Bai, H.; Wang, H.; Li, Y.; Wang, Z. Anti-inflammatory effects and mechanism of Plantago asiatica L. and Lonicera japonica Thunb. extracts based on canine and feline kidney cell models. J. Ethnopharmacol. 2025, 338, 119069. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, A.; Hu, W.; Zhang, Z.; Ruan, Y.; Kuang, H.; Wang, M. Extraction, Purification, structural characteristics, health benefits, and application of the polysaccharides from Lonicera japonica Thunb.: A Review. Molecules 2023, 28, 4828. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Su, X.; Xu, B.; Liu, Y.; Tang, Y.; Sun, S.; Li, P.; Zhao, C. Characterization of the complete chloroplast genome of Lonicera tangutica (Caprifoliaceae), an ornamental and medicinal plant in China. Mitochondrial DNA B Resour. 2022, 7, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Matsi, T.; et al. Integrated nutrient management boosts inflorescence biomass and antioxidant profile of Carlina diae (Asteraceae)—An endangered local Endemic plant of crete with medicinal and ornamental value. Agriculture 2024, 14, 259. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Kong, D.-x.; Li, Y.-q.; Bai, M.; He, H.-j.; Liang, G.-x.; Wu, H. Correlation between the dynamic accumulation of the main effective components and their associated regulatory enzyme activities at different growth stages in Lonicera japonica Thunb. Ind. Crops Prod. 2017, 96, 16–22. [Google Scholar] [CrossRef]
- Li, R.; Kuang, X.; Wang, W.; Wan, C.; Li, W. Comparison of chemical constitution and bioactivity among different parts of Lonicera japonica Thunb. J. Sci. Food Agric. 2020, 100, 614–622. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold Stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, X.; Wang, F.; Li, X.; Qin, Z.; Fan, S.; Yan, N.; Xie, Y.; Zhao, R. Metabolomic response of Zizania latifolia to low-temperature stress and identification of the bZIP transcription factor family. GM. Crops Food. 2025, 16, 413–434. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, X.; Li, S.; Fu, Y.; Xu, J.; Wang, G. The AabHLH35 transcription factor identified from Anthurium andraeanum is involved in cold and drought tolerance. Plants 2019, 8, 216. [Google Scholar] [CrossRef]
- Kamal, K.A.; Shah, F.A.; Zhao, Y.; Chen, Z.; Fu, S.; Zhu, Z.; Ren, J.; Liu, H. Genome-wide identification of the UGT genes family in Acer rubrum and role of ArUGT52 in anthocyanin biosynthesis under cold stress. BMC Plant Biol. 2025, 25, 288. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 1996, 8, 489–503. [Google Scholar] [CrossRef]
- Veremeichik, G.N.; Bulgakov, D.V.; Konnova, Y.A.; Brodovskaya, E.V.; Grigorchuk, V.P.; Bulgakov, V.P. Proteome-level investigation of Vitis amurensis calli transformed with a constitutively active, Ca2+-independent form of the Arabidopsis AtCPK1 gene. Int. J. Mol. Sci. 2023, 24, 13184. [Google Scholar] [CrossRef]
