Basic Characteristics, Superior Individual Selection, and Comprehensive Evaluation of 12 Wild Vernicia fordii (Vernicia fordii (Hemsl.) Airy Shaw) Trees in the Hunan–Guizhou Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Scope and Criteria for Selecting Elite Individuals
2.2. Method for Selecting Elite Individuals
2.2.1. Indicator Measurements and Comprehensive Evaluation for the Preliminary Selection of Elite Individuals
2.2.2. Fatty Acid Composition of the Oil from the Selected Elite Individuals in the Secondary Selection
2.2.3. Physicochemical Properties of the Tung Oil from the Selected Elite Individuals in the Secondary Selection
2.2.4. Evaluating the Floral Phenotypic Characteristics of Selected Superior Trees
2.2.5. Chlorophyll Content of the Selected Elite Individuals in Secondary Selection
2.2.6. Anatomical Structure and Photosynthetic Characteristics of the Leaves from Selected Superior Individuals
2.2.7. Simple Sequence Repeat (SSR) Molecular Marker and Cluster Analyses of the Selected Elite Trees in Secondary Selection
2.2.8. Comprehensive Evaluation Method for the Selected Elite Trees
2.2.9. Data Statistics and Analytical Methods
3. Results
3.1. Screening of the Preliminary Elite Trees
3.2. Comparative Analysis of Oil Quality Among the Selected Elite Trees in Secondary Selection
3.2.1. Comparative Analysis of Fatty Acid Composition Among the Selected Elite Trees in Secondary Selection
3.2.2. Comparative Analysis of the Physicochemical Properties of the Oil from Selected Elite Individuals in Secondary Selection
3.3. Comparative Analysis of Growth Traits Among the Reselected Elite Strains
3.3.1. Comparative Analysis of Floral Phenotypic Characteristics and Pollen Quality
3.3.2. Comparative Analysis of Pigment Contents in the Leaves of Selected Elite Plants
3.3.3. Comparative Analysis of Stomatal Density Among the Reselected Elite Strains
3.3.4. Comparative Analysis of Leaf Anatomical Cross-Sectional Structure Among the Reselected Elite Strains
3.3.5. Comparative Analysis of Photosynthetic Performance Among the Selected Superior Individuals
3.4. Comprehensive Evaluation of the Reselected Superior Plants Using the Entropy Weight–TOPSIS Method
3.5. SSR Molecular Marker and Clustering Analyses of Selected Superior Plants
