Strawberry Performance and Rhizospheric Health Were Efficiently Improved After Long-Term Sheep Manure Organic Fertilizer Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sample Collection
2.3. Strawberry Rhizospheric Soil pH, Bulk Density, Soil Nutrient Content, and Soil Enzyme Activity
2.4. Bacterial Community Structure and qPCR Analysis of P-Related Genes (pqqC and phoD) in Strawberry Rhizospheric Soil
2.5. Determination of Antioxidant Enzyme Activity, MDA, and Photosynthetic Pigment Content in Strawberry Leaves
2.6. Determination of Plant Biomass and Fruit Nutritional Quality of Strawberry
2.7. Data Analysis
3. Results and Discussion
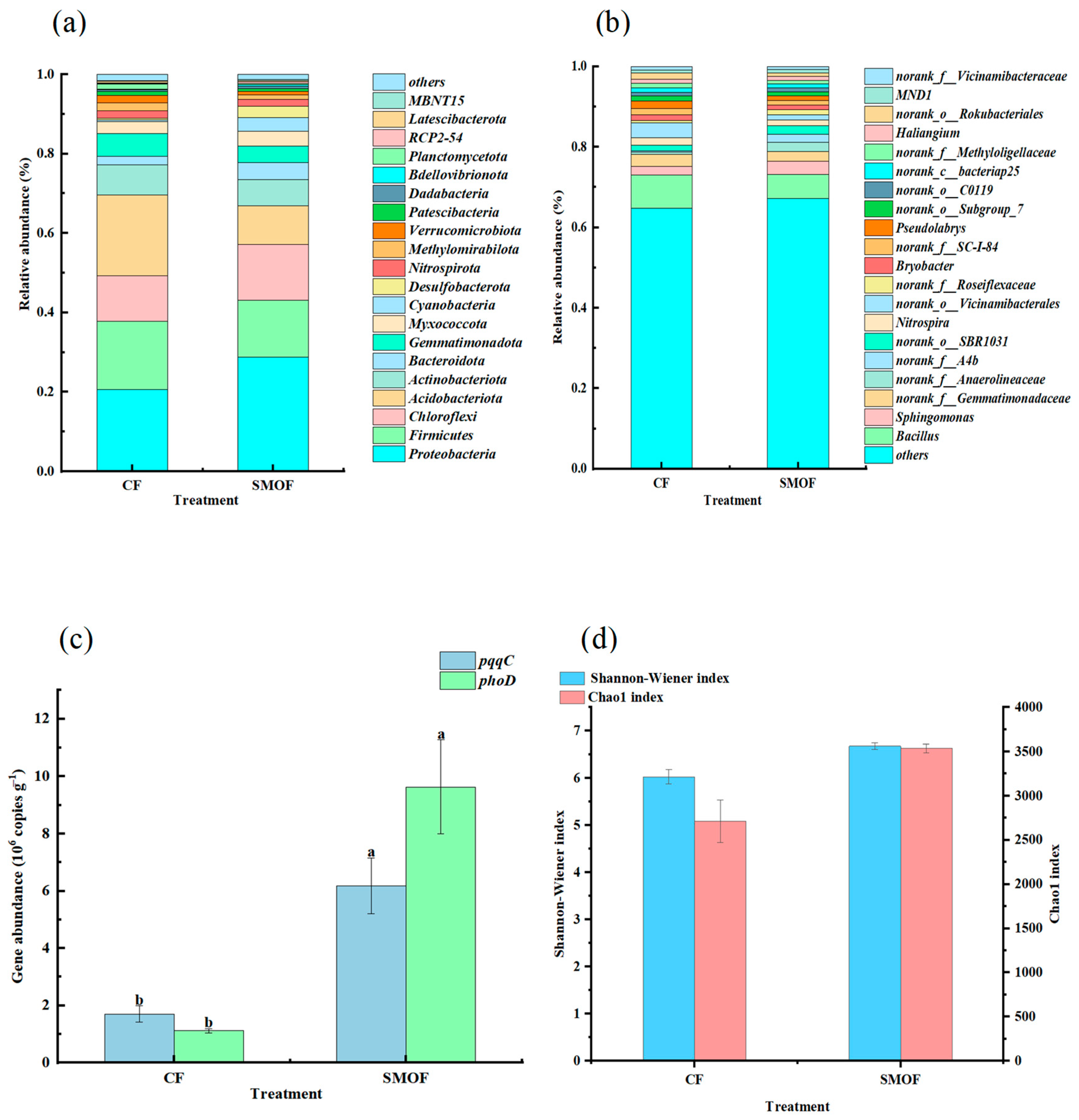
3.1. Effects of Long-Term SMOF on Microbial Community Structure and P-Involved Functional Gene Abundance in Strawberry Rhizospheric Soil
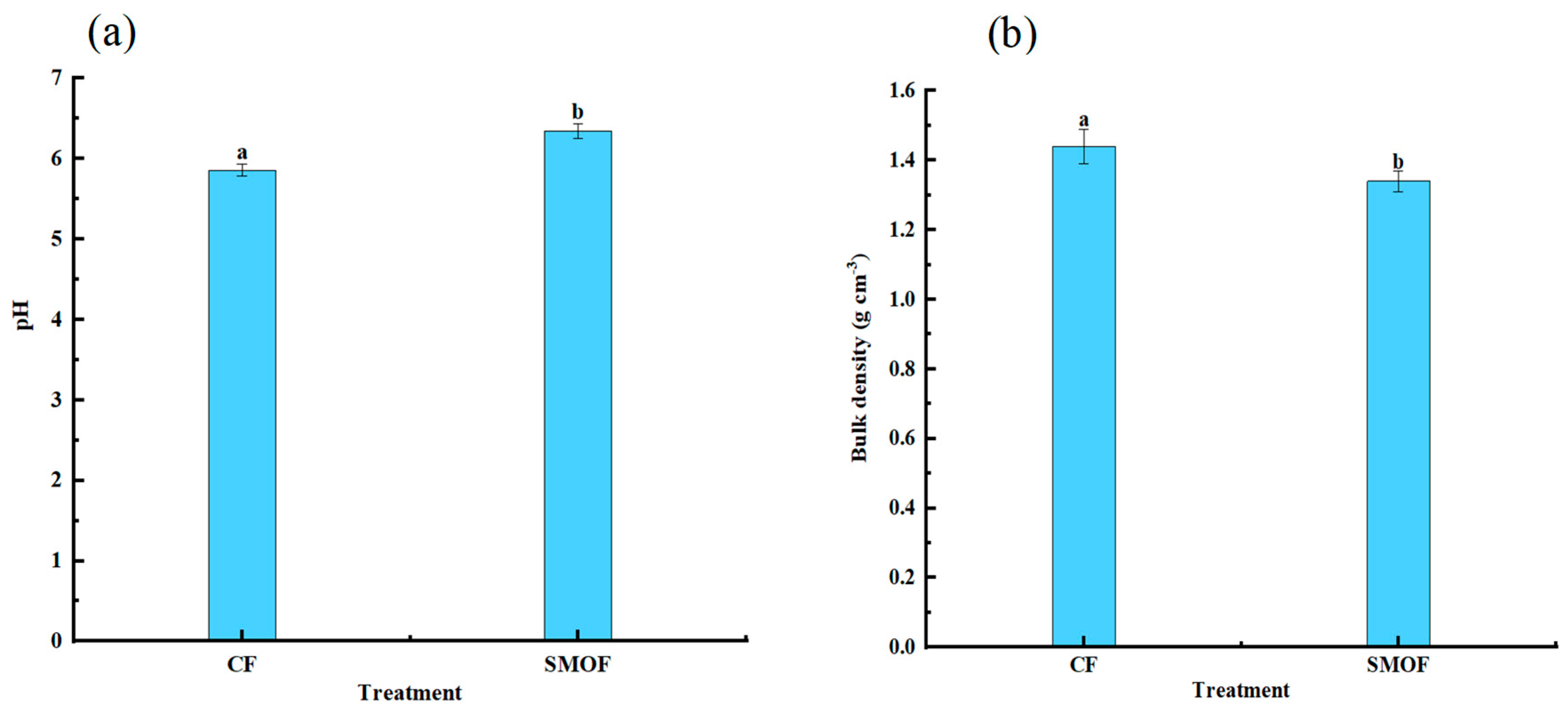
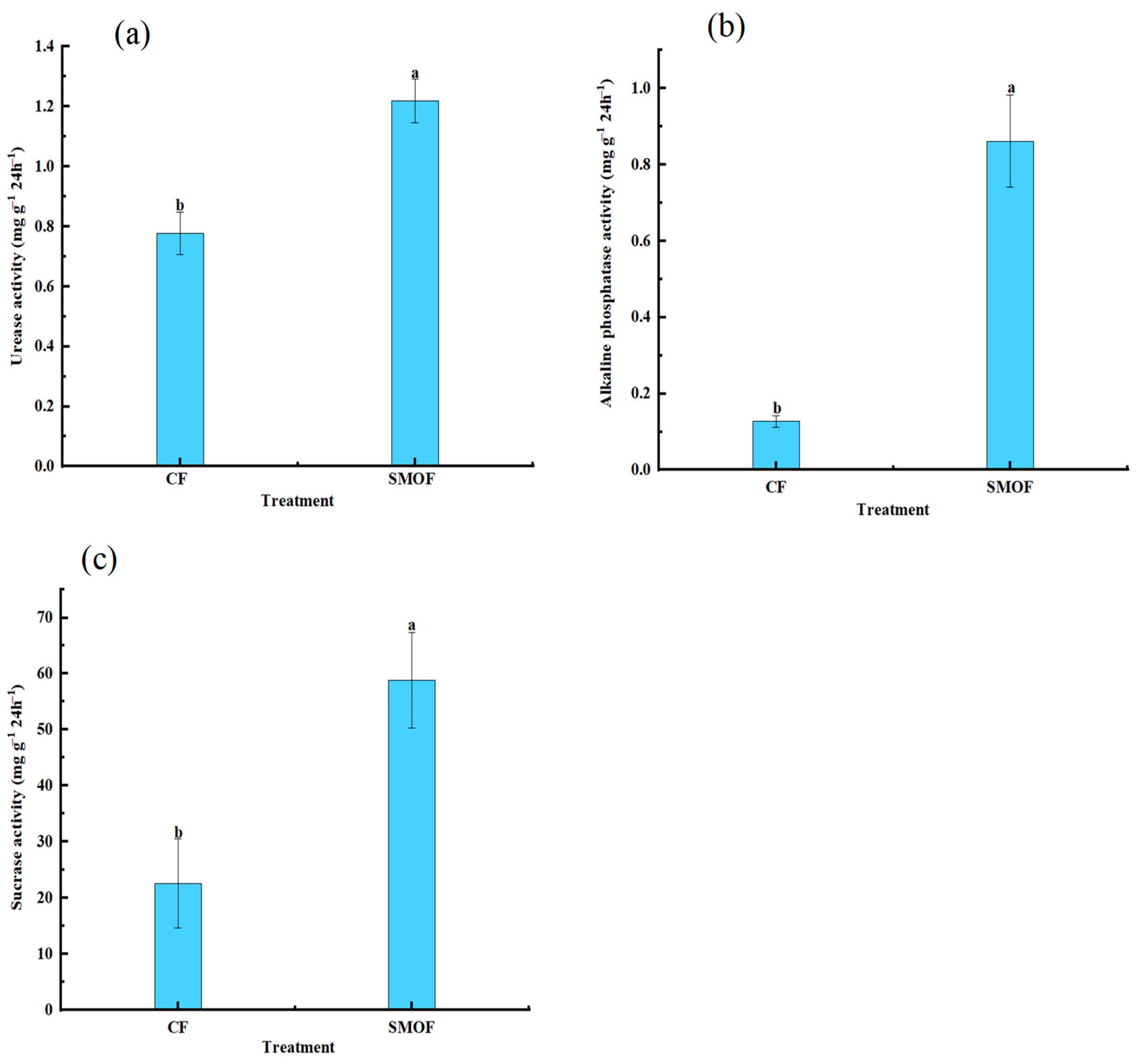
3.2. Soil pH, Bulk Density, Soil Enzyme Activity, and Soil Nutrient Content
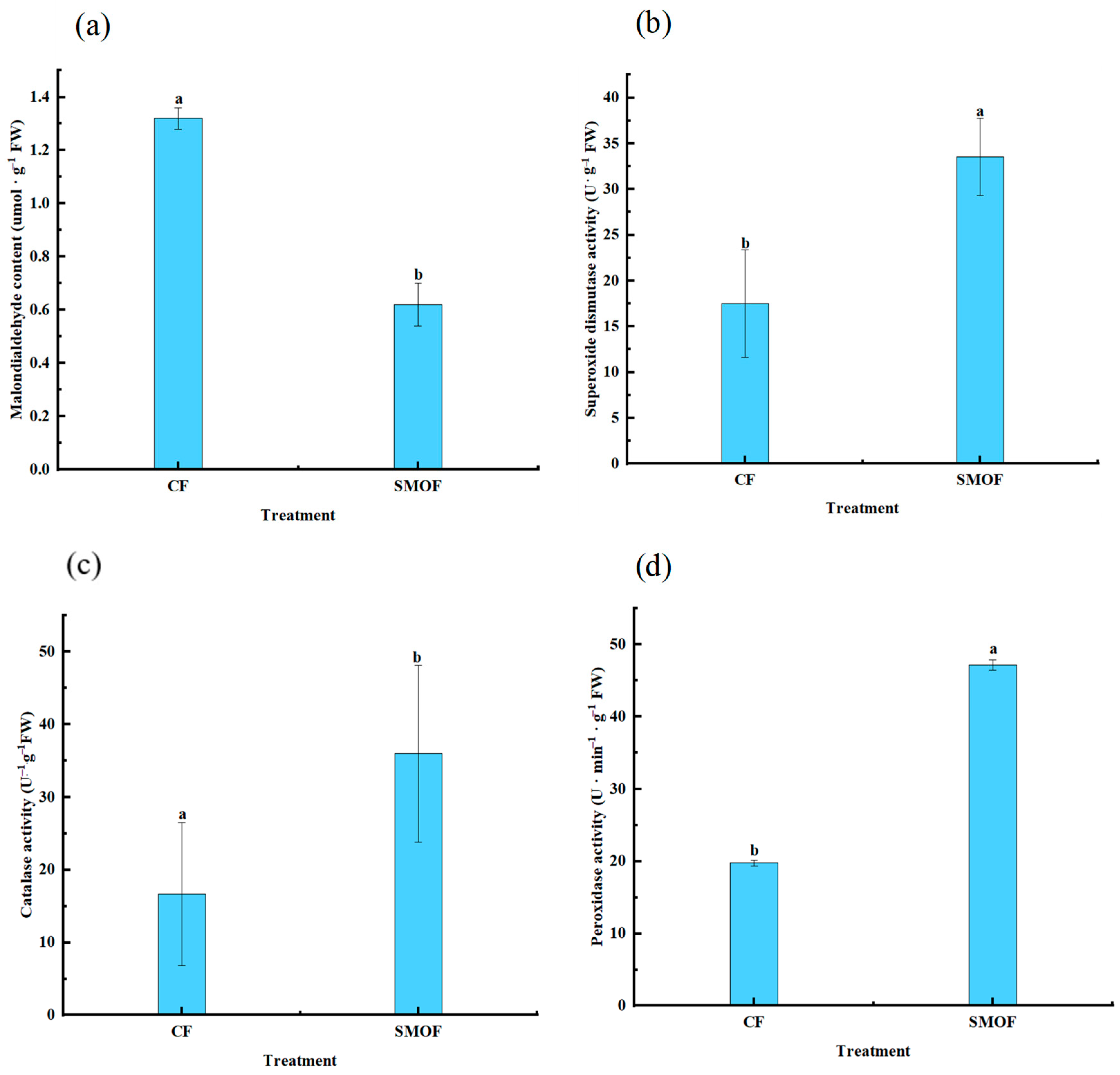
3.3. MDA Content and Antioxidant Enzyme Activity in Strawberry Leaves
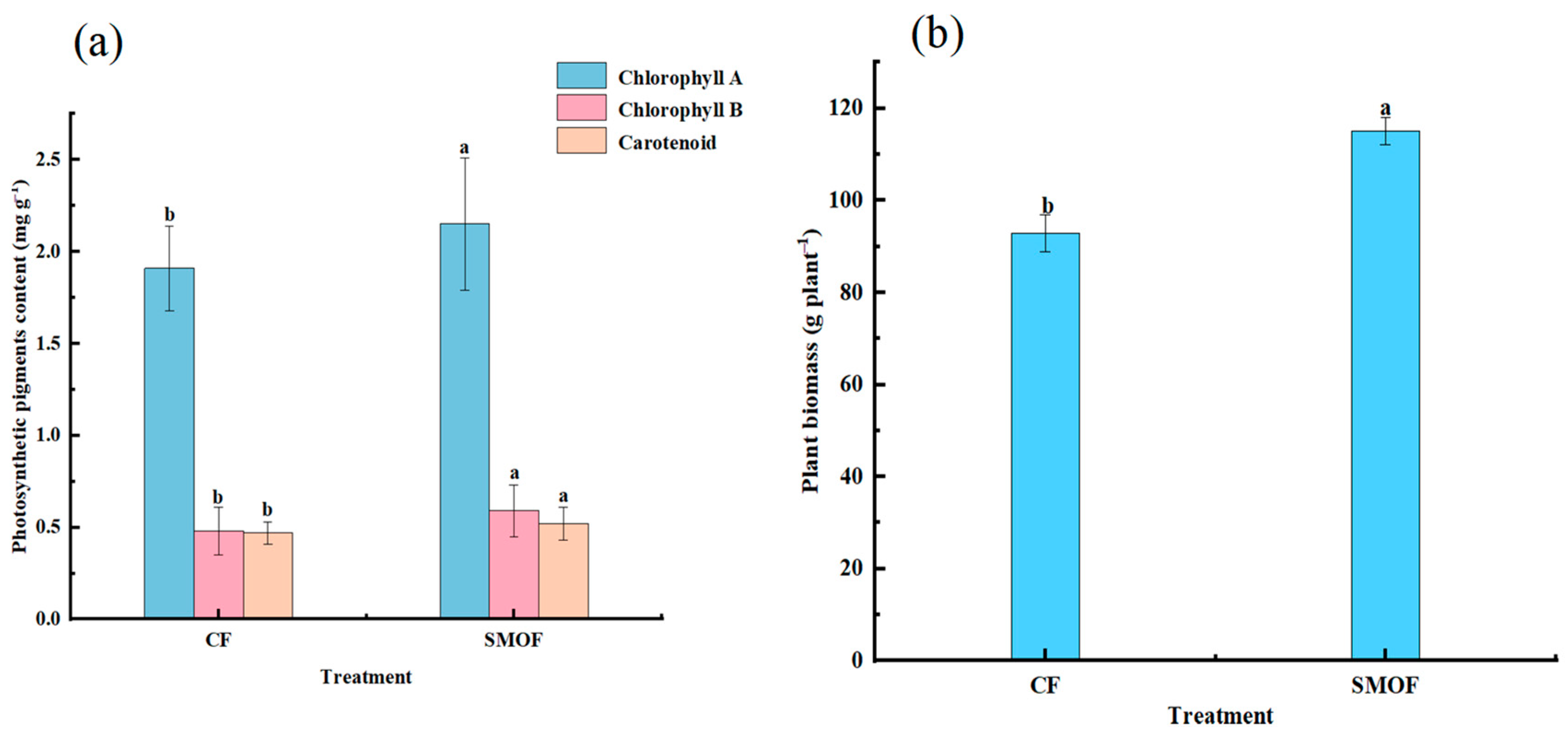
3.4. Strawberry Photosynthetic Pigment Content and Plant Biomass
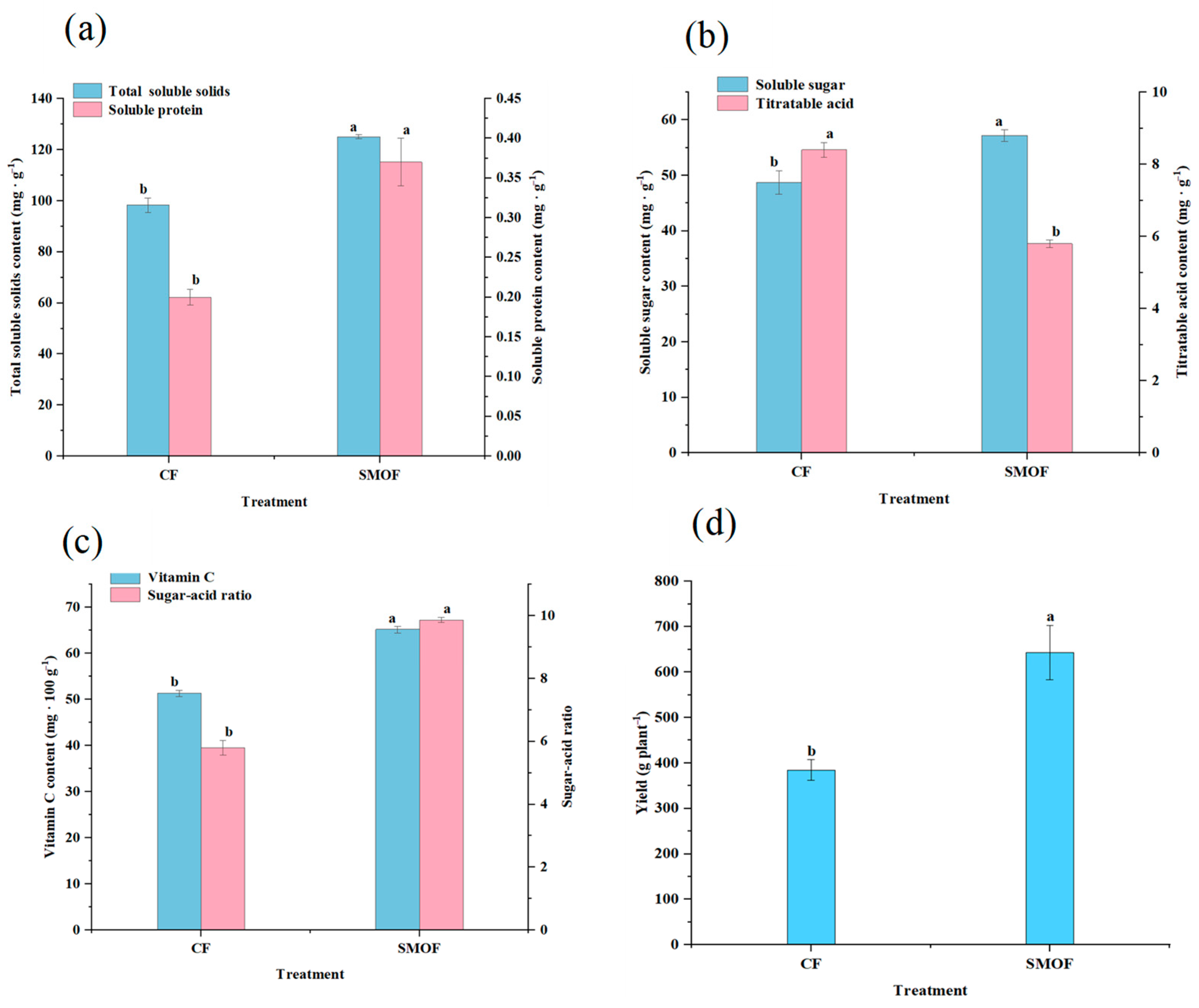
3.5. Strawberry Fruit Nutritional Quality and Yield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Hao, W.; Wang, X.; Wang, Y.; Ma, J.; Tan, J. Harm of excessive fertilization on wheat and ways of reducing fertilizer application. Mod. Agric. Sci. Technol. 2022, 24, 40–44. [Google Scholar]
