Advancements on the Mechanism of Soluble Sugar Metabolism in Fruits
Abstract
1. Introduction
2. Soluble Sugar Composition and Accumulation Patterns in Fruit
2.1. Starch Conversion Type
2.2. Sugar Direct Accumulation Type
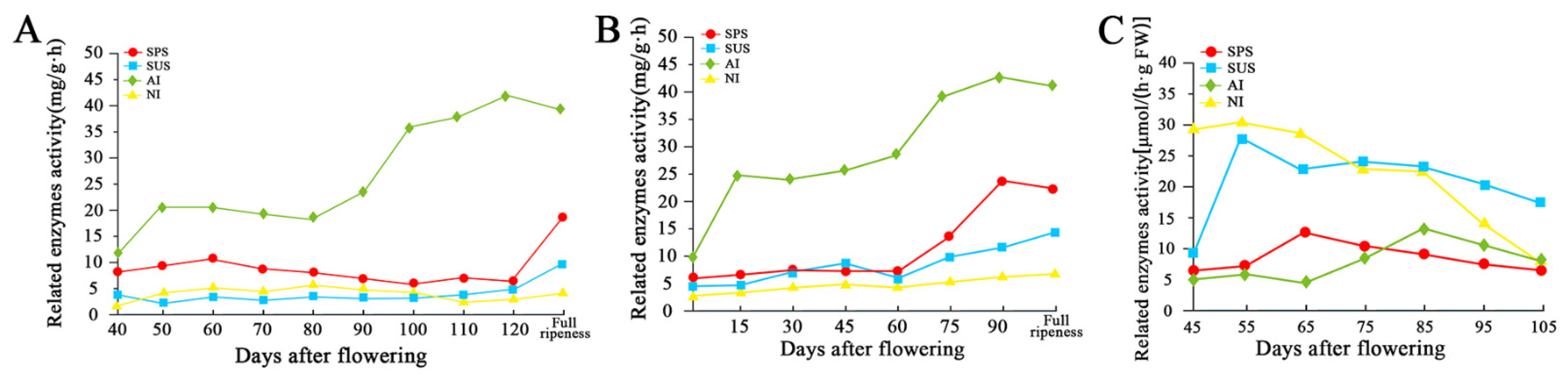
2.2.1. The Accumulation Pattern of Soluble Sugars in Strawberry
2.2.2. The Accumulation Pattern of Soluble Sugars in Citrus
2.2.3. The Accumulation Pattern of Soluble Sugars in Lychee
2.2.4. The Accumulation Pattern of Soluble Sugars in Longan
2.2.5. The Accumulation Pattern of Soluble Sugars in Grape
2.2.6. The Accumulation Pattern of Soluble Sugars in Pitaya
2.3. Mixture Type
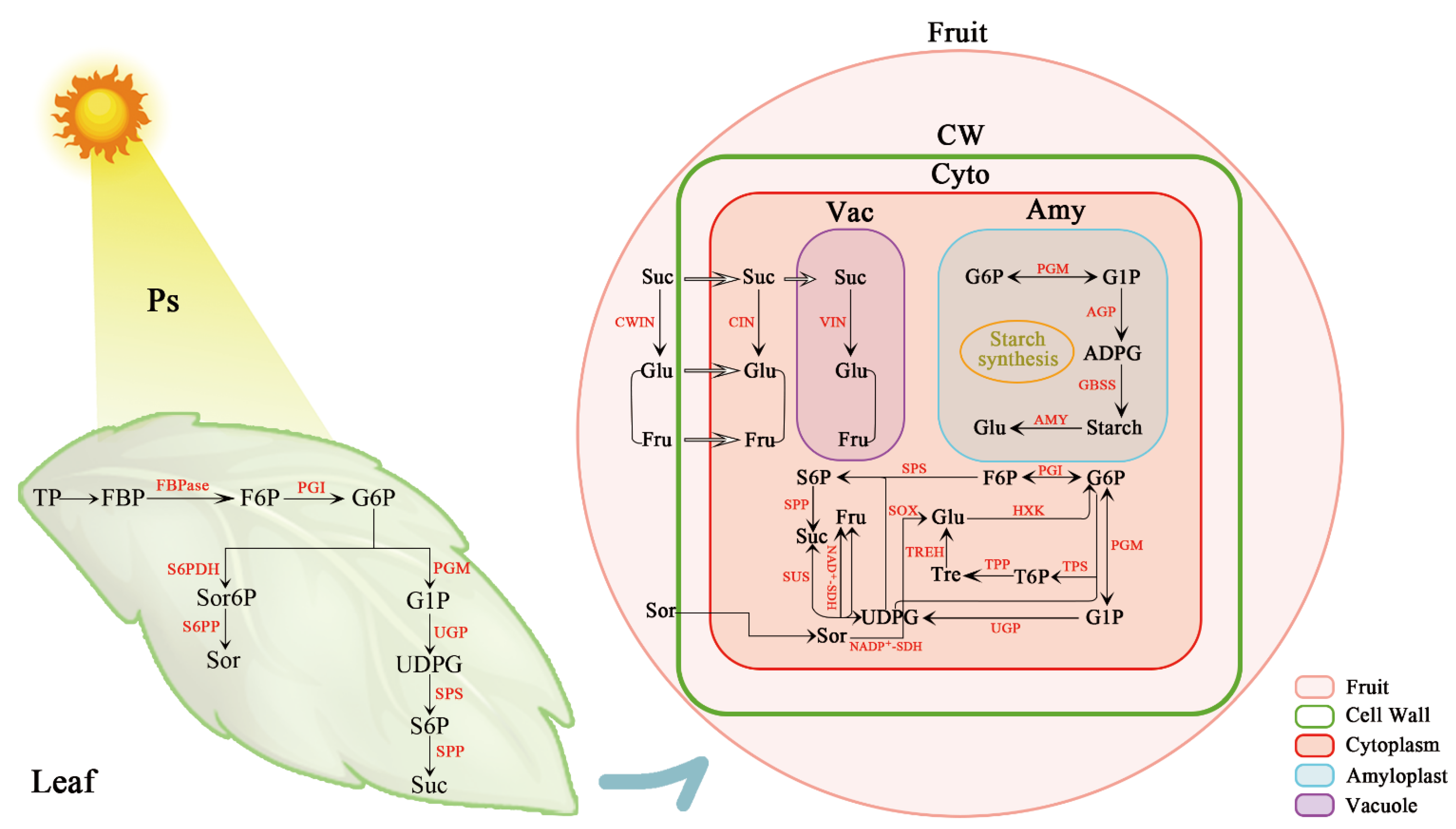
3. Spatiotemporal Distribution of Enzymes Involved in Soluble Sugar Metabolism in Fruits
3.1. Sucrose Metabolism
3.2. Sorbitol Metabolism
3.3. Hexose Metabolism
4. Key Enzymatic Gene Response Signaling Pathways and Genetic Regulatory Networks
4.1. Key Enzyme Genes Involved in Soluble Sugar Metabolism in Fruits
| Enzyme | Fruit | Gene Name | Reference |
|---|---|---|---|
| SPS | Plum | PsSPS2 | [48] |
| Mango | MinSPS1 | [49] | |
| Pineapple | AcSPS1-5 | [50] | |
| Litchi | LcSPS1-4 | [51] | |
| Citrus | CsSPS1-4 | [52] | |
| Jackfruit | AhSPS1-4 | [53] | |
| Apple | MdSPSs | [54] | |
| SUS | Apple | MdSUSs | [55] |
| Pear | PbSSs | [56] | |
| Litchi | LeSUS1-5 | [61] | |
| Yellow-skinned pitaya | SuSys | [62] | |
| Passion fruit | PeSUS1-5 | [63] | |
| Muskmelon | CmSUS1, CmSUS2 | [64] | |
| INV | Pineapple | AcNINV1-6 | [65] |
| Red-fleshed pitaya | HpVIN1-4 | [66] | |
| Blueberry | VcAINs | [68] | |
| Plum | PsNINV1, PsNINV3, PsNINV4 | [48] | |
| Kiwi fruit | ArINVs | [58] | |
| Pomegranate | PgINV1-11 | [69] | |
| SDH | Peach | PpSDH1-3 | [71] |
| Apple | MdSDHs | [70] | |
| S6PDH | Peach | PpS6PDH1, PpS6PDH2 | [71] |
| Plum | PsS6PDH4 | [72] | |
| Apple | MdS6PDHs | [71] | |
| FRK | Apple | MdFRK2 | [73] |
| Bayberry | MrFRK2 | [74] | |
| Strawberry | FaFRK3 | [75] | |
| Pear | PpyFRK5 | [76] | |
| Pitaya | HpFRK1 | [77] | |
| HXK | Apple | MdHXK1 | [78] |
| Pear | PbHXK1 | [79] | |
| Grape | VvHXK3 | [80] | |
| Longan | DlHXK | [81] |
4.2. The Signal Response Mechanism of Key Enzyme Genes
4.3. Key Enzyme Gene Genetic Regulatory Network
5. Conclusions and Outlook
- (1)
