Transcriptomic and Metabolomic Analyses Reveal Differing Phytohormone Regulation in Rhododendron Cultivars in Response to Azalea Lace Bug (Stephanitis pyrioides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Insect Infestation
2.2. Transcriptome Sequencing and DEG Analysis
2.3. Sample Extraction and Non-Targeted Metabolite Detection
2.4. Statistical Analysis
3. Results
3.1. Transcriptomic Sequencing Data Analysis and Screening of DEGs
3.2. KEGG Enrichment Analysis of Differentially Expressed Genes
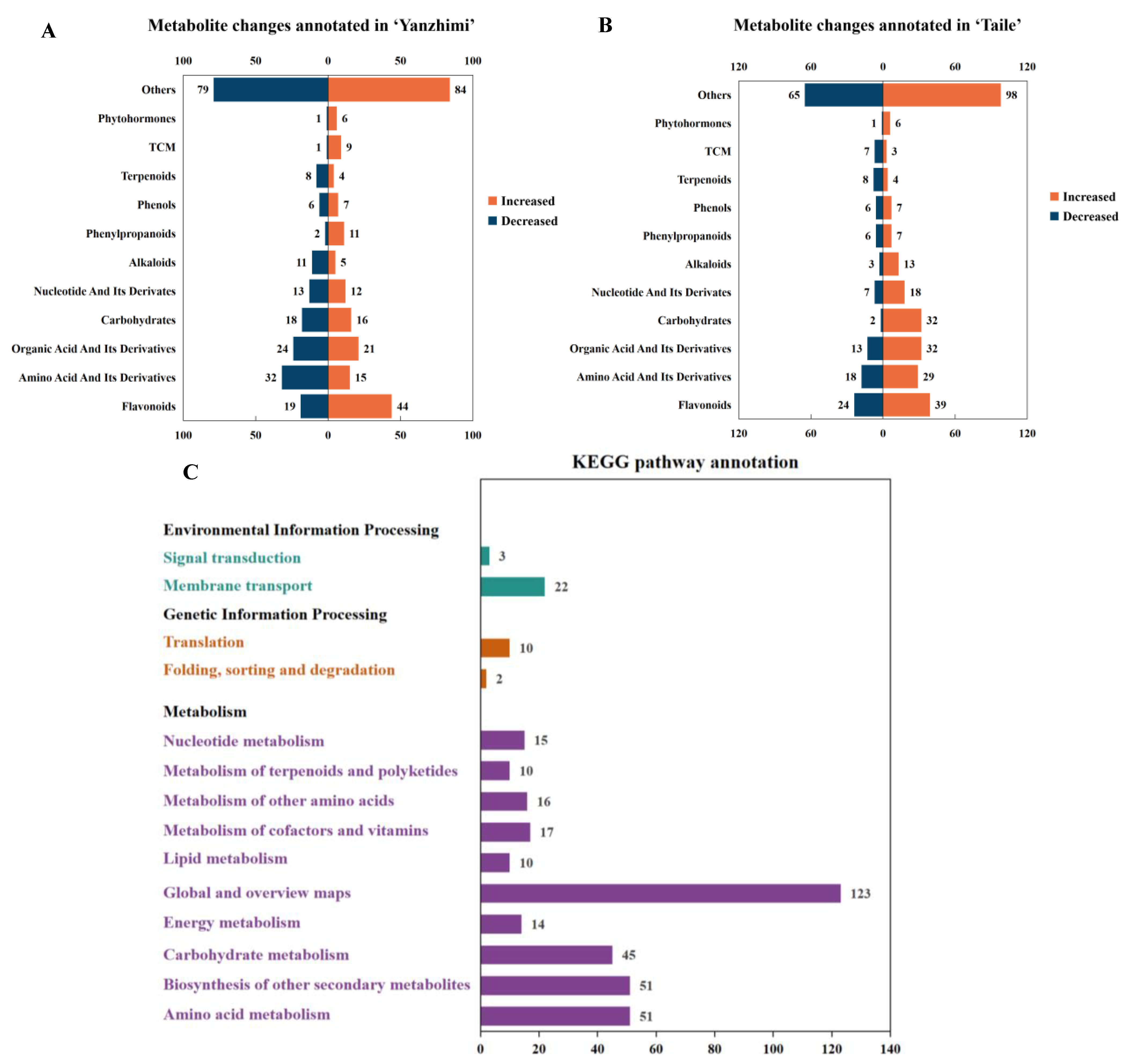
3.3. Metabolite Profile and KEGG Pathway Annotation
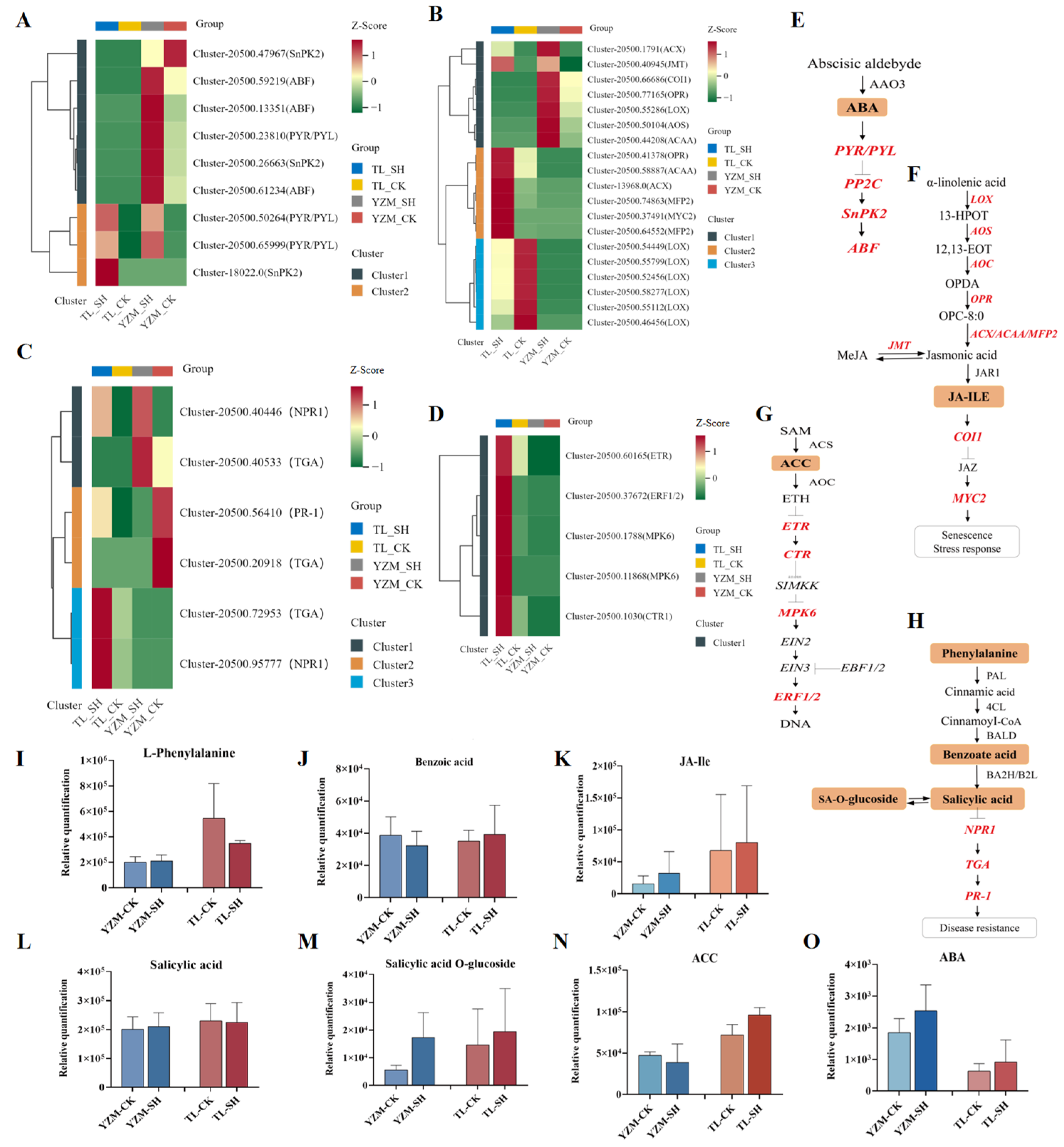
3.4. Joint Analysis of Transcriptome and Metabolome in Plant Hormone Signal Changes
3.4.1. Changes in Genes Related to Auxin, Cytokinin, and Gibberellin
3.4.2. Changes in ABA-, Ethylene-, Jasmonic Acid-, and Salicylic Acid-Related Genes
4. Discussion
4.1. Differences in Defense Responses and Hormonal Regulation Between Cultivars
4.1.1. Auxin and Cytokinin Signals Negatively Regulated in Pest-Susceptible Cultivar ‘YZM’
4.1.2. Auxin and Gibberellin Signals Positively Regulated in Resistant Cultivar ‘TL’
4.2. Possible Regulation Mechanisms of Hormonal Synthesis
4.2.1. ABA and JA Synthesis Regulation
4.2.2. SA Synthesis Regulation and Defense Priming
4.2.3. Ethylene Synthesis Regulation in ‘TL’
4.2.4. Crosstalk Among Multiple Hormonal Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nair, S.; Braman, K.S. A Scientific review on the ecology and management of the azalea lace bug (Stephanitis pyrioides Scott) (Tingidae: Hemiptera). J. Entomol. Sci. 2012, 47, 247–263. [Google Scholar] [CrossRef]
- Garrison, R.R.; Tobin, P.C. Development of azalea lace bug, Stephanitis pyrioides, on susceptible and resistant rhododendron species in western Washington. J. Econ. Entomol. 2021, 115, 233–239. [Google Scholar] [CrossRef]
- Baliji, S.; Lacatus, G.; Sunter, G. The interaction between geminivirus pathogenicity proteins and adenosine kinase leads to increased expression of primary cytokinin-responsive genes. Virology 2010, 402, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef]