3.5.1. Screening of SSR Primers and Polymorphism Analysis of the Amplified Products
3.5.2. Comparative Analysis of Genetic Diversity Between Selected Superior Plants
3.5.3. Cluster Analysis Based on SSR Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Liao, Y.; Tan, X. Discussion on coordinated development of Vernicia fordii industry and future environment-friendly coatings industry in China. Nonwood For. Res. 2018, 36, 188–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, Z.; Luo, K. Advances in biological research of tung tree (Vernicia fordii). Nonwood For. Res. 2020, 38, 238–245. [Google Scholar] [CrossRef]
- Cao, L.; Zhong, Q.; Zou, Y.; Chen, Y.; Wan, X.; Lu, Y. Comparison and Cluster Analysis of Photosynthetic Characteristics of Different Vernicia fordii Germplasm Resources. For. Sci. Technol. 2023, 9, 43–47. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, R.; Tan, X.; Li, Z. Establishing an efficient callus genetic transformation system for the tung tree (Vernicia fordii). Ind. Crops Prod. 2024, 211, 118264. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Ban, Q.; Cheng, T. Screening and Evaluation of Vernicia fordii Plus Trees with 3 Years Old in Guizhou. Guizhou Agric. Sci. 2017, 45, 5–8. [Google Scholar]
- Sun, Y. Comprehensive Evaluation of Fruit Quality of Vernicia fordii Based on Principal Component Analysis in Yunyang County. J. Anhui Agric. Sci. 2024, 52, 140–142+156. [Google Scholar]
- Shuang, D.; An, L.; Wang, F.; Dan, H.; Kong, F.; He, Y.; Xu, Y. Fruit Analysis and Pedigree Screening of Vernicia fordii. Hubei For. Sci. Technol. 2019, 48, 7–10. [Google Scholar]
- Cai, X.; Huang, Z.; Zhang, X.; Zhang, W.; Xiao, Z. Selection of superior trees of Vernicia fordii in the northern distribution margin description of the major traits. J. Anhui Agric. Univ. 2015, 42, 775–779. [Google Scholar] [CrossRef]
- Wu, H.; Gu, Y.; Chen, J.; Gao, J.; Luo, J. A Study of Variation of Seed Character in Natural Populations of Aleuvites fordii in Sichuan and Chongqing Areas. J. Sichuan For. Sci. Technol. 2014, 35, 6–13. [Google Scholar] [CrossRef]
- Wen, S. Research on Main Economic Traits of 36 Elite Families of Tung Oil Tree. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2013. [Google Scholar]
