The ClTFL1-ClGRFs Module Regulates Lateral Branch Number and Flowering Time via Auxin-Mediated Pathway in Watermelon (Citrullus lanatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Phenotype Measurement
2.2. Hormone Measurement
2.3. Transcriptome Analysis
2.4. qRT-PCR Analysis
2.5. Yeast Two-Hybrid Assay
2.6. Bimolecular Fluorescence Complementation (BIFC) Assay
2.7. Watermelon Transformation
2.8. Statistical Analysis
3. Results
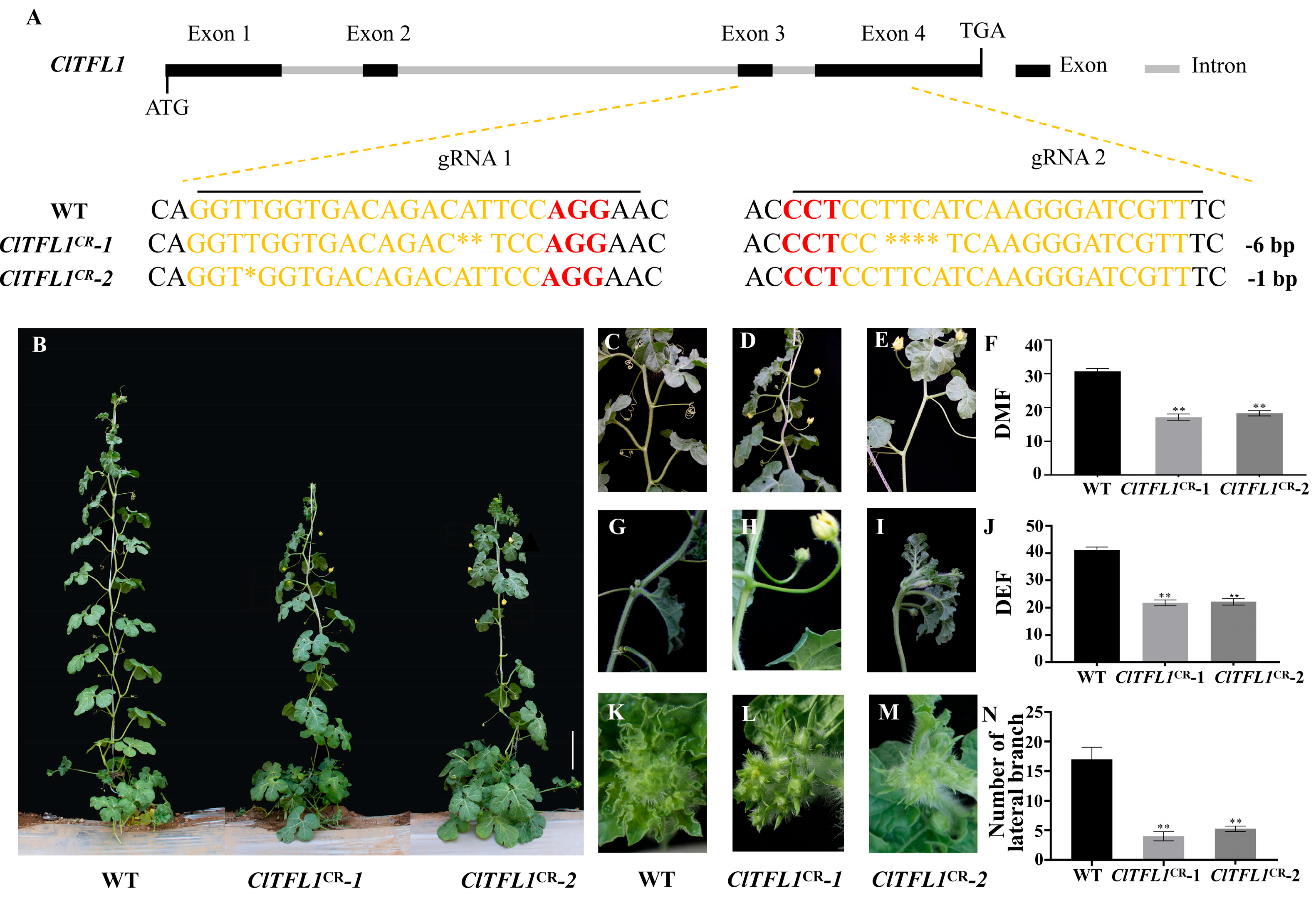
3.1. ClTFL1 Regulates Flowering Time and Number of Lateral Branches in Watermelon
3.2. Function Validation of ClTFL1 in Watermelon
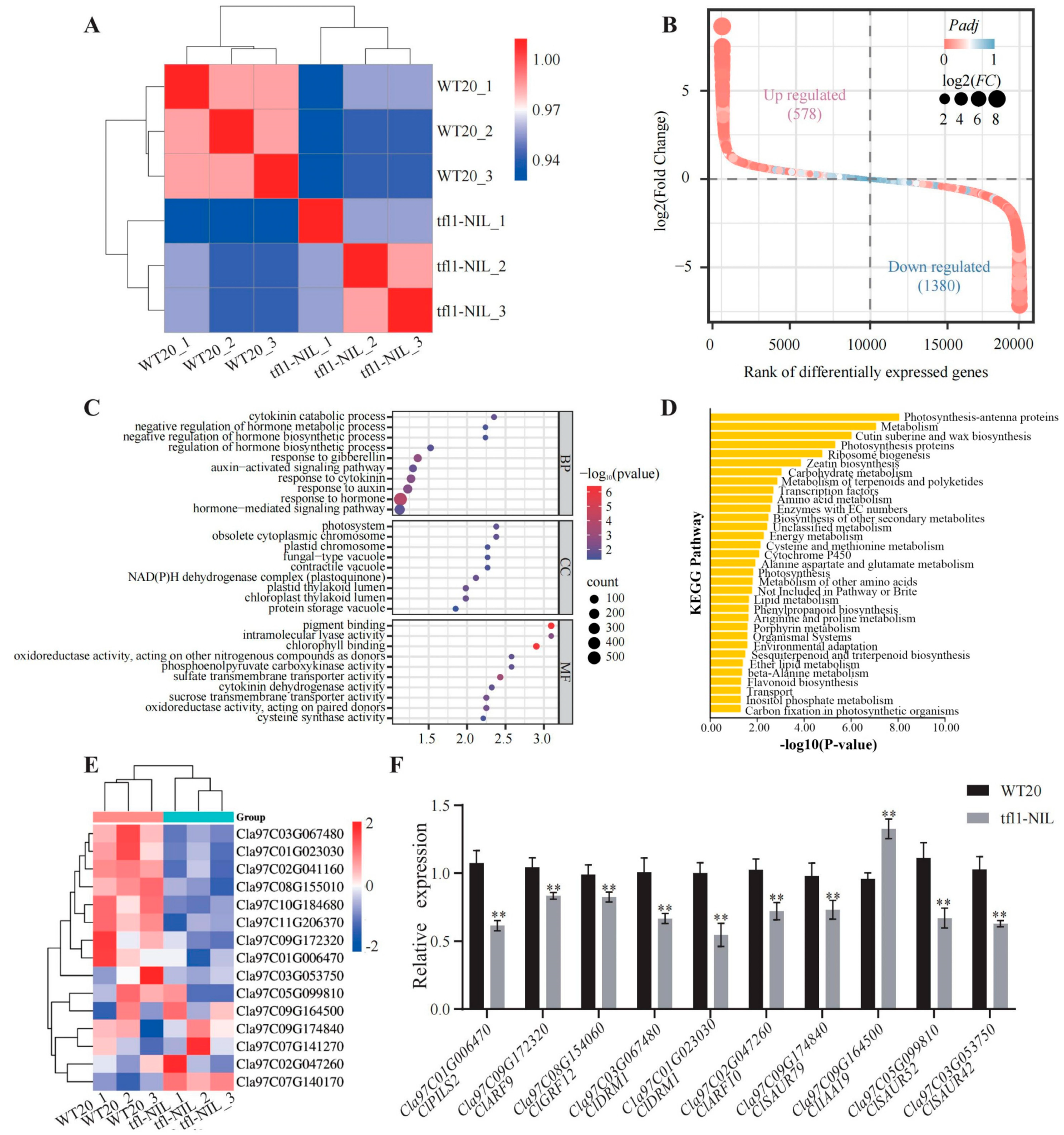
3.3. ClTFL1 Participates in Auxin-Mediated Pathway to Regulate the Flowering Time and the Number of Lateral Branches
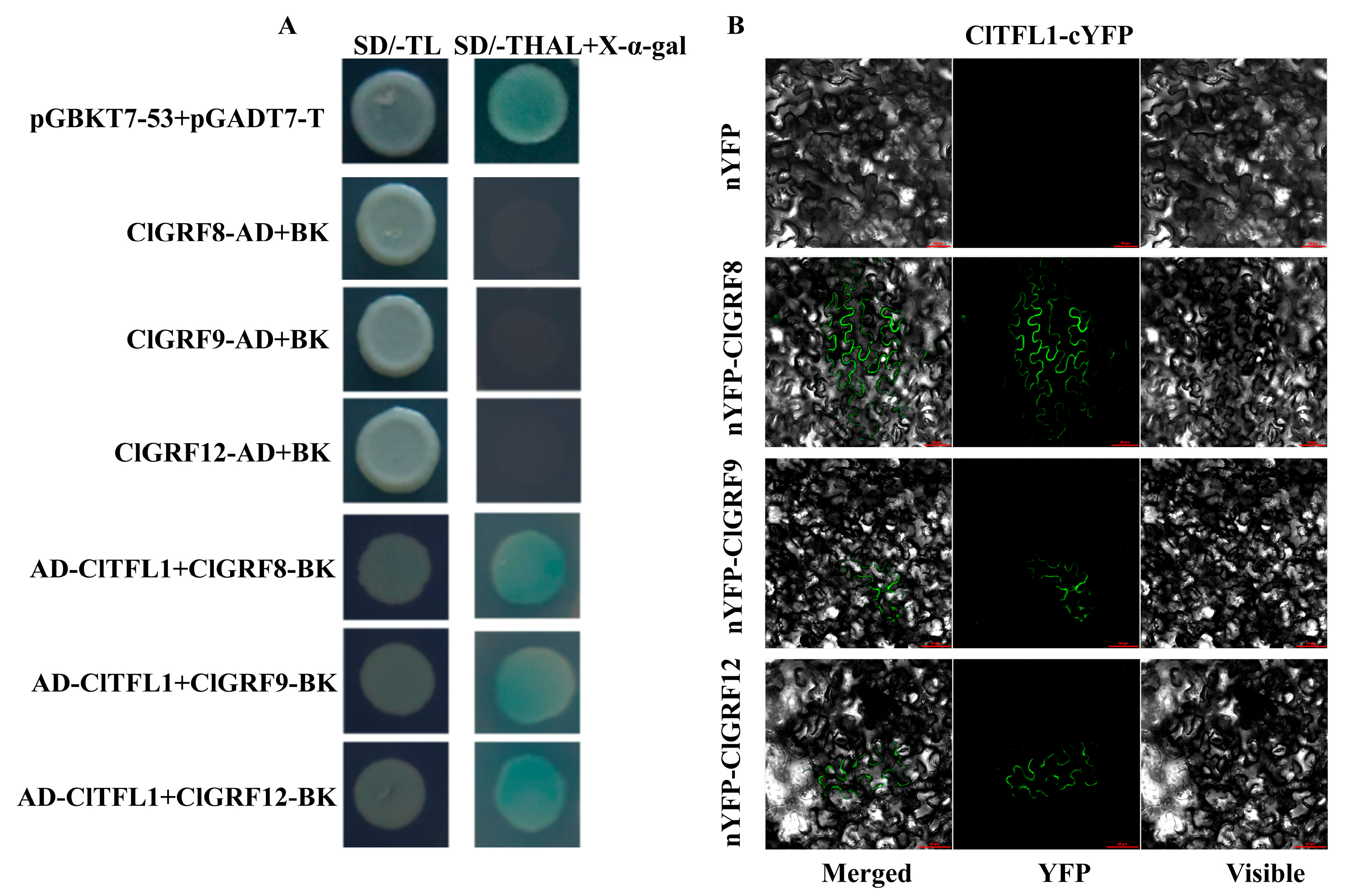
3.4. ClTFL1 Interacts with ClGRF8, ClGRF9, and ClGRF12
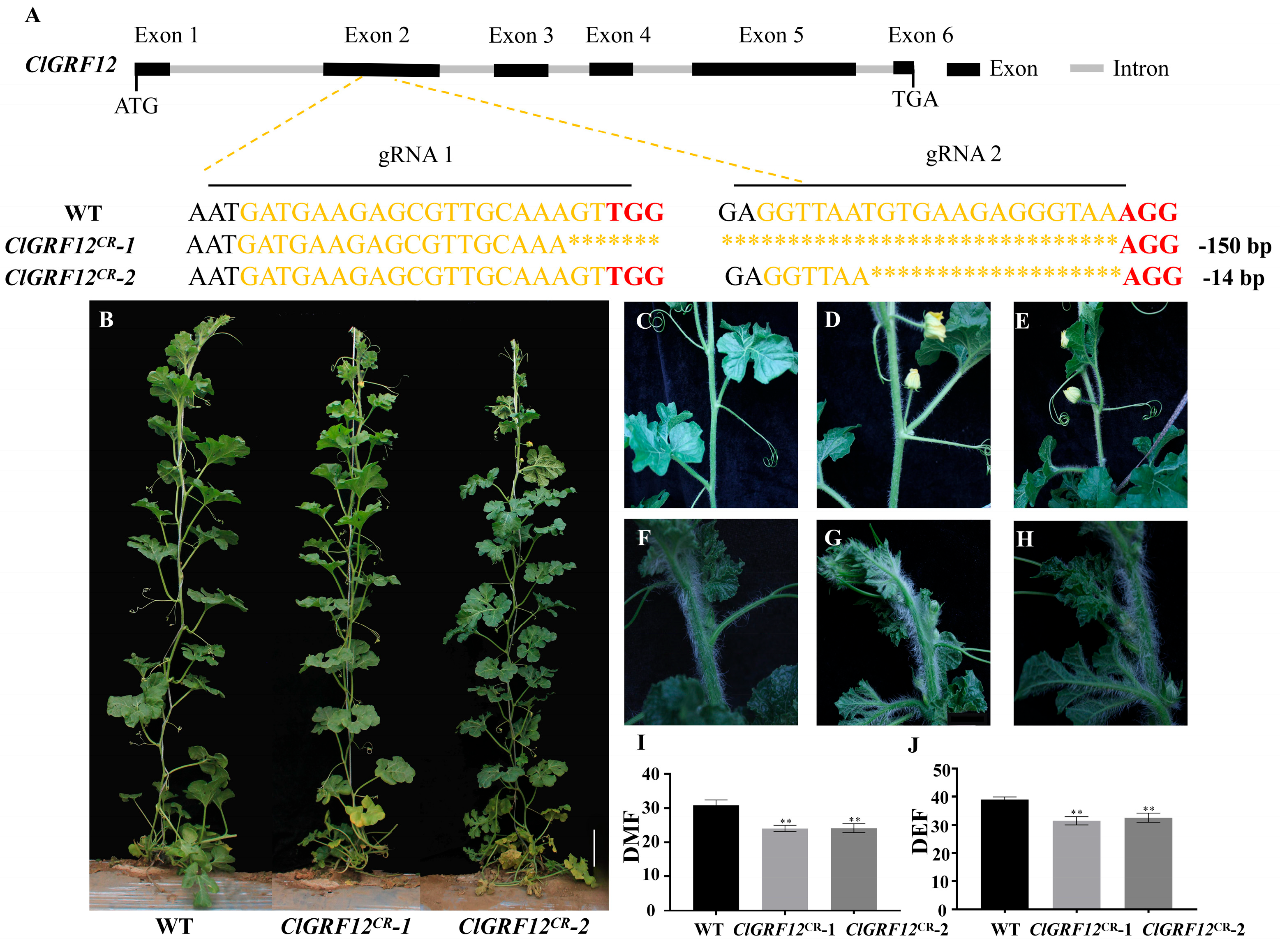
3.5. Knockout of ClGRF12 Can Lead to Earlier Flowering Time
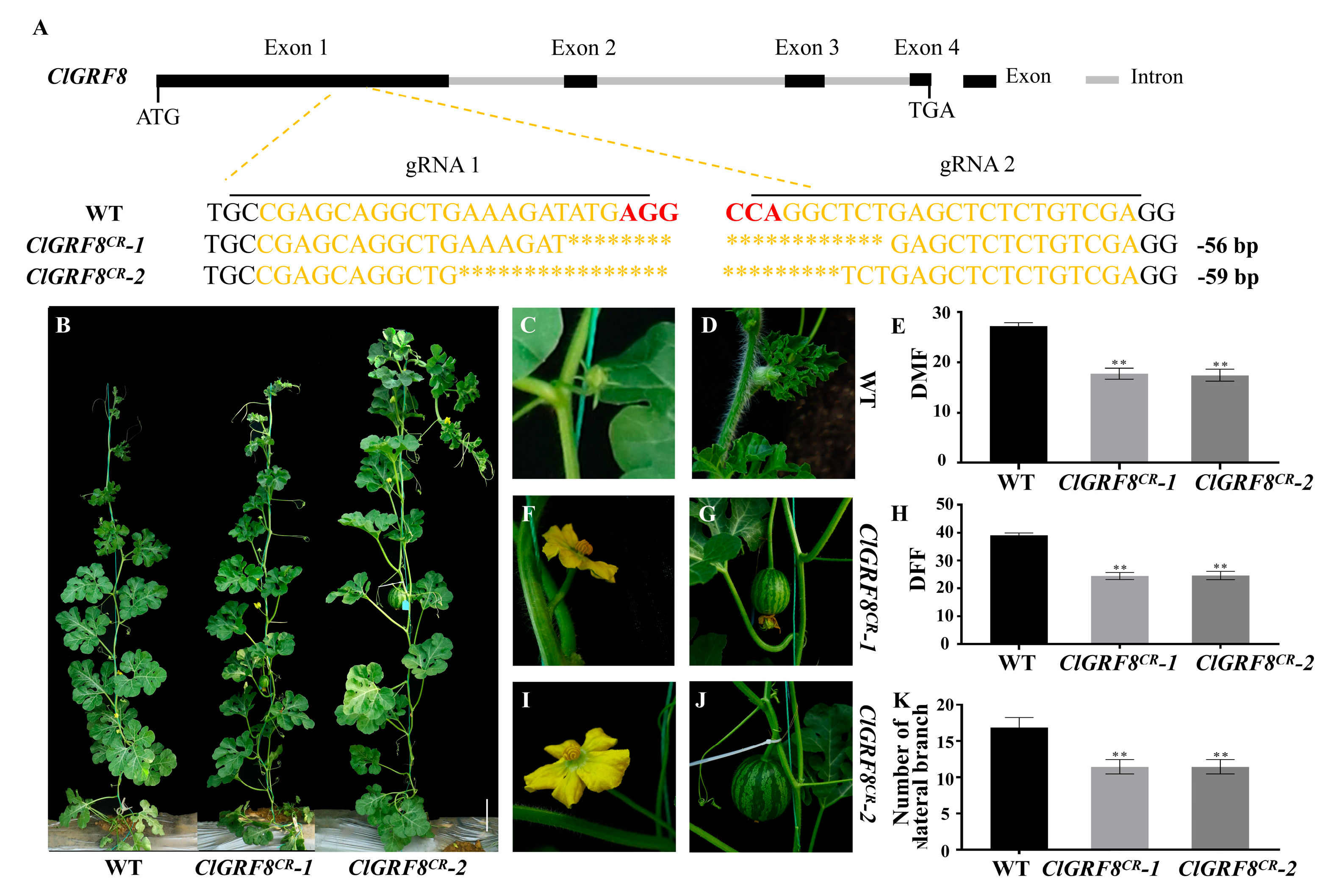
3.6. Knockout of ClGRF8 Can Lead to Earlier Flowering Time and Reduced Number of Lateral Branches
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Biological Process |
| CC | Cellular Component |
| MF | Molecular Function |
References
- Wang, B.; Smith, S.M.; Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef]