- Du, Y.D.; Cui, B.J.; Zhang, Q.; Wang, Z.; Sun, J.; Niu, W.Q. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Liu, M.J.; Cheng, C.A.; Qiao, L.; Li, Y.Q.; Li, Z. Responses of soil available nutrients and microbial performance in a newly established apple orchard after five-year fertilization with different sources of livestock manure. J. Agric. Food Res. 2025, 19, 101635. [Google Scholar] [CrossRef]
- Kai, T.; Adhikari, D. Effect of organic and chemical fertilizer application on apple nutrient content and orchard soil condition. Agriculture 2021, 11, 340. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, Y.C.; Chen, L.L.; Li, R.N.; Zhai, C.X.; Li, Y.Q. Effects of different kinds of manure combination with chemical fertilizer on yield, quality and soil nutrient content in greenhouse tomato. Acta Agric. Boreali-Sin. 2011, 26, 152–156. [Google Scholar]
- Li, P.; Kong, D.N.; Zhang, H.J.; Xu, L.Y.; Li, C.K.; Wu, M.C.; Jiao, J.G.; Li, D.M.; Xu, L.; Li, H.X.; et al. Different regulation of soil structure and resource chemistry under animal-and plant-derived organic fertilizers changed soil bacterial communities. Appl. Soil Ecol. 2021, 165, 104020. [Google Scholar] [CrossRef]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Crop Sand Livestock Products. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 February 2023).
- Nakielska, M.; Berbec, A.K.; Madej, A.; Feledyn-Szewczyk, B. Microbial Fertilizing Products Impact on Productivity and Profitability of Organic Strawberry Cultivars. Horticulturae 2024, 10, 1112. [Google Scholar] [CrossRef]