- Perera, R.; Huerta, M.C.; Barris, C.; Baena, M. Clustering classifier of FRP strengthened concrete beams using superpixels and principal component analysis. Constr. Build. Mater. 2024, 453, 139019. [Google Scholar] [CrossRef]
- Zhao, J. Evaluation of Main Traits and Selection of Superior Individuals of Camellia gauchowensis in Jieyang Region of Guangdong Province. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2021. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Zhang, Y.; Lao, J.; Liu, Y.; Wang, H.; Jiang, B. Effects and interaction of meteorological factors on influenza: Based on the surveillance data in Shaoyang, China. Environ. Res. 2019, 172, 326–332. [Google Scholar] [CrossRef]
- Ye, J.X.; Sun, Y.Z.; Yao, T.M. A Keichousaurus-bearing regurgitalite from the Middle Triassic Xingyi Fauna, Dingxiao of Xingyi City, Guizhou, South China. Palaeoworld 2024, 33, 363–373. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, B.; Tan, X.; Thammina, C.S.; Long, H.; Liu, M.; Wen, S.; Song, X.; Cao, H.; Prasad, M. Fatty acid profile and unigene-derived simple sequence repeat markers in tung tree (Vernicia fordii). PLoS ONE 2017, 9, e105298. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.; Zhang, Z.; Liu, C.; Yang, X.; Long, L.; Chen, L.; Wang, R. Analysis of Fruit Economic Characters and Fatty Traits of Camellia oleifera Cultivars. J. Chin. Cereals Oils Assoc. 2025, 40, 112–118. [Google Scholar] [CrossRef]
- Janik-Zabrotowicz, E.; Arczewska, M.; Hamera, J.; Sofińska-Chmiel, W.; Łastawiecka, E.; Gagoś, M. Epoxy resin-mediated transformation of chlorophyll-rich photosynthetic pigments extract into high-yield fluorescent products. J. Lumin. 2025, 277, 120962. [Google Scholar] [CrossRef]
- Sijin, L.; Nan, Y.; Longqing, C. Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J. 2022, 8, 221–229. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, R.; Chen, H. The Establishment of Isolation and Transient Transformation Methods of Protoplasts of Vernicia fordii Mesophyll Cells. J. For. Sci. 2018, 54, 46–53. [Google Scholar]
- Li, Z.; Ma, F.; Zhang, H.; Luo, C.R.; Liu, Y.Y.; Mi, X.Q.; Li, L.S.; Shu, H.Y.; Tan, X.F. Grafting Techniques of Tung Tree Seedlings with Hypocotyle Rootstocks and the Anatomical Structure. J. For. Sci. 2024, 60, 80–92. [Google Scholar]