- Liu, D.M.; Yang, H.H.; Yuan, Y.; Zhu, H.Y.; Zhang, M.; Wei, X.; Sun, D.; Wang, X.; Yang, S.; Yang, L. Comparative transcriptome analysis provides insights into yellow rind formation and preliminary mapping of the Clyr (yellow rind) gene in watermelon. Front. Plant Sci. 2020, 11, 192. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Zhang, A.R.; He, W.J.; Li, Q.Q.; Zhao, B.S.; Zhao, H.J.; Ke, X.B.; Guo, Y.L.; Sun, P.Y.; Yang, T.W.; et al. GRAS family member LATERAL SUPPRESSOR regulates the initiation and morphogenesis of watermelon lateral organs. Plant Physiol. 2023, 193, 2592–2604. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Tang, Z.; Ge, J.; Wei, D.; Wang, Z.; Tang, Q. BjuBBX6-1 interacts with BjuNF-YB2/3 to regulate flowering time and drought tolerance in Brassica juncea. Hortic. Plant J. 2025. [Google Scholar] [CrossRef]
- Dou, J.L.; Yang, H.H.; Sun, D.L.; Yang, S.; Sun, S.R.; Zhao, S.J.; Lu, X.Q.; Zhu, H.Y.; Liu, D.M.; Ma, C.S.; et al. The branchless gene Clbl in watermelon encoding a TERMINAL FLOWER1 protein regulates the number of lateral branches. Theor. Appl. Genet. 2022, 135, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.L.; Wang, Y.P.; Yang, H.H.; Niu, H.H.; Liu, D.M.; Yang, S.; Zhu, H.Y.; Sun, S.R.; Yang, L.M. Development of branchless watermelon near isogenic lines by marker assisted selection. Hortic. Plant J. 2022, 8, 627–636. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Irwin, J.; Dean, C. Remembering the prolonged cold of winter. Curr. Biol. 2013, 23, R807–R811. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.W.; Song, S.; Wang, Y.; Liu, J.; Yu, H. New insights into the regulation of inflorescence architecture. Trends Plant Sci. 2014, 19, 158–165. [Google Scholar] [CrossRef]
- Périlleux, C.; Bouché, F.; Randoux, M.; Orman-Ligeza, B. Turning meristems into fortresses. Trends Plant Sci. 2019, 24, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jiang, K.; Tal, L.; Yichie, Y.; Lippman, Z.B. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat. Genet. 2014, 46, 1337–1342. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Erasmus, Y.; Lane, B.; Harder, L.D.; Coen, E. Evolution and development of inflorescence architectures. Science 2007, 316, 1452–1456. [Google Scholar] [CrossRef]
- Eshed, Y.; Lippman, Z.B. Revolutions in agriculture chart a course fortargeted breeding of old and new crops. Science 2019, 366, eaax0025. [Google Scholar] [CrossRef]
- Kaneko-Suzuki, M.; Kurihara-Ishikawa, R.; Okushita-Terakawa, C.; Kojima, C.; Nagano-Fujiwara, M.; Ohki, I.; Tsuji, H.; Shimamoto, K.; Taoka, K.I. TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol. 2018, 59, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Klasfeld, S.; Jeong, C.W.; Jin, R.; Goto, K.; Yamaguchi, N.; Wagner, D. TERMINAL FLOWER 1-FD complex target genes and competition with FLOWERING LOCUS T. Nat. Commun. 2020, 11, 5118. [Google Scholar] [CrossRef]