- Further investigation is required into the specific signaling pathways regulating key enzymes involved in sugar metabolism, particularly the interactions among environmental factors, endogenous hormones, transcription factors, and epigenetic modifications. Experimental designs could incorporate environmental and hormonal interactions to monitor phenotypic responses, combined with multi-omics approaches such as methylomics, transcriptomics, and metabolomics to dynamically capture changes in relevant regulatory factors. Integrative multi-omics analysis can identify key regulatory nodes, thereby elucidating interaction networks that may serve as a foundation for improving fruit quality.
- (2)
- A comprehensive understanding of the molecular network of sugar metabolism requires integrating multi-omics technologies, genetic engineering, and computational modeling. For the same fruit material, transcriptomic, proteomic, and metabolomic data should be collected simultaneously across different developmental stages or in mutant lines. Using Weighted Gene Co-expression Network Analysis (WGCNA), we can identify gene modules and protein–metabolite interaction pairs significantly correlated with sugar content. Functional validation of core genes can then be achieved through gene knockout or overexpression experiments. Based on multi-omics datasets, systems biology tools can be applied to construct dynamic models incorporating key enzymes, transporters, and regulatory factors. These models simulate shifts in sugar metabolic flux under varying conditions and are then validated through in vitro metabolic assays.
- (3)
- Given the close relationship between sugar metabolism and other plant metabolic pathways, targeted analysis should focus on identifying key intersection points between sugar metabolism and secondary or energy metabolism. Isotope labeling techniques can trace carbon flux, which, combined with metabolite correlation analysis, helps pinpoint critical cross-regulatory nodes. This approach facilitates the identification of co-regulatory factors influencing both sugar metabolism and other pathways. Subsequently, yeast two-hybrid screening can detect interacting proteins. Chromatin immunoprecipitation sequencing (ChIP-seq) can determine the binding sites of these regulators on genes within different metabolic pathways, thereby clarifying the molecular mechanisms by which a single regulatory factor coordinates multiple pathways. This will deepen our understanding of the interplay between sugar metabolism and other metabolic processes.