- Tian, K.; Xu, J.; Dai, L.; Li, Z.; Geng, X.; Liu, Z.; Wang, Y. Physiological Response of Idesia polycarpa Seedlings to Extreme High Temperature and High Temperature Plus Drought Stress. Sci. Silvae Sin. 2024, 60, 84–92. [Google Scholar]
- Ahmed, A.; Abdalla, A.A.A.; Elsafy, M.; Ezzeldin, A.; Rahmatov, M.; Abdelhalim, T. Harnessing genetic diversity in Sudanese sorghum wild relatives for stay-green drought tolerance via microsatellite (SSR) marker assessment. Genet. Resour. Crop Evol. 2024, 72, 4699–4711. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Huai, H.; Liu, A. Development of EST-SSR markers and investigation of genetic relatedness in tung tree. Tree Genet. Genomes 2012, 8, 933–940. [Google Scholar] [CrossRef]
- Shen, Z.; Min, H.; Wang, L.; Zhang, Y. Evaluation of Carbon Neutrality Capacity of Regional Construction Industry Based on the Entropy Weight TOPSIS Model. Buildings 2024, 14, 2363. [Google Scholar] [CrossRef]
- Wang, X.; Shang, H. Cultivation and Processing Utilization Techniques of Tung Oil Tree. For. Ecol. 2023, 8, 39–40. [Google Scholar] [CrossRef]
- Liu, X.; Liu, N.; Shang, H.; Pan, X.; Wang, X.; Zeng, Y. Identification and differential analysis of metabolomic composition ofleaves, flowers and shells of Vernicia fordii. Plant Physiol. J. 2021, 57, 2366–2378. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Ge, C.; Zhang, L.; Tan, X.; Liu, M. Effects of exogenous hormones on the fiowering and fruit characteristics of male tung trees of ‘putaotong’ (Vernicia fordii Hemsl.). J. Cent. South Univ. For. Technol. 2023, 43, 20–32+110. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, X.; Dai, X.; Wang, D.; Ma, L.; Li, L.; Zeng, B. Comprehensive evaluation of resistance of Aleurites fordii superior family based on principal component analysis and clustering analysis. J. Cent. South Univ. For. Technol. 2012, 32, 24–27. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Zhang, L.; Tan, X.; Zhang, F.; Wang, Z. Study of major economic traits in 4 superior families of tung tree. Nonwood For. Res. 2018, 36, 29–34. [Google Scholar] [CrossRef]
- Li, S.; He, S.; Long, H.; Tan, X. Oil contents and fatty acid compositions of Vernicia fordii seeds from one hundred excellent families. China Oils Fats 2017, 42, 123–127. [Google Scholar]