- Collum, T.D.; Padmanabhan, M.S.; Hsieh, Y.C.; Culver, J.N. Tobacco mosaic virus-directed reprogramming of auxin/indole acetic acid protein transcriptional responses enhances virus phloem loading. Proc. Natl. Acad. Sci. USA 2016, 113, E2740–E2749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.; Gao, Z.; Wang, Y.; Liu, Y.; Ma, Z.; Chen, Y.; Zhang, Y.; Yan, F.; Li, J. Analysis of phytohormone signal transduction in sophora alopecuroides under salt stress. Int. J. Mol. Sci. 2021, 22, 7313. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant responses to herbivory, wounding, and infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Rosa-Díaz, I.; Rowe, J.; Cayuela-López, A.; Arbona, V.; Díaz, I.; Jones, A.M. Spider mite herbivory induces an ABA-driven stomatal defense. Plant Physiol. 2024, 195, 2970–2984. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yang, X.; Shi, Z.; Miao, X. Novel crosstalk between ethylene- and jasmonic acid-pathway responses to a piercing–sucking insect in rice. New Phytol. 2020, 225, 474–487. [Google Scholar] [CrossRef]
- Zhao, S.; Hong, W.; Wu, J.; Wang, Y.; Ji, S.; Zhu, S.; Wei, C.; Zhang, J.; Li, Y. A viral protein promotes host SAMS1 activity and ethylene production for the benefit of virus infection. Elife 2017, 10, e27529. [Google Scholar] [CrossRef]
- Fabian, S.; Patricia, F.; Mark, Z.; Monica, D.D.; Sandra, F.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef]
- Guo, Q.; Major, I.T.; Kapali, G.; Howe, G.A. MYC transcription factors coordinate tryptophan-dependent defence responses and compromise seed yield in Arabidopsis. New Phytol. 2022, 236, 132–145. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Xiong, Z.; Robert, C.; Li, B.; He, S.; Chen, W.; Bi, J.; Zhai, G.; Guo, S.; et al. Trade-offs between the accumulation of cuticular wax and jasmonic acid-mediated herbivory resistance in maize. J. Integr. Plant Biol. 2024, 66, 143–159. [Google Scholar] [CrossRef]
- Costarelli, A.; Bianchet, C.; Ederli, L.; Salerno, G.; Piersanti, S.; Rebora, M.; Pasqualini, S. Salicylic acid induced by herbivore feeding antagonizes jasmonic acid mediated plant defenses against insect attack. Plant Signal. Behav. 2019, 15, 1704517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Carrillo, J.; Siemann, E.; Ding, J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 2019, 11, plz003. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Lei, B.; Luo, Z.; Li, Z.; Xiu, C.; Fu, N.; Cai, X.; Chen, Z. Enhanced volatile emissions and anti-herbivore functions mediated by the synergism between jasmonic acid and salicylic acid pathways in tea plants. Hortic. Res. 2022, 9, uhac144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Jin, W.; Fang, Y.; Yang, Q.; Xiang, J. Transcriptome analysis and identification of genes associated with flower development in Rhododendron pulchrum Sweet (Ericaceae). Gene 2018, 679, 108–118. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Park, Y.E.; Park, S.Y.; Kim, J.K.; Park, S.U. Metabolomic profiling of the white, violet, and red flowers of Rhododendron schlippenbachii maxim. Molecules 2018, 23, 827. [Google Scholar] [CrossRef]