- (4)
- The distinct patterns of sugar metabolism observed across fruit species reflect their evolutionary strategies for environmental adaptation. Future studies should explore the role of sugar metabolism in ecological and evolutionary adaptation in plants. Comparative analyses of sugar metabolism phenotypes between cultivated varieties and their wild relatives can reveal evolutionarily conserved, quality-associated genes. These genes can serve as molecular markers in hybrid breeding programs. Consequently, marker-assisted selection can be applied to precisely improve fruit sugar content and environmental resilience, thus offering novel insights for fruit tree breeding and cultivation practices.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.W.; Zhang, S.L.; Zhang, L.C. Sugar Transport, Metabolism, Accumulation and Their Regulation in Fruits. J. Plant Physiol. Mol. Biol. 2004, 30, 1–10. (In Chinese) [Google Scholar]
- Ma, X.W.; Xing, S.S.; Li, L.; Liu, L.Q.; Yao, Q.S.; Wang, S.B.; Wu, H.X.; Zhan, R.L. Characteristics of Soluble Sugars Contents in Fruit of Mango Cultivars. Chin. J. Trop. Crops 2011, 32, 1648–1652. (In Chinese) [Google Scholar]
- Guo, X.J.; Tian, H.; Ma, C.; Zhang, Q.; Wang, P.; Yang, X.F. Analysis of the Content Characteristics and Soluble Sugar Components of Mango Fruits from Various Cultivars. Food Res. Dev. 2021, 42, 125–132. (In Chinese) [Google Scholar]
- Ran, X.Y.; Huang, W.J.; Zhong, C.H. Advance in starch metabolism research of kiwifruit. J. Fruit Sci. 2024, 41, 325–337. (In Chinese) [Google Scholar]
- Lin, X.X.; Peng, M.; Wu, S.P.; Yi, G.J.; Dong, T.; Zhong, X.H.; Gao, H.J. A comparative analysis of the differences in starch degradation and soluble sugar accumulation between ‘Zhongjiao No. 9’ and ‘Baxijiao’ during fruit ripening. J. Fruit Sci. 2019, 36, 1524–1539. (In Chinese) [Google Scholar]
- Jia, H.F.; Wang, Y.H.; Sun, M.Z.; Li, B.B.; Han, Y.; Zhao, Y.X.; Li, X.L.; Ding, N.; Li, C.; Ji, W.L.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Z.; Mao, W.W.; Jia, M.R.; Xing, S.N.; Ali, U.; Zhao, Y.Y.; Chen, Y.T.; Cao, M.L.; Dai, Z.R.; Zhang, K.; et al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J. Exp. Bot. 2018, 69, 4805–4820. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit Sugar and Organic Acid Were Significantly Related to Fruit Mg of Six Citrus Cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Zhang, S.L.; Chen, J.W.; Tao, J.; Wu, Y.J. The Physiological Mechanism on the Difference of Sugar Accumulation in Citrus Varieties. Sci. Agric. Sin. 2002, 35, 541–545. (In Chinese) [Google Scholar]
- Huang, H.B.; Cheng, G.W.; Gao, F.F. Studies on the Development of Lychee Fruit II. Some Physiological and Biochemical Characteristics During Maturation. Acta Hortic. Sin. 1981, 8, 123–128. (In Chinese) [Google Scholar]
- Li, J.G.; Luo, S.; Yuan, W.Q. Changes of Sugar Accumulation and Enzyme Activity Related to Sugar Metabolism During Litchi Fruit Maturation. J. South China Agric. Univ. (Nat. Sci. Ed.) 2003, 24, 87–88. (In Chinese) [Google Scholar]
- Cai, X.L.; Pan, J.C.; Zhou, Y.M.; Liu, H.H. Advances in Research on Sugar and Acid Metabolism and Its Regulation in Lychee. Jiangsu Agric. Sci. 2018, 46, 17–22. (In Chinese) [Google Scholar]
- Wang, H.C.; Huang, H.B.; Huang, X.M. Sugar Accumulation and Related Enzyme Activities in the Litchi Fruit of ‘Nuomici’ and ‘Feizixiao’. Acta Hortic. Sin. 2003, 30, 1–5. (In Chinese) [Google Scholar]
- Yu, D.; Wei, X.Q.; Xu, L.; Zhang, X.J.; Xu, J.H. Carbohydrate Accumulation and Variagtion of Relative Enzyme Activities During Fruit Development of Longan. Fujian Agric. Sci. Technol. 2014, 43, 3–10. (In Chinese) [Google Scholar]
- Zhou, M.; Li, Y.F.; Liu, T.X.; Yang, G.S. Research Progress in Sugar Accumulation in Grape Berries. Hunan Agric. Sci. 2020, 56, 91–95. (In Chinese) [Google Scholar]
- Sun, P.G.; Cheng, Z.H.; Sun, C.J.; Guo, S.X.; Guo, G.; Wu, Q.; Li, H.L.; Wang, Y.R. Changes of Starch and Soluble Sugar Contents and Their Correlation Analysis During Fruit Development of Pitaya. China Fruits 2022, 9, 51–54. (In Chinese) [Google Scholar]
- Teo, G.; Suzuki, Y.; Uratsu, S.L.; Lampinen, B.; Ormonde, N.; Hu, W.K.; DeJong, T.M.; Dandekar, A.M. Silencing Leaf Sorbitol Synthesis Alters Long-Distance Partitioning and Apple Fruit Quality. Proc. Natl. Acad. Sci. USA 2006, 103, 18842–18847. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Li, X.W.; Ma, Y.P.; Liu, P.; Su, M.S.; Zhou, J.Y.; Du, J.H. Research Progress in Sugar Metabolism of Peach Fruits. Shanghai J. Agric. Sci. 2019, 35, 144–150. (In Chinese) [Google Scholar]
- Wang, H.; Wang, H.; Han, W.; Wang, W.; Yu, L. Research Progress on Metabolic Pathways and Physiological Mechanisms of Sugar Accumulation in Peach Fruit. Northern Hortic. 2024, 15, 128–133. (In Chinese) [Google Scholar]
- Huang, Y.H.; Zeng, M. Research Advances in Sugar Metabolism and Regulatory Factors in Pear Fruits. Plant Physiol. J. 2013, 49, 709–714. (In Chinese) [Google Scholar]
- Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Molecular Basis for Optimizing Sugar Metabolism and Transport During Fruit Development. aBIOTECH 2021, 2, 330–340. [Google Scholar] [CrossRef]
- Moshchenskaya, Y.L.; Galibina, N.A.; Novitskaya, L.L.; Nikerova, K.M. The Role of Sucrose Synthase in Sink Organs of Woody Plants. Russ. J. Plant Physiol. 2019, 66, 10–21. [Google Scholar] [CrossRef]
- Schmölzer, K.; Gutmann, A.; Diricks, M.; Desmet, T.; Nidetzky, B. Sucrose Synthase: A Unique Glycosyltransferase for Biocatalytic Glycosylation Process Development. Biotechnol. Adv. 2016, 34, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, S.; Tyagi, P.; Sindhi, V.; Yadavilli, K.S. Invertase and its applications—A brief review. J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Xie, J.; Cai, K.; Hu, H.X.; Jiang, Y.L.; Yang, F.; Hu, P.F.; Cao, D.D.; Li, W.F.; Chen, Y.; Zhou, C.Z. Structural Analysis of the Catalytic Mechanism and Substrate Specificity of Anabaena Alkaline Invertase InvA Reveals a Novel Glucosidase. J. Biol. Chem. 2016, 291, 25667–25677. [Google Scholar] [CrossRef]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef]
- Ruan, Y.L. Signaling Role of Sucrose Metabolism in Development. Mol. Plant 2012, 5, 764–765. [Google Scholar] [CrossRef]
- Deng, S.; Mai, Y.; Niu, J. Fruit characteristics, soluble sugar compositions and transcriptome analysis during the development of Citrus maxima “seedless”, and identification of SUS and INV genes involved in sucrose degradation. Gene 2019, 689, 131–140. [Google Scholar] [CrossRef]
- Murayama, S.; Handa, H. Genes for alkaline/neutral invertase in rice: Alkaline/neutral invertases are located in plant mitochondria and also in plastids. Planta 2007, 225, 1193–1203. [Google Scholar] [CrossRef]
- Wu, H.X.; Yao, Q.S.; Wang, S.B.; Ma, X.W.; Zhan, R.L. The Relationship between Sugar Accumulation and Related Enzyme Activities during the Development Process of ‘KRS’ Mango Fruits. J. Anhui Agri. Sci. 2016, 44, 24–26. (In Chinese) [Google Scholar]
- Lin, Y. Characteristics of Sugar Accumulation and Related Metabolic Enzyme Activities in Mango Fruits at Different Developmental Stages. South China Fruits 2021, 50, 4–8. (In Chinese) [Google Scholar]
- Zhang, H.N.; Li, J.G.; Shu, B.; Yang, W.H.; Deng, X.; Jue, D.W.; Shi, S.Y.; Liu, L.Q. Changes of Sugar Metabolism and Related Enzyme Activities in the Development of Pericarp of ‘Shixia’ Longan. Chin. J. Trop. Crops 2016, 37, 1065–1068. (In Chinese) [Google Scholar]
- Ma, Y.L.; Qi, Y.; Cheng, Y.; Liu, Y.; Ma, J.; Chen, Y. Study on the Regulation Characteristics of Source-sink Sugar Accumulation and Sucrose Metabolism-related Enzyme Activities during Fruit Expansion of Muskmelon. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2023, 44, 23–28. (In Chinese) [Google Scholar]
- Liu, H.; Zhang, C.; Li, C.; Le, F.; Liu, Y. Effects of Different Rootstocks on Sugar Accumulation and Sucrose Metabolism of Watermelon Fruit. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2025, 46, 1–12. (In Chinese) [Google Scholar]
- Su, J.; Zhu, L.C.; Liu, X.; Peng, Y.J.; Ma, B.Q.; Ma, F.W.; Li, M.J. Research Progress on Sugar Metabolism and Concentration Regulation in Fruit. J. Fruit Sci. 2022, 39, 266–279. (In Chinese) [Google Scholar]
- Maimaiti, A.; Zhang, X.L.; Mei, C.; Ma, K.; Yan, P.; Wang, J.X. Soluble Sugar Accumulation and Related Enzyme Activity in the Fruits of Korla Fragrant Pear. Xinjiang Agric. Sci. 2018, 55, 664–673. (In Chinese) [Google Scholar]
- Wang, D.F.; Yang, Z.J.; Sun, J.M.; Cao, Y.F.; Huang, X.S.; Zhang, S.L.; Wu, J. Difference in Soluble Sugar Accumulation and Related Enzyme Activity of Fruits among Different Pear Cultivars. J. Fruit Sci. 2014, 31, 30–38. (In Chinese) [Google Scholar]
- Yang, W.Y.; Xie, H.J.; Tao, L.; Huan, Y.M.; Chen, S.B.; Lin, L.J.; Liao, M.A. Study on Sugar Accumulation and Metabolism Related Enzyme Activities in Fruit of ‘Golden Delicious’ Apple and Its Excellent Variation (SGP-1). Acta Agric. Boreali-Occident. Sin. 2022, 31, 1112–1120. (In Chinese) [Google Scholar]
- Wu, J.C.; Wu, B.S.; Chen, D.Q.; Lin, S.Q.; Wu, L.J.; Feng, F.T.; Cai, M.H.; Lin, X. Changes of Sorbitol Content and Related Enzymes During the Development of Loquat Fruit. Chin. J. Trop. Crops 2014, 35, 1997–2001. (In Chinese) [Google Scholar]
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose Kinases and Their Role in Sugar-Sensing and Plant Development. Front. Plant Sci. 2013, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Claeyssen, E.; Rivoal, J. Isozymes of Plant Hexokinase: Occurrence, Properties and Functions. Phytochemistry 2007, 68, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial Roles of Hexokinase and Fructokinase in the Effects of Sugars on Plant Physiology and Development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. Plant Fructokinases: Evolutionary, Developmental, and Metabolic Aspects in Sink Tissues. Front. Plant Sci. 2018, 9, 339. [Google Scholar] [CrossRef]
- Damari-Weissler, H.; Kandel-Kfir, M.; Gidoni, D.; Mett, A.; Belausov, E.; Granot, D. Evidence for Intracellular Spatial Separation of Hexokinases and Fructokinases in Tomato Plants. Planta 2006, 224, 1495–1502. [Google Scholar] [CrossRef]