- Yang, G.; Shen, S.; Yang, S.; Komatsu, S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced in response to cold and gibberellin. Plant Physiol. Biochem. 2003, 41, 369–374. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous calcium: Its mechanisms and research advances involved in plant stress tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef] [PubMed]
- Junyan, W.; Qiaowen, P.; Fahim, A.M.; Lulu, Z.; Hui, G.; Lijun, L.; Gang, Y.; Wangtian, W.; Yuanyuan, P.; Yan, F.; et al. Effects of exogenous calcium and calcium inhibitor on physiological characteristics of winter turnip rape (Brassica rapa) under low temperature stress. BMC Plant Biol. 2024, 24, 937. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, H.; Xiao, Q.; Li, X.; Shao, Q. Combined analysis of metabolome and transcriptome provides insights into metabolisms of chlorophylls, carotenoids, and flavonoids in the yellowing leaves of ‘HAES344’ macadamia. Sci. Hortic. 2023, 308, 111600. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Fu, H.; Wang, K.; Tian, X.; Qiu, R.; Liu, J.; Gao, S.; Zhong, Z.; Yang, B.; et al. Transcriptomic analysis unravels the molecular response of Lonicera japonica leaves to chilling stress. Front. Plant Sci. 2022, 13, 1092857. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Li, Z.; Tian, Y.; Gao, R.; Hao, L.; Hu, Y.; He, C.; Sun, W.; Xu, M.; Peters, R.J.; et al. The honeysuckle genome provides insight into the molecular mechanism of carotenoid metabolism underlying dynamic flower coloration. New Phytol. 2020, 227, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids. Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Chen, C.; Zou, L.; Yin, S.; Liu, S.; Yuan, J.; Wu, N.; Liu, X. Comparative transcriptome analysis reveals variations of bioactive constituents in Lonicera japonica flowers under salt stress. Plant Physiol. Biochem. 2022, 173, 87–96. [Google Scholar] [CrossRef]
- Brady, K.; Talbot, C.C., Jr.; Long, J.A.; Welch, G.; French, N.; Nicholson, D.; Bakst, M.R. Transcriptome analysis of blastoderms 481 exposed to prolonged egg storage and short periods of incubation during egg storage. BMC Genomics 2022, 23, 262. [Google Scholar] [CrossRef]
- Hormaetxe, K.; Hernández, A.; Becerril, J.M.; García-Plazaola, J.I. Role of red carotenoids in photoprotection during winter acclimation in Buxus sempervirens leaves. Plant Biol. 2004, 6, 325–332. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in Corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Tang, W.; Fan, S.; Bai, M.; Chen, M.; Yang, G. Role of calcium in regulating anthocyanin accumulation in ‘Manicure Finger’ grape berries. Sci. Hortic. 