- Wang, S.; Tian, L.; Zhou, S.; Chu, Y.; Mei, D.; Yuan, J.; Yu, Y.; Fu, X. Effects of Polyploidization on Leaf Morphology, Photosynthetic Performance and Accumulation of Secondary Metabolites in Cyclocarya paliurus. Sci. Silvae Sin. 2024, 60, 120–131. [Google Scholar]
- Rajasree, S.S.; Lal, M.G. Assessment of Genetic Diversity in Greengram (Vigna radiata L.) Using Metroglyph Analysis. Arch. Curr. Res. Int. 2024, 24, 128–144. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, B.; Lee, C.; Shin, D.; Jung, K.-H. Identification of core drought-responsive genes for developing drought-tolerant rice varieties through meta-analysis of RNA-Seq data. Plant Biotechnol. Rep. 2024, 18, 705–718. [Google Scholar] [CrossRef]
- Jia, B.; Lin, Q.; Tan, X.; Li, Z.; Long, X.; Xiang, H.; Zhang, L. Development of EST-SSR Markers and Their Use for Genetic Diversity Analysis in Tung Tree (Vernicia fordii (Hemsl.) Airy Shaw). J. Plant Genet. Resour. 2016, 17, 625–636. [Google Scholar] [CrossRef]
- Fu, J.; Nan, L.; Zhang, Z.; Wu, S.; Chen, X. Evaluation of Morphological Characteristics and SSR Genetic Diversity of 21 Accessions of Timothy Seeds. Chin. J. Grassl. 2024, 46, 23–33. [Google Scholar] [CrossRef]

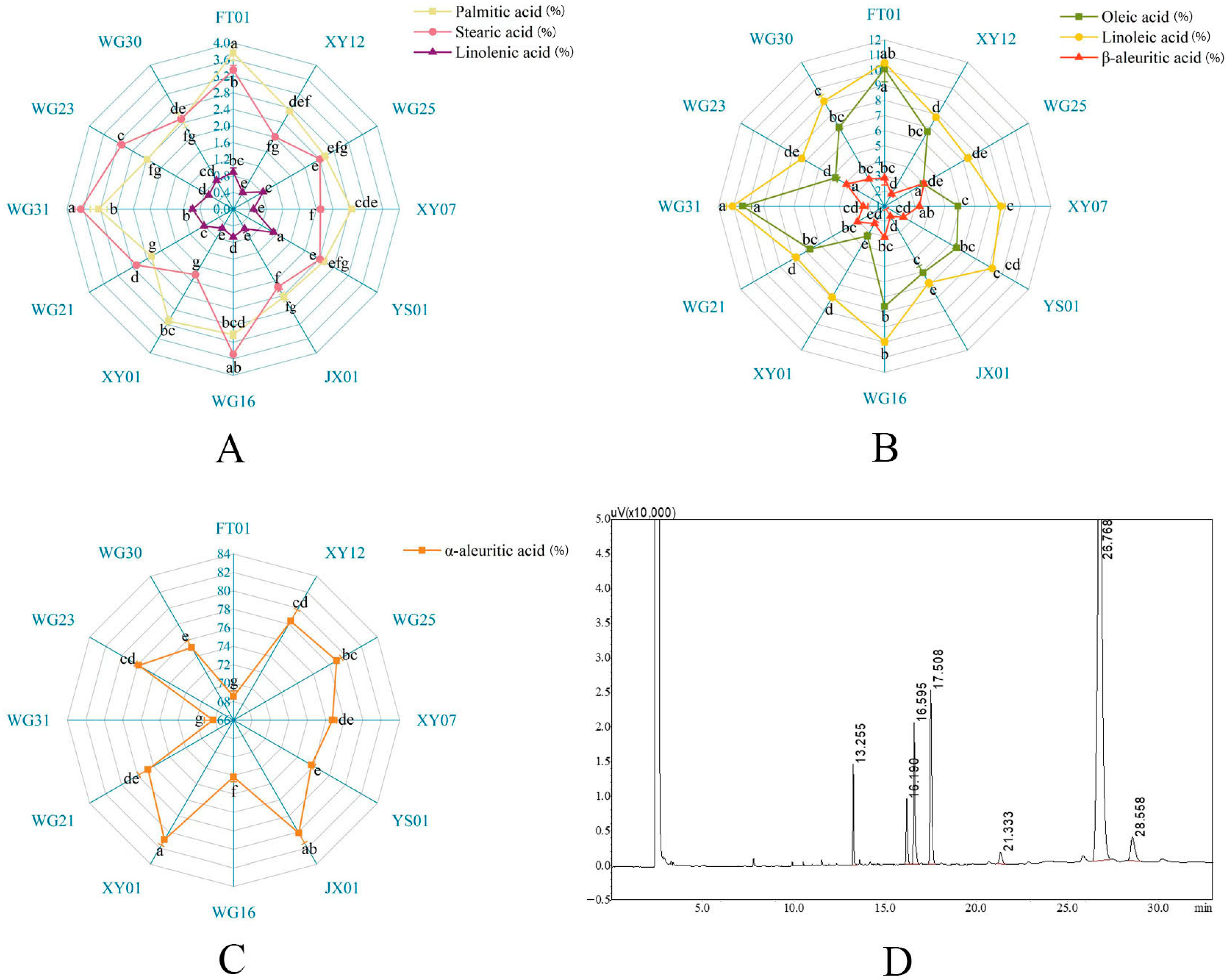


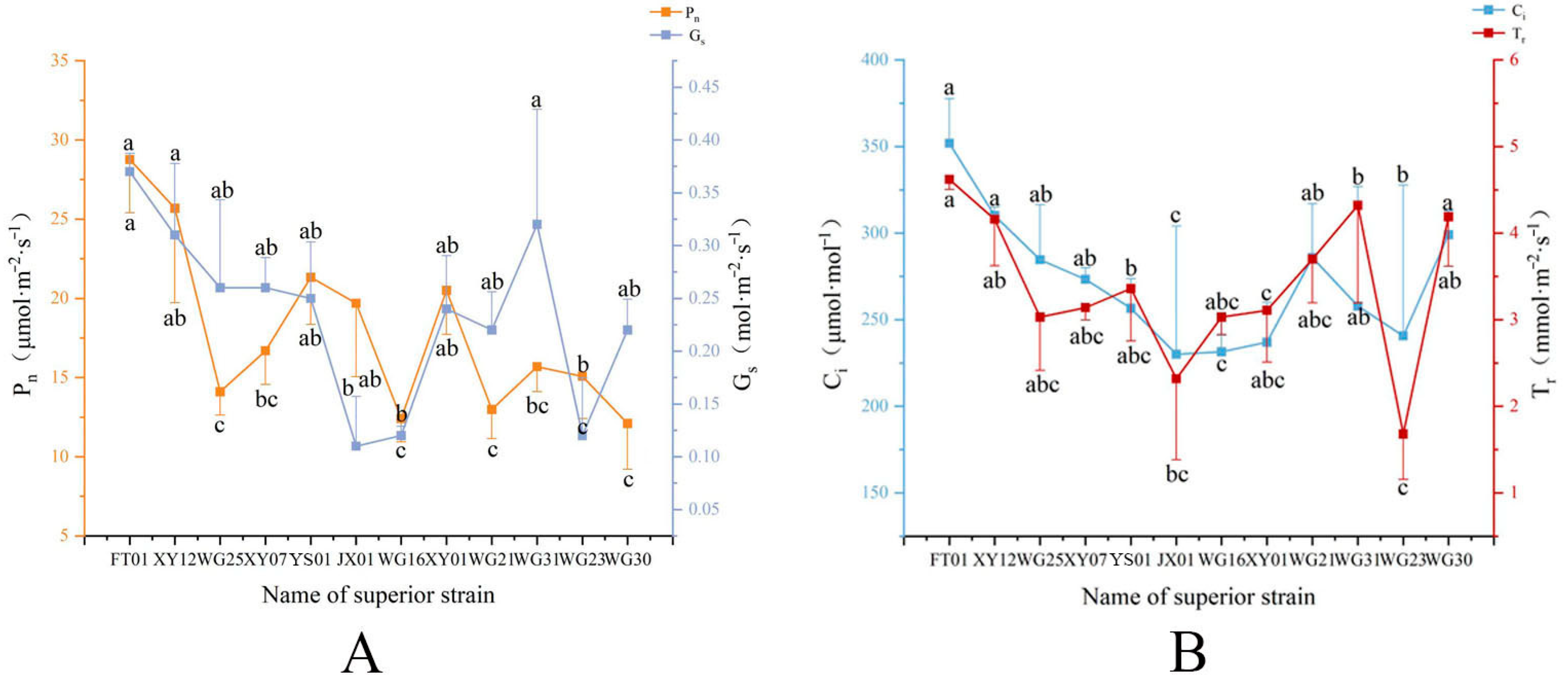
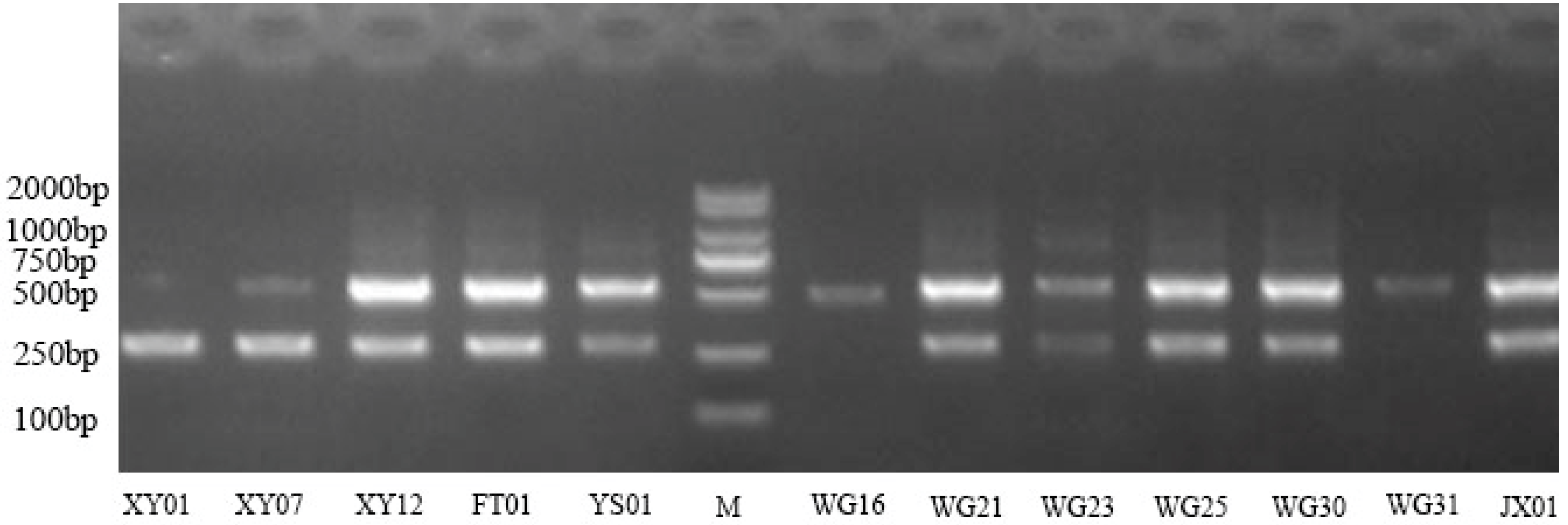
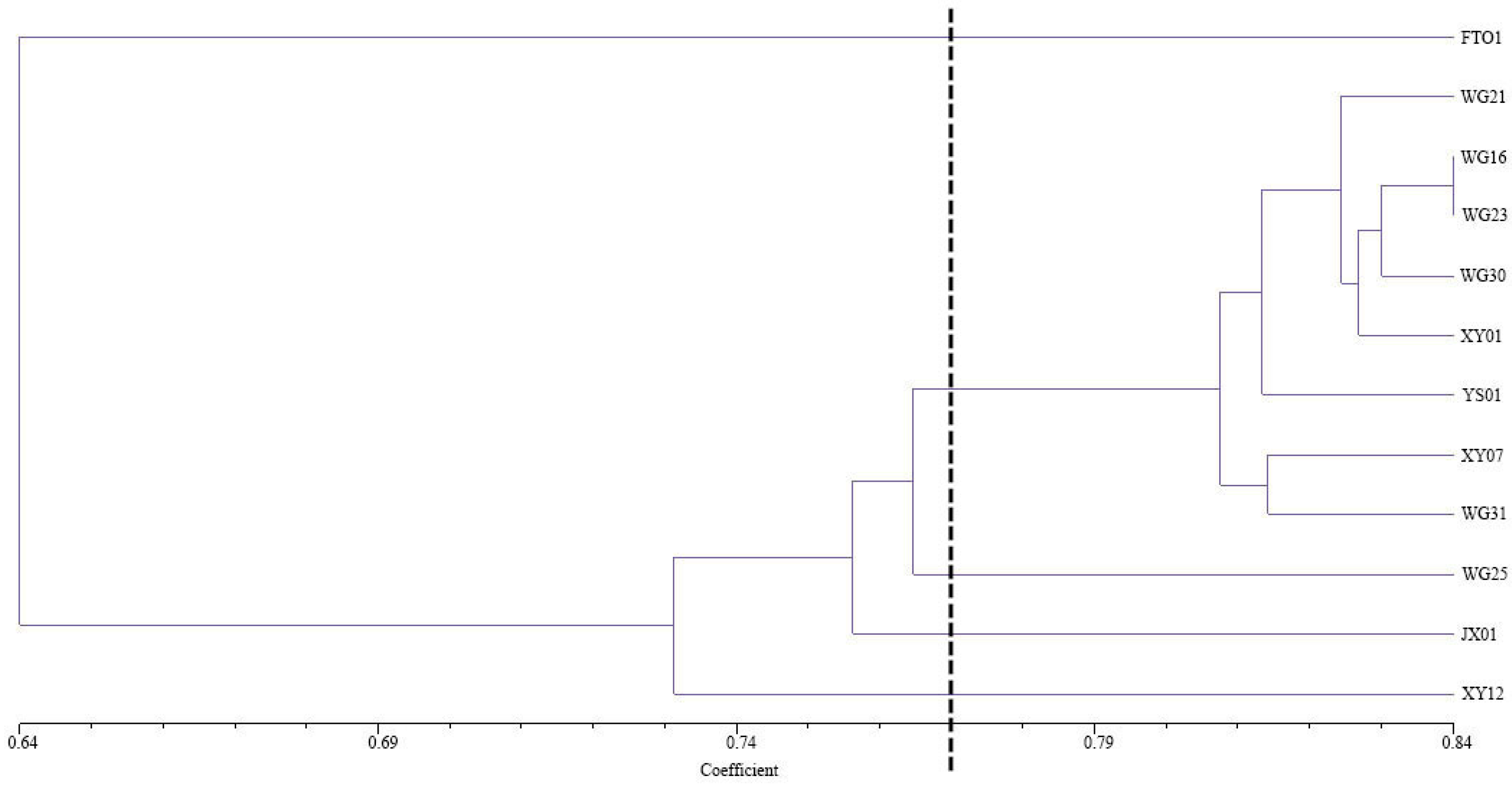
| Number | Codes | Yield Per Plant (kg) | Oil Yield per Plant (kg) | Peel Thickness (mm) | Fruit Transverse Diameter (mm) | Fruit Vertical Diameter (mm) | Fruit Shape Index | Single Fruit Weight (g) | Fresh Seed Rate (%) | Rate of Dryness (%) | Oil Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FT01 | 111.7 | 12.14 | 8.49 ± 0.11 | 64.22 ± 0.25 | 52.70 ± 0.11 | 0.82 ± 0.05 | 93.90 ± 0.55 | 31.84 ± 0.16 | 65.35 ± 0.19 | 52.25 ± 0.05 |
| 2 | XY12 | 61.4 | 9.78 | 6.48 ± 0.64 | 48.80 ± 0.61 | 48.80 ± 1.03 | 1.05 ± 0.07 | 59.06 ± 0.28 | 39.33 ± 0.33 | 65.50 ± 0.33 | 61.85 ± 0.84 |
| 3 | WG25 | 53.6 | 8.29 | 7.59 ± 0.62 | 55.39 ± 0.45 | 57.00 ± 0.43 | 1.03 ± 0.11 | 79.26 ± 1.47 | 42.10 ± 0.46 | 62.55 ± 0.53 | 58.74 ± 0.03 |