- Dong, W.J.; Chen, M.; Xiang, M.L.; Chen, J.Y.; Zeng, J.K. Expression Analysis of Hexokinase Genes in Kiwifruit during Postharvest Softening. Food Sci. 2024, 45, 189–196. (In Chinese) [Google Scholar]
- Shuai, L.; Xue, X.; Niu, J.; Cui, Y.; Han, D.; Wu, Z. Analyses of the Fructokinase Activity and Its Gene Expression during the Development of Longan Fruits. J. South China Agric. Univ. 2015, 36, 99–104. (In Chinese) [Google Scholar]
- Nie, X.S.; Hong, C.; Wang, Q.Y.; Lu, M.; An, H.M. Sugar Composition and Transcriptome Analysis in Developing ‘Fengtang’ Plum (Prunus salicina Lindl.) Reveal Candidate Genes Regulating Sugar Accumulation. Plant Physiol. Biochem. 2023, 202, 107955. [Google Scholar] [CrossRef]
- Bai, B.B.; Geng, H.Y.; Jing, Y.L.; Zhao, Z.C.; Chen, Y.Y. Cloning and Expression Vector Construction of MinSPS1 Gene in Mango. Mol. Plant Breed. 2019, 17, 855–861. (In Chinese) [Google Scholar]
- Wu, J.Y.; Chen, M.; Yao, Y.L.; Fu, Q.; Zhu, Z.Y.; Zhang, X.M. Identification, characterisation, and expression profile analysis of the sucrose phosphate synthase gene family in pineapple (Ananas comosus). J. Hortic. Sci. Biotechnol. 2021, 97, 201–210. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.T.; Hu, B.; Li, J.Q.; Qin, Y.Q.; Chen, L.H.; Qin, Y.H.; Hu, G.B. Identification and Expression Profile Analysis of the Sucrose Phosphate Synthase Gene Family in Litchi chinensis Sonn. PeerJ 2018, 6, e4379. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ma, Z.; Le, S.; Lei, C.; Ma, Q.; Gu, Q. Identification and Expression Analysis of Sucrose-phosphate Synthase (SPS) Genes in Citrus. Acta Hortic. Sin. 2020, 47, 334–344. (In Chinese) [Google Scholar]
- Duan, X.Q. Analysis on Activities of Sugar-metabolization Related Enzymes and Its Gene Expressions during Fruit Ripening of Jackfruit. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2024. (In Chinese). [Google Scholar]
- Zhang, L.H.; Zhu, L.C.; Xu, Y.; Lü, L.; Li, X.G.; Li, W.H.; Liu, W.D.; Ma, F.W.; Li, M.J.; Han, D.G. Genome-Wide Identification and Function Analysis of the Sucrose Phosphate Synthase MdSPS Gene Family in Apple. J. Integr. Agric. 2023, 22, 2080–2093. [Google Scholar] [CrossRef]
- Tong, X.L.; Wang, Z.Y.; Ma, B.Q.; Zhang, C.X.; Zhu, L.C.; Ma, F.; Li, M.J. Structure and Expression Analysis of the Sucrose Synthase Gene Family in Apple. J. Integr. Agric. 2018, 17, 847–856. [Google Scholar] [CrossRef]
- Abdullah, M.; Cao, Y.; Cheng, X.; Meng, D.; Chen, Y.; Shakoor, A.; Gao, J.; Cai, Y. The Sucrose Synthase Gene Family in Chinese Pear (Pyrus bretschneideri Rehd.): Structure, Expression, and Evolution. Molecules 2018, 23, 1144. [Google Scholar] [CrossRef]
- Islam, M.Z.; Hu, X.M.; Jin, L.F.; Liu, Y.Z.; Peng, S.A. Genome-Wide Identification and Expression Profile Analysis of Citrus Sucrose Synthase Genes: Investigation of Possible Roles in the Regulation of Sugar Accumulation. PLoS ONE 2014, 9, e113623. [Google Scholar] [CrossRef]
- Jia, Y.; Qiang, X.; Dong, P.; Ren, T.; Zhang, Y.; Yang, Y. Genome-Wide Identification and Characterization of Sucrose Metabolism Genes Involved in Actinidia rufa and Their Expression Profiling during the Fruit Developmental Stages. Horticulturae 2024, 10, 772. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, M.; Li, X.; Jiu, S.; Wang, C.; Fang, J. Genome-Wide Analysis of the Sucrose Synthase Gene Family in Grape (Vitis vinifera): Structure, Evolution, and Expression Profiles. Genes 2017, 8, 111. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yu, M.L.; Ma, R.J.; Shen, Z.J.; Zhang, B.B.; Korir, N.K. Structure, Expression Profile, and Evolution of the Sucrose Synthase Gene Family in Peach (Prunus persica). Acta Physiol Plant 2015, 37, 81. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.T.; Qin, Y.Q.; Qin, Y.H.; Hu, G.B. Molecular Cloning, Characterization and Expression Profile of the Sucrose Synthase Gene Family in Litchi chinensis. Hortic. Plant J. 2021, 7, 520–528. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.; Chen, J.; Yuan, Y.; Hua, Q.; Zhang, Z.; Zhao, J.; Hu, G.; Chen, J.; Qin, Y. Metabolic Profiling of Sugars and Organic Acids, and Expression Analyses of Metabolism-Associated Genes in Two Yellow-Peel Pitaya Species. Plants 2022, 11, 694. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Peng, X.R.; Pan, R.Y.; Ren, R.; Fang, T. Genomic Identification and Expression Analysis of the SUS Gene Family in Passion Fruit. J. Jiangsu Agric. Sci. 2024, 52, 45–51. (In Chinese) [Google Scholar]
- Stroka, M.A.; Reis, L.; Souza Los, K.K.; Pinto, C.A.; Gustani, F.M.; Forney, C.F.; Etto, R.M.; Galvão, C.W.; Ayub, R.A. The Maturation Profile Triggers Differential Expression of Sugar Metabolism Genes in Melon Fruits. Plant Physiol. Biochem. 2024, 207, 108418. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, M.; Yao, Y.L.; Zhang, X.M. Genome-wide identification and expression analysis of AcNINV family in pineapple. J. Fruit Sci. 2024, 41, 598–610. (In Chinese) [Google Scholar]