- Bradley, D.; Carpenter, R.; Copsey, L.; Vincent, C.; Rothstein, S.; Coen, E. Control of inflorescence architecture in Antirrhinum. Nature 1996, 379, 791–797. [Google Scholar] [CrossRef]
- Kaur, H.; Banga, S.S. Discovery and mapping of Brassica juncea Sdt1 gene associated with determinate plant growth habit. Theor. Appl. Genet. 2015, 128, 235–245. [Google Scholar] [CrossRef]
- Kotoda, N.; Wada, M. MdTFL1, a TFL1-like gene of apple, retards the transition from the vegetative to reproductive phase in transgenic Arabidopsis. Plant Sci. 2005, 168, 95–104. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, W.; Wang, L.; Lin, C.; Cong, B.; Sun, C.; Da, L. TFL1/CEN-like genes control intercalary meristem activity and phase transition in rice. Plant Sci. 2005, 168, 1393–1408. [Google Scholar] [CrossRef]
- Huang, X.; Ding, J.; Effgen, S.; Turck, F.; Koornneef, M. Multiple loci and genetic interactions involving flowering time genes regulate stem branching among natural variants of Arabidopsis. New Phytol. 2013, 199, 843–857. [Google Scholar] [CrossRef]
- Azevedo, L.M.; De Oliveira, R.R.; Chalfun-Junior, A. The role of FT/TFL1 clades and their hormonal interactions to modulate plant architecture and flowering time in perennial crops. Plants 2025, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, C.; Li, Y.; Yu, H. Mobile TERMINAL FLOWER1 determines seed size in Arabidopsis. Nat. Plants 2020, 6, 1146–1157. [Google Scholar] [CrossRef]