- Vishwakarma, G.; Shukla, A.K.; Zaman, F.; Singh, A.; Shukla, S.K. Assessment of Strawberry to Integrated Nutrient Management for Different Yield Attributes and Quality Parameters. Appl. Fruit Sci. 2024, 66, 833–841. [Google Scholar] [CrossRef]
- Zha, Y.; Liu, A.C.; Lai, W.G.; Wang, J.R.; Li, X.Y.; Yu, H.; Xiao, W.F. Sheep manure organic fertilizer is an effective strategy to promote strawberry growth by improving soil physicochemical properties and microbiota. Front. Environ. Sci. 2024, 12, 1414010. [Google Scholar] [CrossRef]
- Quddus, M.A.; Ahmed, R.; Islam, M.; Haque, M.E.; Islam, M.A.; Alam, A.; Rahman, M.Z.; Fahad, Z.H.; Islam, M.K.; Gaber, A.; et al. Organic and inorganic fertilizers influence the productivity, fruit quality and nutrient use efficiency of strawberry (Fragaria × ananassa Duch.). Sci. Rep. 2025, 15, 26252. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, C.L.; Dai, H.L. Soil Agricultural Chemistry Analysis and Environmental Monitoring, 1st ed.; China Land Press: Beijing, China, 2008; pp. 18–20. [Google Scholar]
- Lu, R.K. Methods of Soil Agricultural Chemical Analysis, 2nd ed.; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 12–185. [Google Scholar]
- Guan, S.Y. Soil Enzymes and Their Research Methods, 1st ed.; Agriculture Press: Beijing, China, 1986; pp. 260–334. [Google Scholar]