2019, 256, 108585. [Google Scholar] [CrossRef]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low Temperature Promotes Anthocyanin Biosynthesis and Related Gene Expression in the Seedlings of Purple Head Chinese Cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Qi, J.; Su, L.; Wang, X.; Wang, M.; Chen, B.; Yu, X.; Zhao, X.; Gao, W.; Guo, X.; et al. Chalcone synthase 2 (BpCHS2), a structural gene, was activated by low temperature to promote anthocyanin synthesis in Broussonetia papyrifera to improve its cold tolerance. Plant Physiol. Biochem. 2025, 222, 109656. [Google Scholar] [CrossRef]
- Petridis, A.; Döll, S.; Nichelmann, L.; Bilger, W.; Mock, H.P. Arabidopsis thaliana G2-LIKE FLAVONOID REGULATOR and BRASSINOSTEROID ENHANCED EXPRESSION1 are low-temperature regulators of flavonoid accumulation. New Phytol. 2016, 211, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.H.; Park, T.K.; Kim, Y.P.; Lee, J.W.; Kim, T.W. Transcription factors BZR1 and PAP1 cooperate to promote anthocyanin biosynthesis in Arabidopsis shoots. Plant Cell 2024, 36, 3654–3673. [Google Scholar] [CrossRef]
- Lucchin, A.; Fouassier, H.; Robe, E.; Mbengue, M.; Aguilar, M.; San Clemente, H.; Vert, G.; Galaud, J.P.; Aldon, D. The calcium sensor AtCML8 contributes to Arabidopsis plant cell growth by modulating the brassinosteroid signaling pathway. Plant J. 2025, 121, e17179. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Gerós, H. Calcium- and hormone-driven regulation of secondary metabolism and cell wall enzymes in grape berry cells. J. Plant Physiol. 2018, 231, 57–67. [Google Scholar] [CrossRef]
- Martins, V.; Billet, K.; Garcia, A.; Lanoue, A.; Gerós, H. Exogenous calcium deflects grape berry metabolism towards the production of more stilbenoids and less anthocyanins. Food Chem. 2020, 313, 126123. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- López-Maury, L.; Marguerat, S.; Bähler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008, 9, 583–593. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Ren, H.; Zhao, T.; Li, Q.; Wang, S.; Zhang, Y.; Xiao, F.; Wang, X. BAK1 Mediates light intensity to phosphorylate and activate catalases to regulate plant growth and development. Int. J. Mol. Sci. 2020, 21, 1437. [Google Scholar] [CrossRef]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002, 2, 505–513. [Google Scholar] [CrossRef]
- Huang, C.-K.; Lo, P.-C.; Huang, L.-F.; Wu, S.-J.; Yeh, C.-H.; Lu, C.-A. A single-repeat MYB transcription repressor, MYBH, participates in regulation of leaf senescence in Arabidopsis. Plant Mol. Biol. 2015, 88, 269–286. [Google Scholar] [CrossRef]
- Su, C.-F.; Wang, Y.-C.; Hsieh, T.-H.; Lu, C.-A.; Tseng, T.-H.; Yu, S.-M. A Novel MYBS3-Dependent Pathway Confers Cold Tolerance in Rice. Plant Physiol. 2010, 153, 145–158. [Google Scholar] [CrossRef]