| 4 | XY07 | 50.5 | 7.84 | 6.52 ± 0.82 | 50.23 ± 0.48 | 45.70 ± 0.08 | 1.00 ± 0.09 | 62.18 ± 0.25 | 42.12 ± 0.44 | 58.13 ± 0.23 | 63.41 ± 0.04 |
| 5 | YS01 | 49.5 | 6.59 | 6.72 ± 0.25 | 56.74 ± 0.45 | 57.97 ± 0.64 | 1.02 ± 0.02 | 71.70 ± 0.57 | 44.54 ± 0.71 | 66.07 ± 0.72 | 45.22 ± 0.03 |
| 6 | JX01 | 52.9 | 5.25 | 8.64 ± 0.35 | 61.00 ± 0.42 | 70.40 ± 0.62 | 1.15 ± 0.05 | 96.84 ± 0.26 | 40.65 ± 0.52 | 64.97 ± 0.47 | 37.56 ± 0.05 |
| 7 | WG16 | 42.7 | 4.88 | 9.07 ± 0.43 | 66.47 ± 1.52 | 60.01 ± 0.03 | 0.91 ± 0.02 | 105.33 ± 3.12 | 39.82 ± 0.09 | 65.01 ± 0.11 | 44.12 ± 0.72 |
| 8 | XY01 | 58.9 | 6.49 | 7.96 ± 0.86 | 68.63 ± 0.74 | 1.27 ± 0.10 | 1.27 ± 0.04 | 70.37 ± 1.23 | 30.62 ± 0.35 | 59.88 ± 0.33 | 60.10 ± 0.41 |
| 9 | WG21 | 47.2 | 5.86 | 8.87 ± 0.37 | 57.44 ± 0.35 | 57.47 ± 0.87 | 1.00 ± 0.03 | 78.27 ± 1.38 | 41.21 ± 0.24 | 62.50 ± 0.55 | 48.21 ± 0.03 |
| 10 | WG31 | 62.7 | 6.49 | 7.36 ± 0.26 | 56.70 ± 0.24 | 59.25 ± 0.83 | 1.04 ± 0.05 | 68.94 ± 0.57 | 34.53 ± 0.72 | 58.20 ± 0.65 | 51.51 ± 0.05 |
| 11 | WG23 | 45.7 | 5.91 | 6.96 ± 0.38 | 57.26 ± 0.92 | 58.06 ± 0.65 | 1.02 ± 0.09 | 78.24 ± 1.09 | 43.42 ± 0.63 | 58.78 ± 0.82 | 50 ± 67 ± 0.11 |
| 12 | WG30 | 32.3 | 3.83 | 8.42 ± 0.63 | 62.75 ± 0.36 | 69.14 ± 0.47 | 1.10 ± 0.04 | 92.36 ± 1.84 | 38.56 ± 0.37 | 67.19 ± 0.23 | 45.76 ± 0.32 |
| Code | Acid Value (mg/g) | Iodine Value (g/100 g) | Saponification Value (mg/g) |
|---|---|---|---|
| FT01 | 1.15 ± 0.04 f | 170.98 ± 0.23 e | 196.96 ± 0.38 c |
| XY12 | 0.98 ± 0.03 gh | 172.84 ± 0.25 d | 195.51 ± 0.21 d |
| WG25 | 1.40 ± 0.06 e | 159.39 ± 0.47 g | 177.48 ± 0.06 g |
| XY07 | 1.64 ± 0.03 d | 160.08 ± 0.21 g | 167.59 ± 0.28 i |
| YS01 | 0.87 ± 0.02 h | 176.74 ± 0.22 b | 208.53 ± 0.27 a |
| JX01 | 0.69 ± 0.01 i | 182.59 ± 0.10 a | 201.70 ± 0.12 b |
| WG16 | 2.25 ± 0.10 c | 153.73 ± 0.32 h | 187.55 ± 0.21 e |
| XY01 | 2.46 ± 0.05 b | 146.89 ± 0.18 i | 152.06 ± 0.11 l |
| WG21 | 2.23 ± 0.04 c | 162.79 ± 0.18 f | 169.78 ± 0.14 h |
| WG31 | 1.12 ± 0.08 fg | 175.96 ± 0.19 c | 180.29 ± 0.50 f |
| WG23 | 2.81 ± 0.03 a | 140.91 ± 0.18 k | 153.75 ± 0.15 k |
| WG30 | 2.82 ± 0.02 a | 141.72 ± 0.25 j | 163.47 ± 0.18 j |
| Min | 0.69 | 140.91 | 152.06 |
| Max | 2.82 | 182.59 | 208.53 |
| Mean value | 1.7 | 162.05 | 179.56 |
| Standard deviation | 0.78 | 14.14 | 18.77 |
| Coefficient of variation/% | 45.56 | 8.72 | 10.45 |