- Li, H.L.; Zhu, H.T.; Li, H.B.; Zhang, Y.Q.; Xu, S.X.; Cai, S.M.; Sulaiman, A.A.; Kuzyakov, Y.; Rengel, Z.; Zhang, D.S. Dynamics of root-microbe interactions governing crop phosphorus acquisition after straw amendment. Soil Biol. Biochem. 2023, 181, 109039. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments, 1st ed.; Higher Education Press: Beijing, China, 2000; pp. 134–248. [Google Scholar]
- Zhang, X.; Li, J.; Shao, L.; Qin, F.; Yang, J.; Gu, H.R.; Zhai, P.; Pan, X.Q. Effects of organic fertilizers on yield, soil physico-chemical property, soil microbial community diversity and structure of Brassica rapa var. Chinensis. Front. Microbiol. 2023, 14, 1132853. [Google Scholar] [CrossRef]
- Wan, J.X.; Wang, X.F.; Yang, T.J.; Wei, Z.; Banerjee, S.; Friman, V.P.; Mei, X.L.; Xu, Y.C.; Shen, Q.R. Livestock manure type affects microbial community composition and assembly during composting. Front. Microbiol. 2021, 12, 621126. [Google Scholar] [CrossRef]
- Azene, B.; Zhu, R.H.; Pan, K.W.; Sun, X.M.; Nigussie, Y.; Gruba, P.; Raza, A.; Guadie, A.; Wu, X.G.; Zhang, L. Land use change alters phosphatase enzyme activity and phosphatase-harboring microbial abundance in the subalpine ecosystem of southeastern Qinghai-Tibet Plateau, China. Ecol. Indic. 2023, 153, 110416. [Google Scholar] [CrossRef]
- Hu, M.J.; Penuelas, J.; Sardans, J.; Tong, C.; Chang, C.T.; Cao, W.Z. Dynamics of phosphorus speciation and the phoD phosphatase gene community in the rhizosphere and bulk soil along an estuarine freshwater-oligohaline gradient. Geoderma 2020, 365, 114236. [Google Scholar] [CrossRef]