- Yang, G.X.; Qin, Y.; Jia, Y.H.; Xie, X.H.; Li, D.B.; Jiang, B.X.; Wang, Q.; Feng, S.Y.; Wu, Y.Y. Transcriptomic and metabolomic data reveal key genes that are involved in the phenylpropanoid pathway and regulate the floral fragrance of Rhododendron fortunei. BMC Plant Biol 2023, 23, 8. [Google Scholar] [CrossRef]
- Zhang, X.M.; Duan, S.G.; Xia, Y.; Li, J.T.; Liu, L.X.; Tang, M.; Tang, J.; Sun, W.; Yi, Y. Transcriptomic, physiological, and metabolomic response of an alpine plant, Rhododendron delavayi, to waterlogging stress and post-waterlogging recovery. Int. J. Mol. Sci. 2023, 24, 10509. [Google Scholar] [CrossRef]
- Xu, S.; Geng, X.; Mao, L.; Yi, Y.; Gong, J.; Xu, X. Transcriptome analysis and identification of the genes associated with the heat stress response in four rhododendron species. Sci. Hortic. 2022, 303, 111176. [Google Scholar] [CrossRef]
- He, B.; Zhou, Y.; Peng, Y.; Xu, D.Y.; Tong, J.; Dong, Y.F.; Fang, L.C.; Mao, J. Comparative metabolomic responses of three rhododendron cultivars to the azalea lace bug (Stephanitis pyrioides). Plants 2024, 13, 2569. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lv, J.; Hu, Z.; Wang, J.; Wang, P.; Yu, J.; Foyer, C.H.; Shi, K. Phytosulfokine peptide optimizes plant growth and defense via glutamine synthetase GS2 phosphorylation in tomato. EMBO J. 2022, 42, e111858. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O.; et al. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dai, Y.; Wang, M.; Yang, W.; Zhao, D. Transcriptome Dynamics of Double Recessive Mutant, o2o2o16o16, Reveals the Transcriptional Mechanisms in the Increase of Its Lysine and Tryptophan Content in Maize. Genes 2019, 10, 316. [Google Scholar] [CrossRef]
- Sharma, A.; Prakash, S.; Chattopadhyay, D. Killing two birds with a single stone—Genetic manipulation of cytokinin oxidase/dehydrogenase (CKX) genes for enhancing crop productivity and amelioration of drought stress response. Front. Genet. 2022, 13, 941595. [Google Scholar] [CrossRef]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.M.; et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef]