- Yang, Q.S.; Gao, J.; He, W.D.; Dou, T.X.; Ding, L.J.; Wu, J.H.; Li, C.Y.; Peng, X.X.; Zhang, S.; Yi, G.J. Comparative transcriptomics analysis reveals difference of key gene expression between banana and plantain in response to cold stress. BMC Genomics 2015, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, R.; Yang, X.; Ju, Q.; Li, W.; Lü, S.; Tran, L.P.; Xu, J. The R2R3-MYB transcription factor AtMYB49 modulates salt tolerance in Arabidopsis by modulating the cuticle formation and antioxidant defence. Plant Cell Environ. 2020, 43, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Shi, C.; Peng, Y.; Tan, H.; Xin, P.; Yang, Y.; Wang, F.; Li, X.; Chu, J.; Huang, J.; et al. Brassinosteroid-activated BRI1-EMS-SUPPRESSOR 1 inhibits flavonoid biosynthesis and coordinates growth and UV-B stress responses in plants. Plant Cell 2020, 32, 3224–3239. [Google Scholar] [CrossRef] [PubMed]

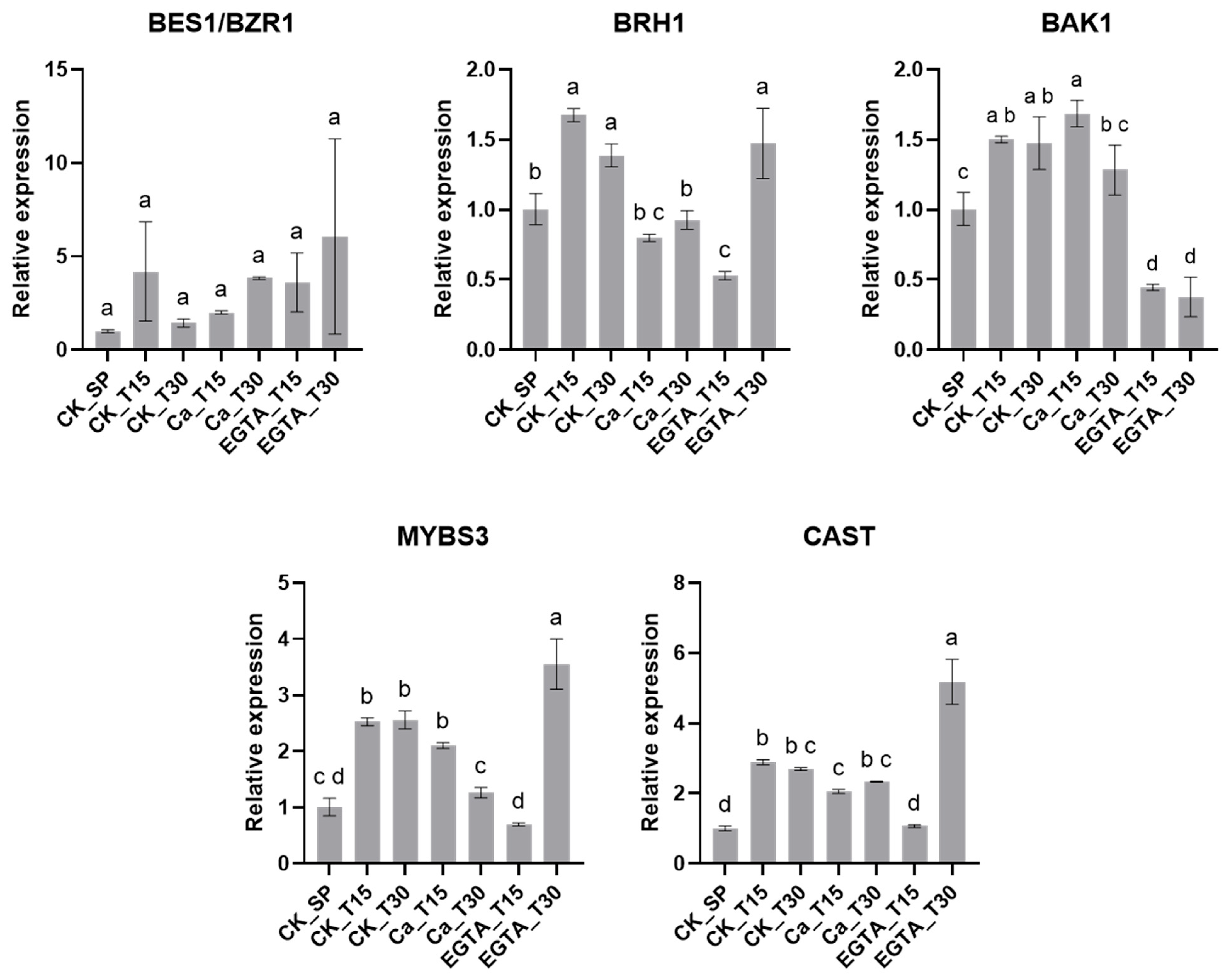
| No. | Description a | Function b | FPKM Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CK_SP | CK_T15 | CK_T30 | Ca_T15 | Ca_T30 | EGTA_T15 | EGTA_T30 | |||
| 1 | MYBS3 | RNA | 157.39 | 167.39 | 84.15 | 148.52 | 155.14 | 246.83 | 215.14 |
| 2 | BRH1 | Hormone | 46.15 | 46.15 | 54.00 | 41.18 | 44.84 | 104.06 | 106.58 |
| 3 | BAK1 | Hormone | 32.47 | 32.47 | 31.20 | 32.68 | 30.06 | 39.85 | 37.43 |
| 4 | BES1/BZR1 | Hormone | 25.93 | 24.81 | 24.14 | 26.08 | 31.11 | 38.54 | 28.36 |
| 5 | CAST | Signaling | 43.86 | 80.32 | 66.40 | 68.52 | 55.27 | 124.24 | 113.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zheng, W.; Que, R.; Lv, J.; Wang, P.; Li, J.; Zhang, L.; Yang, B. Transcriptomic Analysis Reveals the Regulation Function of Calcium Ions Regarding Anthocyanin Biosynthesis in Lonicera japonica Under Cold Stress. Horticulturae 2025, 11, 1023. https://doi.org/10.3390/horticulturae11091023
Chen J, Zheng W, Que R, Lv J, Wang P, Li J, Zhang L, Yang B. Transcriptomic Analysis Reveals the Regulation Function of Calcium Ions Regarding Anthocyanin Biosynthesis in Lonicera japonica Under Cold Stress. Horticulturae. 2025; 11(9):1023. https://doi.org/10.3390/horticulturae11091023
Chicago/Turabian StyleChen, Jie, Wenxi Zheng, Ruonan Que, Junle Lv, Pei Wang, Jiachen Li, Lin Zhang, and Bingxian Yang. 2025. "Transcriptomic Analysis Reveals the Regulation Function of Calcium Ions Regarding Anthocyanin Biosynthesis in Lonicera japonica Under Cold Stress" Horticulturae 11, no. 9: 1023. https://doi.org/10.3390/horticulturae11091023
APA StyleChen, J., Zheng, W., Que, R., Lv, J., Wang, P., Li, J., Zhang, L., & Yang, B. (2025). Transcriptomic Analysis Reveals the Regulation Function of Calcium Ions Regarding Anthocyanin Biosynthesis in Lonicera japonica Under Cold Stress. Horticulturae, 11(9), 1023. https://doi.org/10.3390/horticulturae11091023