- Shih, P.M.; Ward, L.M.; Fischer, W.W. Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proc. Natl. Acad. Sci. USA 2017, 114, 10749–10754. [Google Scholar] [CrossRef]
- Qin, S.M.; Zhang, H.Y.; He, Y.H.; Chen, Z.J.; Yao, L.G.; Han, H. Improving radish phosphorus utilization efficiency and inhibiting Cd and Pb uptake by using heavy metal-immobilizing and phosphate-solubilizing bacteria. Sci. Total Environ. 2023, 868, 161685. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Hu, Y.J.; Cai, G.; Yao, H.Y.; Ye, J.; Sun, Q.; Veresoglou, S.D.; Li, Y.Y.; Zhu, Z.K.; Guggenberger, G.; et al. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil Biol. Biochem. 2021, 161, 108364. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.L.; Zhao, W.Q.; Pang, J.Y.; Hu, W.; Zhou, Z.G.; Meng, Y.L. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P-related enzymes and the abundance of phoC and phoD genes. Soil Tillage Res. 2022, 220, 105390. [Google Scholar] [CrossRef]
- Luo, G.W.; Sun, B.; Li, L.; Li, M.H.; Liu, M.Q.; Zhu, Y.Y.; Guo, S.W.; Ling, N.; Shen, Q.R. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Zheng, B.X.; Hao, X.L.; Ding, K.; Zhou, G.W.; Chen, Q.L.; Zhang, J.B.; Zhu, Y.G. Long-term nitrogen fertilization decreased the abundance of inorganic phosphate solubilizing bacteria in an alkaline soil. Sci. Rep. 2017, 7, 42284. [Google Scholar] [CrossRef]
- Meyer, J.B.; Frapolli, M.; Keel, C.; Maurhofer, M. Pyrroloquinoline quinone biosynthesis gene pqqC, a novel molecular marker for studying the phylogeny and diversity of phosphate-solubilizing pseudomonads. Appl. Environ. Microbiol. 2011, 77, 7345–7354. [Google Scholar] [CrossRef]