- Kurepa, J.; Li, Y.; Smalle, J.A. Cytokinin signaling stabilizes the response activator ARR1. Plant J. 2014, 78, 157–168. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, W.; Chan, Z.; Wu, Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis Thaliana. Front. Plant Sci. 2015, 6, 1004. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Li, Z.; Staswick, P.E.; Wang, M.; Zhu, Y.; He, Z. Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol. 2007, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Kirungu, J.N.; Magwanga, R.O.; Lu, P.; Cai, X.; Zhou, Z.; Wang, X.; Peng, R.; Wang, K.; Liu, F. Functional characterization of GH-A08G1120 (GH3.5) gene reveal their significant role in enhancing drought and salt stress tolerance in cotton. BMC Genet. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Wang, D.; Ma, G.; Shen, J.; Xu, X.; Shou, W.; Xuan, Z.; He, Y. A samll AUXIN up-regulated RNA gene isolated from watermelon (ClSAUR1) positively modulates the chilling stress response in tobacco via multiple signaling pathways. Horticulturae 2025, 11, 52. [Google Scholar] [CrossRef]
- Youseif, S.H.; El-Megeed, F.H.A.; Soliman, M.S.; Ageez, A.; Mohamed, A.H.; Ali, S.A.; EI-Kholy, A.A. Nodules-associated Klebsiella oxytoca complex: Genomic insights into plant growth promotion and health risk assessment. BMC Microbiol. 2025, 25, 294. [Google Scholar] [CrossRef]
- Magome, H.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; Oda, K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 2008, 56, 613–626. [Google Scholar] [CrossRef]
- Shan, C.; Mei, Z.; Duan, J.; Chen, H.; Feng, H.; Cai, W. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS ONE 2014, 9, e87110. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J. Exp. Bot. 2020, 71, 1160–1170. [Google Scholar] [CrossRef]
- He, H.; Liang, G.; Lu, S.; Wang, P.; Liu, T.; Ma, Z.; Zuo, C.; Sun, X.; Chen, B.; Mao, J. Genome-wide identification and expression analysis of GA2ox, GA3ox, and GA20ox are related to gibberellin oxidase genes in grape (Vitis vinifera L.). Genes 2019, 10, 680. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Wang, N.; Zhou, H.; Xu, Q.; Yan, G. Overexpression of stga2ox1 gene increases the tolerance to abiotic stress in transgenic potato (Solanum tuberosum L.) plants. Appl. Biochem. Biotechnol. 2019, 187, 1204–1219. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, J.; Chen, R.J.; Luo, Y.Y.; Yao, Y.H.; Nan, L.L. Genome-wide identification of pyrabactin resistance 1-like (PYL) gene family under phytohormones and drought stresses in alfalfa (Medicago sativa). BMC Genom. 2025, 26, 383. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, G.; Wang, Y.; Huang, Y.; Marchant, D.B.; Wang, Y.; Yang, Q.; Dai, F.; Hills, A.; Blatt, M.R. Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol. 2017, 174, 732–747. [Google Scholar] [CrossRef]
- Manuel, J.C.; Esther, L.; Gemma, F.; Esther, C.; Gloria, G.C.; Manuel, F.Z.J.; Philippe, H.; Zamarreño, A.M.; García, M.J.M.; Rubio, V.; et al. CUL3BPM E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl. Acad. Sci. USA 2020, 117, 6205–6215. [Google Scholar] [CrossRef]
- Hu, G.; Liu, B.; Yang, K.; Zheng, W.; Zhang, Y.; Teng, C.; Huang, D.; Yan, R.; Notaguchi, M.; Lin, Z.; et al. CsCOI1 regulates plant growth and defense in citrus. Hortic. Res. 2025, 7, 8190184. [Google Scholar] [CrossRef]
- Mu, T.; Luo, S.; Li, L.; Zhang, R.; Wang, P.; Zhang, G. A review of the interaction mechanisms between jasmonic acid (JA) and various plant hormones, as well as the core regulatory role of MYC2. Plant Sci. 2025, 353, 112407. [Google Scholar] [CrossRef] [PubMed]
- Maruri-Lopez, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.J.; Seo, P.J.; Kim, J.H.; Sim, H.J.; Kim, S.G.; Park, C.M. Systemic immunity requires SnRK2.8-mediated nuclear import of NPR1 in arabidopsis. Plant Cell 2015, 27, 3425–3438. [Google Scholar] [CrossRef]
- Raul, Z.; Xinnian, D. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 2023, 84, 131–141. [Google Scholar] [CrossRef]
- Silva, K.J.P.; Mahna, N.; Mou, Z.; Folta, K.M. NPR1 as a transgenic crop protection strategy in horticultural species. Hortic. Res. 2018, 5, 15. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.H.; Ma, L.Y.; Li, X.; Zhao, F.Y.; Tan, X.L. Overexpression of Brassica napus NPR1 enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Physiological and Molecular Plant Pathology 2020, 110, 101460. [Google Scholar] [CrossRef]
- Dutt, M.; Barthe, G.; Irey, M.; Grosser, J. Transgenic citrus expressing an Arabidopsis NPR1 gene exhibit enhanced resistance against huanglongbing (HLB; citrus greening). PLoS ONE 2015, 10, e0137134. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.J.; Zhang, X.; Gowda, S.; Orbovic, V.; Dawson, W.O.; Mou, Z. Overexpression of the Arabidopsis NPR1 protein in citrus confers tolerance to Huanglongbing. J. Citrus Pathol. 2018, 5, 38911. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Li, K.; Wu, M.; Zhuang, Q.; Li, M.; Garcia-Caparros, P.; Xie, Y.; Hu, C.; Liu, M. Glycerol treatment enhances resistance to soft rot disease and maintains postharvest quality in kiwifruit. Postharvest Biol. Technol. 2025, 228, 113630. [Google Scholar] [CrossRef]