- Martí, E.; Gisbert, C.; Bishop, G.J.; Dixon, M.S.; García-Martínez, J.L. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef]
- Lifschitz, E.; Ayre, B.G.; Eshed, Y. Florigen and anti-florigen—A systemic mechanism for coordinating growth and termination in flowering plants. Front. Plant Sci. 2014, 5, 465. [Google Scholar] [CrossRef]
- Wen, C.; Zhao, W.; Liu, W.; Yang, L.; Wang, Y.; Liu, X.; Xu, Y.; Ren, H.; Guo, Y.; Li, C.; et al. CsTFL1 inhibits determinate growth and terminal flower formation through interaction with CsNOT2a in cucumber. Development 2019, 146, dev180166. [Google Scholar] [CrossRef]
- Njogu, M.K.; Yang, F.; Li, J.; Wang, X.; Chen, J. A novel mutation in TFL1 homolog sustaining determinate growth in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2020, 133, 3323–3332. [Google Scholar] [CrossRef]
- Bi, X.; Esse, W.V.; Mulki, M.A.; Kirschner, G.; Korff, M.V. CENTRORADIALIS interacts with FLOWERING LOCUS T-like genes to control spikelet initiation, floret development and grain number. Plant Physiol. 2019, 180, 1013–1030. [Google Scholar] [CrossRef]
- Prewitt, S.F.; Ayre, B.G.; McGarry, R.C. Cotton CENTRORADIALIS/TERMINAL FLOWER 1/ SELF-PRUNING genes functionally diverged to differentially impact plant architecture. J. Exp. Bot. 2018, 69, 5403–5417. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Liu, H.; Zhu, J.; Chen, J.; Wang, Q.; Fang, L.; Gao, F.; Tian, Y.; Chen, Y.; Chang, L.; et al. Mutation of SELF-PRUNING homologs in cotton promotes short-branching plant architecture. J. Exp. Bot. 2018, 69, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- RRuiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Zhang, M.J.; Sun, S.R.; Yang, S.; Li, J.X.; Li, H.; Yang, H.H.; Zhang, K.G.; Hu, J.B.; Liu, D.M.; et al. A Single Nucleotide Deletion in an ABC Transporter Gene Leads to a Dwarf Phenotype in Watermelon. Front. Plant Sci. 2019, 10, 1399. [Google Scholar]
- Duan, S.X.; Guo, Y.M.; Wang, Y.P.; Muhammad, J.U.; Liu, D.M.; Yang, S.; Niu, H.H.; Sun, S.R.; Yang, L.M.; Dou, J.L.; et al. HD-Zip Transcription Factor is Responsible for No-Lobed Leaf in Watermelon (Citrullus lanatus L.). Phyton-Int. J. Exp. Bot. 2023, 92, 1311–1328. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.L.; Zhu, H.Y.; Zhang, M.J.; Wang, D.K.; Xie, K.X.; Fan, P.F.; Dou, J.L.; Liu, D.M.; Liu, B.; et al. A novel HD-Zip I/C2H2-ZFP/WD-repeat complex regulates the size of spine base in cucumber. New Phytol. 2022, 233, 2643–2658. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhu, H.Y.; Zhang, K.G.; Wang, Y.L.; Wu, L.; Chen, C.H.; Liu, X.W.; Yang, S.; Ren, H.Z.; Yang, L.M. The MIXTA-LIKE transcription factor CsMYB6 regulates fruit spine and tubercule formation in cucumber. Plant Sci. 2020, 300, 110636. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A.; Yang, Y.; Schmidt, R.J.; Jackson, D. The Relationship between auxin transport and maize branching. Plant Physiol. 2008, 147, 1913–1923. [Google Scholar] [CrossRef]