| Code | D+ | D− | C | Sorting Results |
|---|---|---|---|---|
| FT01 | 0.1064 | 0.2298 | 0.6836 | 1 |
| XY12 | 0.1386 | 0.2013 | 0.5922 | 2 |
| WG25 | 0.1533 | 0.1695 | 0.5251 | 4 |
| XY07 | 0.1775 | 0.1663 | 0.4838 | 6 |
| YS01 | 0.1858 | 0.1548 | 0.4544 | 7 |
| JX01 | 0.1610 | 0.1918 | 0.5436 | 3 |
| WG16 | 0.1961 | 0.1411 | 0.4184 | 10 |
| XY01 | 0.1831 | 0.1399 | 0.4332 | 9 |
| WG21 | 0.1726 | 0.1434 | 0.4537 | 8 |
| WG31 | 0.1671 | 0.1697 | 0.5039 | 5 |
| WG23 | 0.2046 | 0.1398 | 0.4059 | 11 |
| WG30 | 0.2141 | 0.1361 | 0.3887 | 12 |
| Primer ID | Primer Sequence | Na | Ne | I | Ho | He | PIC |
|---|---|---|---|---|---|---|---|
| VFEST12 | F:TTATGTGTGTTGATGTGGCT R:TTCTCTGCTTCTCCCTCTC | 3 | 1.6969 | 0.5937 | 0.3792 | 0.4857 | 0.4506 |
| VFEST18 | F:GCCAAAGAAACCTAAGAC R:ACAAGCAAAACAAAGAGAC | 2 | 1.4279 | 0.4831 | 0.2263 | 0.3728 | 0.3391 |
| VFEST20 | F:TGGCATTGGCACTCACTACAG R:TAAGTTCACAAAAGCGGTCACA | 2 | 1.2236 | 0.3159 | 0.1925 | 0.2794 | 0.2563 |
| VFEST3 | F:TGGGAAACAATAATGGGAGG R:CGGGAACTAATAAAATCAAGCC | 2 | 1.3022 | 0.3932 | 0.2531 | 0.3847 | 0.3495 |
| VFEST41 | F:CACTGCTGGTAACGGAACTG R:ATAAGACTCCACCGACGCT | 2 | 1.5379 | 0.5211 | 0.1908 | 0.3957 | 0.3718 |
| VFEST5 | F:ATTCCAACTCAAAAACTCTG R:TTGATTTACAGAGCAAGTGAT | 2 | 1.0985 | 0.1973 | 0.1296 | 0.1748 | 0.1486 |
| VFEST51 | F:AGCGGCAACACCAGCAACT R:TGGGTAGAGGGAGGAGGCAT | 2 | 1.3726 | 0.4127 | 0.2625 | 0.3708 | 0.3252 |
| VFEST55 | F:GTCTCTCTCTTTCTATCTGTAACC R:GCTTCAGGCTCTAAATCTTC | 2 | 1.4365 | 0.5155 | 0.3124 | 0.4598 | 0.4199 |
| VFEST58 | F:ATCCCTATTGATGAGACC R:TTAACACTAACTATACTTGACACT | 3 | 1.7501 | 0.6506 | 0.3684 | 0.5227 | 0.5005 |
| VFEST60 | F:CTCCACCCAGTCTTCTACTTCAC R:ATCCAATAGCGTAAGATGACAAAG | 2 | 1.8734 | 0.7439 | 0.4463 | 0.6051 | 0.5596 |
| VFEST62 | F:TAATCCCATCGCCAAATCC R:TTCCGAAGAAACCGCAGT | 4 | 2.1428 | 0.9723 | 0.4272 | 0.6059 | 0.5833 |
| VFEST65 | F:GGAGGATGATGAAGTCAGAGAG R:GAGTGTGTCAACTGCCCAAC | 2 | 1.8927 | 0.7327 | 0.3894 | 0.5736 | 0.5385 |
| VFEST68 | F:ATCAGGGCTTGGTTTTGGGT R:ATAGGTAGGGGAGGCAGAGGAG | 2 | 1.3573 | 0.4008 | 0.1844 | 0.3581 | 0.3278 |
| VFEST8 | F:GCAATCTTCCCTCCAATGA R:TGTAGTTTTTCCCTGATAGCATTA | 3 | 1.5524 | 0.5114 | 0.3351 | 0.4383 | 0.4025 |
| Mean value | 2.2142 | 1.5461 | 0.5325 | 0.2926 | 0.4305 | 0.3981 |