- Chong, J.; Pierrel, M.A.; Atanassova, R.; Werck-Reichhart, D.; Fritig, B.; Saindrenan, P. Free and Conjugated Benzoic Acid in Tobacco Plants and Cell Cultures. Induced Accumulation upon Elicitation of Defense Responses and Role as Salicylic Acid Precursors. Plant Physiol. 2001, 125, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Meng, X.; Wang, R.; Mao, G.; Han, L.; Liu, Y.; Zhang, S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in arabidopsis. PLOS Genet. 2012, 8, e1002767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Gomila, J.; Takken, F.L.W. Involvement of salicylic acid, ethylene and jasmonic acid signalling pathways in the susceptibility of tomato to Fusarium oxysporum. Mol. Plant Pathol. 2017, 18, 1024–1035. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Peng, Y.; Tong, J.; Xu, D.; Dong, Y.; Zhou, Y.; Tang, Y.; Zhang, S.; Fang, L.; Mao, J. Transcriptomic and Metabolomic Analyses Reveal Differing Phytohormone Regulation in Rhododendron Cultivars in Response to Azalea Lace Bug (Stephanitis pyrioides). Horticulturae 2025, 11, 1005. https://doi.org/10.3390/horticulturae11091005
He B, Peng Y, Tong J, Xu D, Dong Y, Zhou Y, Tang Y, Zhang S, Fang L, Mao J. Transcriptomic and Metabolomic Analyses Reveal Differing Phytohormone Regulation in Rhododendron Cultivars in Response to Azalea Lace Bug (Stephanitis pyrioides). Horticulturae. 2025; 11(9):1005. https://doi.org/10.3390/horticulturae11091005
Chicago/Turabian StyleHe, Bei, Yu Peng, Jun Tong, Dongyun Xu, Yanfang Dong, Yuan Zhou, Yanping Tang, Si Zhang, Linchuan Fang, and Jing Mao. 2025. "Transcriptomic and Metabolomic Analyses Reveal Differing Phytohormone Regulation in Rhododendron Cultivars in Response to Azalea Lace Bug (Stephanitis pyrioides)" Horticulturae 11, no. 9: 1005. https://doi.org/10.3390/horticulturae11091005
APA StyleHe, B., Peng, Y., Tong, J., Xu, D., Dong, Y., Zhou, Y., Tang, Y., Zhang, S., Fang, L., & Mao, J. (2025). Transcriptomic and Metabolomic Analyses Reveal Differing Phytohormone Regulation in Rhododendron Cultivars in Response to Azalea Lace Bug (Stephanitis pyrioides). Horticulturae, 11(9), 1005. https://doi.org/10.3390/horticulturae11091005