- McSteen, P. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001479. [Google Scholar] [CrossRef]
- Gallavotti, A. The role of auxin in shaping shoot architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Ratcliffe, O.J.; Amaya, I.; Vincent, C.A.; Rothstein, S.; Carpenter, R.; Coen, E.S.; Bradley, D.J. A common mechanism controls the life cycle and architecture of plants. Development 1998, 125, 1609–1615. [Google Scholar] [CrossRef]
- Wickland, D.P.; Hanzawa, Y. The FLOWERING LOCUS T/TERMINAL FLOWER1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef]
- Bradley, D. Inflorescence commitment and architecture in Arabidopsis. Science 1997, 275, 80–83. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrion, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Silva, W.B.; Vicente, M.H.; Robledo, J.M.; Reartes, D.S.; Ferrari, R.C.; Bianchetti, R.; Araújo, W.L.; Freschi, L.; Peres, L.E.P.; Zsögön, A. SELF-PRUNING Acts Synergistically with DIAGEOTROPICA to Guide Auxin Responses and Proper Growth Form. Plant Physiol. 2018, 176, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Keicher, J.; Jaspert, N.; Weckermann, K.; Möller, C.; Throm, C.; Kintzi, A.; Oecking, C. Arabidopsis 14-3-3 epsilon members contribute to polarity of PIN auxin carrier and auxin transport-related development. eLife 2017, 6, e24336. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, Y.; Niu, H.; Wang, Y.; Chen, Z.; Cui, J.; Shen, C.; Duan, S.; Kang, Q.; Zhu, H.; et al. The ClTFL1-ClGRFs Module Regulates Lateral Branch Number and Flowering Time via Auxin-Mediated Pathway in Watermelon (Citrullus lanatus). Horticulturae 2025, 11, 1022. https://doi.org/10.3390/horticulturae11091022
Guo Y, Liu Y, Niu H, Wang Y, Chen Z, Cui J, Shen C, Duan S, Kang Q, Zhu H, et al. The ClTFL1-ClGRFs Module Regulates Lateral Branch Number and Flowering Time via Auxin-Mediated Pathway in Watermelon (Citrullus lanatus). Horticulturae. 2025; 11(9):1022. https://doi.org/10.3390/horticulturae11091022
Chicago/Turabian StyleGuo, Yaomiao, Yachen Liu, Huanhuan Niu, Yinping Wang, Zihao Chen, Jiaxin Cui, Changbao Shen, Shixiang Duan, Qishuai Kang, Huayu Zhu, and et al. 2025. "The ClTFL1-ClGRFs Module Regulates Lateral Branch Number and Flowering Time via Auxin-Mediated Pathway in Watermelon (Citrullus lanatus)" Horticulturae 11, no. 9: 1022. https://doi.org/10.3390/horticulturae11091022
APA StyleGuo, Y., Liu, Y., Niu, H., Wang, Y., Chen, Z., Cui, J., Shen, C., Duan, S., Kang, Q., Zhu, H., Yang, S., Liu, D., Yan, W., Dou, J., & Yang, L. (2025). The ClTFL1-ClGRFs Module Regulates Lateral Branch Number and Flowering Time via Auxin-Mediated Pathway in Watermelon (Citrullus lanatus). Horticulturae, 11(9), 1022. https://doi.org/10.3390/horticulturae11091022