| Code | Na | Ne | I | Ho | He |
|---|---|---|---|---|---|
| FT01 | 2.0000 | 1.4399 | 0.5829 | 0.3465 | 0.5114 |
| XY12 | 1.7500 | 1.3674 | 0.4761 | 0.2354 | 0.3202 |
| WG25 | 1.6667 | 1.2537 | 0.3946 | 0.2021 | 0.2883 |
| XY07 | 2.0000 | 1.0021 | 0.1753 | 0.0988 | 0.1136 |
| YS01 | 1.6667 | 1.1476 | 0.3724 | 0.2090 | 0.2948 |
| JX01 | 1.9167 | 1.5985 | 0.6391 | 0.4485 | 0.5638 |
| WG16 | 2.0833 | 1.3625 | 0.5287 | 0.2636 | 0.3817 |
| XY01 | 1.5833 | 0.8758 | 0.2068 | 0.1124 | 0.1693 |
| WG21 | 1.7467 | 0.9544 | 0.2103 | 0.1090 | 0.1582 |
| WG31 | 2.0000 | 1.7514 | 0.7116 | 0.4372 | 0.5641 |
| WG23 | 1.6667 | 1.1323 | 0.4725 | 0.3011 | 0.3561 |
| WG30 | 1.5000 | 0.8127 | 0.2329 | 0.1798 | 0.2003 |
| Mean value | 1.7983 | 1.2249 | 0.4169 | 0.2453 | 0.3268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.-Y.; Liu, Y.-Y.; Luo, C.-R.; Zhang, R.; Deng, L.; Li, L.-S.; Li, Z.; Tan, X.-F. Basic Characteristics, Superior Individual Selection, and Comprehensive Evaluation of 12 Wild Vernicia fordii (Vernicia fordii (Hemsl.) Airy Shaw) Trees in the Hunan–Guizhou Region. Horticulturae 2025, 11, 1024. https://doi.org/10.3390/horticulturae11091024
Shu H-Y, Liu Y-Y, Luo C-R, Zhang R, Deng L, Li L-S, Li Z, Tan X-F. Basic Characteristics, Superior Individual Selection, and Comprehensive Evaluation of 12 Wild Vernicia fordii (Vernicia fordii (Hemsl.) Airy Shaw) Trees in the Hunan–Guizhou Region. Horticulturae. 2025; 11(9):1024. https://doi.org/10.3390/horticulturae11091024
Chicago/Turabian StyleShu, Han-Yu, Ye-Yao Liu, Cheng-Rui Luo, Rong Zhang, Lei Deng, Le-Sheng Li, Ze Li, and Xiao-Feng Tan. 2025. "Basic Characteristics, Superior Individual Selection, and Comprehensive Evaluation of 12 Wild Vernicia fordii (Vernicia fordii (Hemsl.) Airy Shaw) Trees in the Hunan–Guizhou Region" Horticulturae 11, no. 9: 1024. https://doi.org/10.3390/horticulturae11091024
APA StyleShu, H.-Y., Liu, Y.-Y., Luo, C.-R., Zhang, R., Deng, L., Li, L.-S., Li, Z., & Tan, X.-F. (2025). Basic Characteristics, Superior Individual Selection, and Comprehensive Evaluation of 12 Wild Vernicia fordii (Vernicia fordii (Hemsl.) Airy Shaw) Trees in the Hunan–Guizhou Region. Horticulturae, 11(9), 1024. https://doi.org/10.3390/horticulturae11091024