- Zhang, Z.; Xing, Y.; Ramakrishnan, M.; Chen, C.; Xie, F.; Hua, Q.; Chen, J.; Zhang, R.; Zhao, J.; Hu, G.; et al. Transcriptomics-Based Identification and Characterization of Genes Related to Sugar Metabolism in ‘Hongshuijing’ Pitaya. Hortic. Plant J. 2022, 8, 450–460. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Wang, H.L.; Yan, S.; Xie, P. Genome-Wide Isolation of VIN Gene Family and Functional Identification of HpVIN4 in Red Pitaya (Hylocereus polyrhizus). Horticulturae 2024, 10, 833. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.Y.; Zheng, J.X.; Fu, W.Q.; Wang, W.K.; Zheng, Y.Q.; Meng, M.L.; Yang, L.; Guo, W.D. Identification and expression analysis of the VcAIN gene family encoding acid invertase in highbush blueberry. J. Zhejiang Norm. Univ. (Nat. Sci.) 2025, 1–9. (In Chinese) [Google Scholar] [CrossRef]
- Feng, L.; Li, Y.; Wang, C.; Yin, Y.; Guo, L.; Tan, W. Identification and Expression Analysis of Sucrose Phosphate Synthase (SPS) and Invertase (INV) Gene Families in Pomegranate. Shandong Agric. Sci. 2024, 56, 11–18. (In Chinese) [Google Scholar]
- Yamada, K.; Oura, Y.; Mori, H.; Yamaki, S. Cloning of NAD-Dependent Sorbitol Dehydrogenase from Apple Fruit and Gene Expression. Plant Cell Physiol. 1998, 39, 1375–1379. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Wu, J.; Yin, H.; Dunwell, J.M.; Zhang, S. Genome-Wide Identification and Comparative Evolutionary Analysis of Sorbitol Metabolism Pathway Genes in Four Rosaceae Species and Three Model Plants. BMC Plant Biol. 2022, 22, 341. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Gao, L.; Ren, J.; Pan, X.; Zhu, Y. Sorbitol Metabolism Plays a Key Role in the Differential Accumulation of Sugar in Two Plum Cultivars. Physiol. Plant. 2024, 176, e14465. [Google Scholar] [CrossRef]
- Yang, J.J. Function Study of Apple Fructokinase Gene MdFRK2 in Regulating Sugar Metabolism. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2019. (In Chinese). [Google Scholar]
- Chen, X.; Shi, L.Y.; Shao, J.R.; Chen, W.; Zheng, Y.H.; Yang, Z.F. Molecular Cloning and Expression Analysis of MrFRK2 in Chinese Bayberry During Fruit Ripening. Acta Hortic. Sin. 2016, 43, 1585–1592. (In Chinese) [Google Scholar]
- Lü, W.Y.; Zhang, L.Q.; Gao, Q.H.; Duan, K. Cloning and Expression Analysis of Fructokinase Gene FaFRK3 from ‘Benihoppe’ Strawberry. Acta Agric. Shanghai 2020, 36, 1–5. (In Chinese) [Google Scholar]
- Tao, X.; Zhu, R.X.; Gong, X.; Wu, L.; Zhang, S.L.; Zhao, J.R.; Zhang, H.P. Fructokinase Gene PpyFRK5 Plays an Important Role in Sucrose Accumulation of Pear Fruit. Acta Hortic. Sin. 2022, 49, 1429–1440. (In Chinese) [Google Scholar]
- Xie, P.; Yan, S.; Wang, H.L.; Zheng, Q.M. Cloning, Expression, and Enzymatic Activity Analysis of the Fructokinase Gene HpFRK1 in Red Pitaya (Hylocereus polyrhizus). Acta Bot. Boreal. Occident. Sin. 2024, 44, 1589–1596. (In Chinese) [Google Scholar]
- Zhao, J.; Sun, M.H.; Hu, D.G.; Hao, Y.J. Molecular Cloning and Expression Analysis of a Hexokinase Gene, MdHXK1 in Apple. Hortic. Plant J. 2016, 2, 67–74. [Google Scholar] [CrossRef]
- Zhao, B.Y. Cloning, Expression Analysis and Preliminary Function Characterization of Hexokinase Genes, PbHXK1 and PbFRK1, in Pear Fruit. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. (In Chinese). [Google Scholar]
- Çakir, B. Identification and structure of six members of the hexokinase gene family in Vitis vinifera: Cloning, expression, and functional analysis of a putative chloroplast stromal-type hexokinase. J. Hortic. Sci. Biotechnol. 2014, 89, 663–673. [Google Scholar] [CrossRef]
- Shuai, L.; Li, J.; Han, D.; Wu, Z. Cloning and Prokaryotic Expression of Hexokinase Gene from Dimocarpus longan. J. South China Agric. Univ. 2015, 36, 91–97. (In Chinese) [Google Scholar]
- Murcia, G.; Pontin, M.; Piccoli, P. Role of ABA and Gibberellin A3 on gene expression pattern of sugar transporters and invertases in Vitis vinifera cv. Malbec during berry ripening. Plant Growth Regul. 2018, 84, 275–283. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Liu, X.; Wu, Y.; Han, Z.; Yang, G.; Li, S.; Xie, Z.; Liu, L.; Li, B. Effects of Gibberellic Acid on Soluble Sugar Content, Organic Acid Composition, Endogenous Hormone Levels, and Carbon Sink Strength in Shine Muscat Grapes during Berry Development Stage. Horticulturae 2024, 10, 346. [Google Scholar] [CrossRef]
- Hong, P.; Sadeghnezhad, E.; Wang, J.; Yu, W.; Zheng, J.; Zhong, R.; Xu, Y.; Zhang, Y.; Dong, T.; Fang, J.; et al. VvSnRK1-VvSS3 Regulates Sugar Accumulation during Grape Berry Ripening in Response to Abscisic Acid. Sci. Hortic. 2023, 320, 112208. [Google Scholar] [CrossRef]