- Long, X.E.; Yao, H.Y.; Huang, Y.; Wei, W.X.; Zhu, Y.G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R.B.; Sun, T.T.; Liang, Y.T.; Jiang, Y.J.; Sun, B. Organic amendments shift the phosphorus-correlated microbial co-occurrence pattern in the peanut rhizosphere network during long-term fertilization regimes. Appl. Soil Ecol. 2018, 124, 229–239. [Google Scholar] [CrossRef]
- Li, X.Q.; Lu, Q.J.; Li, D.Y.; Wang, D.Z.; Ren, X.X.; Yan, J.L.; Ahmed, T.; Li, B. Effects of Two Kinds of commercial organic fertilizers on growth and rhizosphere soil properties of corn on new reclamation land. Plants 2022, 11, 2553. [Google Scholar] [CrossRef] [PubMed]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.L.; Wang, Y.H.; Zhang, Q.; Lin, S.X.; Zhang, Y.; Du, M.R.; Chen, M.H.; Ye, J.H.; Wu, Z.Y.; Wang, H.B. Reasonable deep application of sheep manure fertilizer to alleviate soil acidification to improve tea yield and quality. Front. Front. Plant Sci. 2023, 14, 1179960. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.K.; Li, Q.; Li, Y.H.; Xie, W.Y.; Zhou, H.P.; Yang, Z.X.; Liu, Z.P.; He, L.Y. Effects of different fertilization measures on microbial community and enzyme activities in brown soil. Environ. Sci. 2025, 1–20. [Google Scholar]
- Sahana, B.J.; Madaiah, D.; Shivakumar, B.S.; Sridhara, S.; Pradeep, S. Influence of organic manures on growth, yield and quality of strawberry (Fragaria × ananassa Duch.) under naturally ventilated polyhouse. J. Pharmacogn. Phytochem. 2020, 9, 3284–3287. [Google Scholar]