- Wang, L.; Brouard, E.; Prodhomme, D.; Hilbert, G.; Renaud, C.; Petit, J.P.; Edwards, E.; Betts, A.; Delrot, S.; Ollat, N. Regulation of Anthocyanin and Sugar Accumulation in Grape Berry through Carbon Limitation and Exogenous ABA Application. Food Res. Int. 2022, 160, 111478. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Shi, Z.D.; Jiang, Y.P.; Zhang, X.H.; Li, X.A.; Li, F. Effects of preharvest regulation of ethylene on carbohydrate metabolism of apple (Malus domestica Borkh cv. Starkrimson) fruit at harvest and during storage. Sci. Hortic. 2021, 276, 109748. [Google Scholar] [CrossRef]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene Regulation of Sugar Metabolism in Climacteric and Non-Climacteric Plums. Postharvest Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Zhang, F.; Ji, S. Ethylene Plays an Important Role in the Softening and Sucrose Metabolism of Blueberries Postharvest. Food Chem. 2020, 310, 125965. [Google Scholar] [CrossRef]
- Zhou, K.; Cheng, Q.; Dai, J.; Liu, Y.; Liu, Q.; Li, R.; Wang, J.; Hu, R.; Lin, L. Effects of Exogenous Melatonin on Sugar and Organic Acid Metabolism in Early-Ripening Peach Fruits. PLoS ONE 2023, 18, e0292959. [Google Scholar] [CrossRef] [PubMed]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Wei, Y.; Shao, X. PpINH1, an Invertase Inhibitor, Interacts with Vacuolar Invertase PpVIN2 in Regulating the Chilling Tolerance of Peach Fruit. Hortic. Res. 2020, 7, 168. [Google Scholar] [CrossRef]
- Jiang, W.; Li, N.; Zhang, D.; Meinhardt, L.; Cao, B.; Li, Y.; Song, L. Elevated Temperature and Drought Stress Significantly Affect Fruit Quality and Activity of Anthocyanin-Related Enzymes in Jujube (Ziziphus jujuba Mill. cv. ‘Lingwuchangzao’). PLoS ONE 2020, 15, e0241491. [Google Scholar] [CrossRef]
- Khanna, S.M. Plant Metabolism during Water Deficit Stress: A Review. Agric. Rev. 2024, 45, 448–455. [Google Scholar] [CrossRef]
- Khan, M.A.; Liu, D.H.; Alam, S.M.; Zaman, F.; Luo, Y.; Han, H.; Ateeq, M.; Liu, Y.Z. Molecular Physiology for the Increase of Soluble Sugar Accumulation in Citrus Fruits under Drought Stress. Plant Physiol. Biochem. 2023, 203, 108056. [Google Scholar] [CrossRef]
- Kanski, L.; Kahle, H.; Naumann, M.; Hagenguth, J.; Ulbrich, A.; Pawelzik, E. Cultivation Systems, Light Intensity, and Their Influence on Yield and Fruit Quality Parameters of Tomatoes. Agronomy 2021, 11, 1203. [Google Scholar] [CrossRef]
- Kishore, K.; Rupa, T.R.; Samant, D. Influence of Shade Intensity on Growth, Biomass Allocation, Yield and Quality of Pineapple in Mango-Based Intercropping System. Sci. Hortic. 2021, 278, 109868. [Google Scholar] [CrossRef]
- Zaman, F.; Liu, D.H.; Liu, Y.Z.; Khan, M.A.; Alam, S.M.; Luo, Y.; Han, H.; Li, Y.T.; Elshahat, A. Short-Day Shading Increases Soluble Sugar Content in Citrus Fruit Primarily through Promoting Sucrose Distribution, Starch Degradation and Sucrose Storage Ability. Plant Physiol. Biochem. 2025, 223, 109779. [Google Scholar] [CrossRef]
- Mascellani, A.; Natali, L.; Cavallini, A.; Mascagni, F.; Caruso, G.; Gucci, R.; Havlik, J.; Bernardi, R. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants 2021, 10, 1861. [Google Scholar] [CrossRef]
- Li, F.; Wei, H.; Qi, J.; Sun, S.; Zou, Y.; Li, M. Effects of Nitrogen Application at Mature Stage on Sugar Content and Related Gene Expression of Fuji Apple. J. Northwest AF Univ. (Nat. Sci. Ed.) 2021, 49, 111–119. (In Chinese) [Google Scholar]
- Xiao, K.; Fan, J.; Bi, X.; Tu, X.; Li, X.; Cao, M.; Liu, Z.; Lin, A.; Wang, C.; Xu, P.; et al. A NAC Transcription Factor and a MADS-Box Protein Antagonistically Regulate Sucrose Accumulation in Strawberry Receptacles. Plant Physiol. 2025, 197, kiaf043. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.J.; Li, Y.N.; Zhu, Z.Z.; Zhao, Z.Y.; Yang, Y.Z. MdNAC5: A Key Regulator of Fructose Accumulation in Apple Fruit. New Phytol. 2024, 244, 2458–2473. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.Y.; Wu, C.J.; Kuang, J.F.; Lu, W.J.; Chen, J.Y.; Shan, W. MaNAC19–MaXB3 Regulatory Module Mediates Sucrose Synthesis in Banana Fruit during Ripening. Int. J. Biol. Macromol. 2023, 253, 127144. [Google Scholar] [CrossRef]
- Lu, W.; Hao, W.; Liu, K.; Liu, J.; Yin, C.; Su, Y.; Hang, Z.; Peng, B.; Liu, H.; Xiong, B.; et al. Analysis of Sugar Components and Identification of SPS Genes in Citrus Fruit Development. Front. Plant Sci. 2024, 15, 1372809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.; Luo, Z.; Lyu, L.; Wang, C.; Zhu, L.; Ma, F.; Li, M.; Han, D. Overexpression of a Transcription Factor MdWRKY126 Altered Soluble Sugar Accumulation in Apple and Tomato Fruit. Hortic. Plant J. 2025, 11, 989–998. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Zhang, L.L.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Wang, C.K.; Wang, W.Y.; Du, M.C.; Hu, D.G. MdbHLH3 Modulates Apple Soluble Sugar Content by Activating Phosphofructokinase Gene Expression. J. Integr. Plant Biol. 2022, 64, 884–900. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, X.; Zhang, Z.; Luo, W.; Nambeesan, S.U.; Li, Q.; Qiao, X.; Yang, B.; Wang, L.; Zhang, M.; et al. PbrbZIP15 Promotes Sugar Accumulation in Pear via Activating the Transcription of the Glucose Isomerase Gene PbrXylA1. Plant J. 2024, 117, 1392–1412. [Google Scholar] [CrossRef]