- Meng, Q.Q.; Shi, Z.J.; Yan, Z.B.; Lambers, H.; Luo, Y.; Han, W.X. Independent and interactive effects of N and P additions on foliar P fractions in evergreen forests of southern China. For. Ecosyst. 2025, 12, 100265. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Yu, X.T.; Li, Y.; Wu, Y.; Gao, H.; Liu, W.; Liu, H.; Gong, S.D.; Wu, H.L. Seasonal changes of prokaryotic microbial community structure in Zhangjiayan Reservoir and its response to environmental factors. Sci. Rep. 2024, 14, 5513. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Hou, Q.; Wang, W.X.; Yang, Y.; Hu, J.; Bian, C.S.; Jin, L.P.; Li, G.C.; Xiong, X.Y. Rhizosphere microbial diversity and community dynamics during potato cultivation. Eur. J. Soil Biol. 2020, 98, 103176. [Google Scholar] [CrossRef]
- Scarlett, K.; Denman, S.; Clark, D.R.; Forster, J.; Vanguelova, E.; Brown, N.; Whitby, C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J. 2021, 15, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Stopnisek, N.; Gubry-Rangin, C.; Höfferle, S.; Nicol, G.W.; Mandic-Mulec, I.; Prosser, J.I. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol. 2010, 76, 7626–7634. [Google Scholar] [CrossRef]