- Meng, D.; Cao, H.; Yang, Q.; Zhang, M.; Borejsza-Wysocka, E.; Wang, H.; Dandekar, A.M.; Fei, Z.; Cheng, L. SnRK1 Kinase-Mediated Phosphorylation of Transcription Factor bZIP39 Regulates Sorbitol Metabolism in Apple. Plant Physiol. 2023, 192, 2123–2142. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Yang, N.; Jin, L.; Wang, L.; Ma, S.; Ruan, Y.-L.; Ma, F.; Li, M. Variation in the Promoter of the Sorbitol Dehydrogenase Gene MdSDH2 Affects Binding of the Transcription Factor MdABI3 and Alters Fructose Content in Apple Fruit. Plant J. 2022, 109, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shi, Y.; Liu, S.; Jin, R.; Sun, J.; Grierson, D.; Li, S.; Chen, K. The Transcription Factor CitZAT5 Modifies Sugar Accumulation and Hexose Proportion in Citrus Fruit. Plant Physiol. 2023, 192, 1858–1876. [Google Scholar] [CrossRef]
- Ma, W.; Li, B.; Zheng, L.; Peng, Y.; Tian, R.; Yuan, Y.; Zhu, L.; Su, J.; Ma, F.; Li, M.; et al. Combined Profiling of Transcriptome and DNA Methylome Reveal Genes Involved in Accumulation of Soluble Sugars and Organic Acid in Apple Fruits. Foods 2021, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, G.; Lian, J.; Garraway, J.; Niu, Y.; Hu, Z.; Yu, J.; Zhang, M. The Chromosome-Scale Genome of Melon Dissects Genetic Architecture of Important Agronomic Traits. iScience 2020, 23, 101422. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Z.; Zhang, S.; Fu, W.; Su, L.; Fang, J.; Jia, H. Effect of the Methylation Level on the Grape Fruit Development Process. J. Agric. Food Chem. 2020, 68, 2099–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, W.; Li, J.; Yue, P.; Bu, H.; Jiang, J.; Liu, W.; Xu, Y.; Yuan, H.; Li, T.; et al. Histone Acetylation at the Promoter for the Transcription Factor PuWRKY31 Affects Sucrose Accumulation in Pear Fruit. Plant Physiol. 2020, 182, 2035–2046. [Google Scholar] [CrossRef]
- Gao, S.; Yin, M.; Xu, M.; Zhang, H.; Li, S.; Han, Y.; Ji, S.; Li, X.; Du, G. Transcription factors PuPRE6/PuMYB12 and histone deacetylase PuHDAC9-like regulate sucrose levels in pear. Plant Physiol. 2024, 194, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Vall-Llaura, N.; Torres, R.; Lindo-García, V.; Muñoz, P.; Munné-Bosch, S.; Larrigaudière, C.; Teixidó, N.; Giné-Bordonaba, J. PbSRT1 and PbSRT2 Regulate Pear Growth and Ripening Yet Displaying a Species-Specific Regulation in Comparison to Other Rosaceae spp. Plant Sci. 2021, 308, 110925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, F.; Wang, X.; Qiu, K.; Sheng, Y.; Xie, Q.; Shi, P.; Zhang, J.; Pan, H. Genome-Wide Identification and Characterization of Long Noncoding RNAs during Peach (Prunus persica) Fruit Development and Ripening. Sci. Rep. 2022, 12, 11044. [Google Scholar] [CrossRef]
- Wang, S.; Guo, M.; Huang, K.; Qi, Q.; Li, W.; Yan, J.; He, J.; Guan, Q.; Ma, F.; Xu, J. Genome-Wide Identification and Characterization of Long Noncoding RNAs Involved in Apple Fruit Development and Ripening. Sci. Horticult. 2022, 295, 110898. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Y.; Pan, L.; Hayward, A.; Wang, Y. Identification and Characterization of miRNAs in Ripening Fruit of Lycium barbarum L. Using High-Throughput Sequencing. Front. Plant Sci. 2015, 6, 778. [Google Scholar] [CrossRef] [PubMed]
| Sugar Accumulation Pattern | Type | Representative Fruit | Forms of Sugar Accumulation |
|---|---|---|---|
| Starch conversion type | Climacteric | Mango Banana Kiwifruit | Starch accumulates during fruit growth, development, and maturation. Post—harvest, starch converts to soluble sugars via sugar metabolism—related enzymes during ripening. |
| Sugar direct accumulation type | Non-climacteric | Strawberry Citrus Lychee Longan Grape Pitaya | Upon entry into fruits, most photosynthates are stored as soluble sugars in vacuoles during early fruit growth, with only a small fraction used for starch accumulation. |
| Mixture type | Climacteric | Apple Pear Peach | Starch accumulates during early growth, while in later stages, either soluble sugars accumulate directly or starch converts to soluble sugars for storage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Lu, L.; Meng, Z.; Qin, Y.; Guo, L.; Ran, M.; Peng, P.; Tang, Y.; Huang, G.; Li, W.; et al. Advancements on the Mechanism of Soluble Sugar Metabolism in Fruits. Horticulturae 2025, 11, 1001. https://doi.org/10.3390/horticulturae11091001
Wu J, Lu L, Meng Z, Qin Y, Guo L, Ran M, Peng P, Tang Y, Huang G, Li W, et al. Advancements on the Mechanism of Soluble Sugar Metabolism in Fruits. Horticulturae. 2025; 11(9):1001. https://doi.org/10.3390/horticulturae11091001
Chicago/Turabian StyleWu, Jiaqi, Liushan Lu, Zixin Meng, Yuming Qin, Limei Guo, Mengyang Ran, Peng Peng, Yingying Tang, Guodi Huang, Weiming Li, and et al. 2025. "Advancements on the Mechanism of Soluble Sugar Metabolism in Fruits" Horticulturae 11, no. 9: 1001. https://doi.org/10.3390/horticulturae11091001
APA StyleWu, J., Lu, L., Meng, Z., Qin, Y., Guo, L., Ran, M., Peng, P., Tang, Y., Huang, G., Li, W., & Li, L. (2025). Advancements on the Mechanism of Soluble Sugar Metabolism in Fruits. Horticulturae, 11(9), 1001. https://doi.org/10.3390/horticulturae11091001