- Madhavi, B.G.K.; Khan, F.; Bhujel, A.; Jaihuni, M.; Kim, N.E.; Moon, B.E.; Kim, H.T. Influence of different growing media on the growth and development of strawberry plants. Heliyon 2021, 7, e07170. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Qu, M.S.; Zhao, Y.Z.; Zhou, J.Z.; Lei, W.W.; Chen, J.; Liu, Y.; Zhao, K.L. Effects of phosphorus fertilizer application rate on the yield and quality of protected strawberries. China Agric. Technol. Ext. 2018, 34, 49–51. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Z.; Lei, C.; Cai, X.; Li, Y.; Zeng, D.; Gong, S.; Wang, J.; Gong, Z. Strawberry Performance and Rhizospheric Health Were Efficiently Improved After Long-Term Sheep Manure Organic Fertilizer Application. Horticulturae 2025, 11, 1000. https://doi.org/10.3390/horticulturae11091000
Chou Z, Lei C, Cai X, Li Y, Zeng D, Gong S, Wang J, Gong Z. Strawberry Performance and Rhizospheric Health Were Efficiently Improved After Long-Term Sheep Manure Organic Fertilizer Application. Horticulturae. 2025; 11(9):1000. https://doi.org/10.3390/horticulturae11091000
Chicago/Turabian StyleChou, Zhengyan, Chenghao Lei, Xinyi Cai, Yong Li, Diya Zeng, Sidan Gong, Jianping Wang, and Zhilian Gong. 2025. "Strawberry Performance and Rhizospheric Health Were Efficiently Improved After Long-Term Sheep Manure Organic Fertilizer Application" Horticulturae 11, no. 9: 1000. https://doi.org/10.3390/horticulturae11091000
APA StyleChou, Z., Lei, C., Cai, X., Li, Y., Zeng, D., Gong, S., Wang, J., & Gong, Z. (2025). Strawberry Performance and Rhizospheric Health Were Efficiently Improved After Long-Term Sheep Manure Organic Fertilizer Application. Horticulturae, 11(9), 1000. https://doi.org/10.3390/horticulturae11091000




