Abstract
The primary challenge facing modern agriculture, including viticulture, is the impact of climate change. The scientific community recommends exploring and utilizing both inter-varietal and intra-varietal variability of local grapevines within each region. The goal is to prioritize planting local varieties over international and imported ones to mitigate the effects of climate change. Within this context, La Palma Island has undertaken a comprehensive assessment evaluating its viticultural heritage. A total of 96 individuals were collected and subjected to genotyping utilizing 20 simple sequence repeats (SSRs). This analysis yielded 44 unique molecular profiles, of which 3 represent new varieties reported for the first time (Aromatica Eufrosina, Cagarruta de oveja, and Viñarda rosada). Additionally, fourteen previously unreported mutations were identified, of which two contain triallelic SSRs. Consequently, the present population of local grapevines on La Palma Island comprises seven varieties (Albillo criollo, Aromatica Eufrosina, Bienmesabe tinto, Cagarruta de oveja, Gual Mazo, Sabro, and Viñarda rosada). The Bienmesabe tinto variety is possibly an interspecific cross. The varieties Aromatica Eufrosina and Viñarda rosada also presented somewhat particular behavior. The distinctiveness of this grapevine population from La Palma Island reinforces the notion that the Canary Archipelago represents a significant center of grapevine biodiversity. The volcanic activity of Tajogaite (2021) did not have a significant impact on grapevine biodiversity on the island.
1. Introduction
Vitis vinifera L., a diploid species with 19 chromosome pairs and a genome of approximately 500 Mb [1], is among the oldest and most globally cultivated crops. Over centuries, human selection and adaptation to diverse environments have endowed grapevine populations with remarkable genetic diversity [1,2]. This genetic variability is increasingly important in the context of climate change, as rising global temperatures threaten traditional cultivation patterns, increase vulnerability to pests and diseases, and alter grape and wine quality [3,4]. Traditional or lesser-known grapevine varieties are therefore a critical resource—not only for environmental resilience and breeding but also for producing wines with distinct profiles that can provide commercial differentiation [5].
The Canary Islands (IC), a Spanish volcanic archipelago in the Macaronesia region (which also includes the Azores, Madeira, the Savage Islands, and Cape Verde), are of special interest for viticultural research due to their geological and geographic isolation (Figure 1a). The archipelago includes eight main islands: El Hierro (HI), La Palma (LP), La Gomera (LG), Tenerife, Gran Canaria, Fuerteventura (FT), Lanzarote (LZ), and La Graciosa (Figure 1b). Viticulture in this region began in the 15th century with the arrival of European settlers [6]. The islands’ volcanic soils, inhospitable to phylloxera (Daktulosphaira vitifoliae), have allowed for the preservation of ungrafted vines and local varieties that have evolved uniquely over centuries [7]. These conditions make the IC, and LP in particular, a unique site for the study of grapevine biodiversity.
Figure 1.
(a) Map of Macaronesia [8]; (b) Canary Islands Archipelago and La Palma Island [9].
La Palma (28°40′ N, 17°52′ W) is the second highest island in the archipelago, covering 708.32 km2 and reaching elevations of 2426 m at Roque de los Muchachos [10] (Figure 2a). Its complex topography and climatic variability create diverse viticultural terroirs [11], with influences such as altitude, prevailing trade winds, variable rainfall, and sun exposure contributing to wines with unique organoleptic qualities [12]. The island’s Appellation d’Origine Contrôlée/Protégée (AOC/AOP La Palma), established in 1994 [13], organizes viticulture into three subzones based on geographic and microclimatic traits (Figure 2b). The North subzone has fertile, non-sandy soils and uses terrace cultivation on steep slopes, where vines are often grown in goblet form at elevations ranging from 100 to 2000 m [14]. In Hoyo de Mazo (eastern LP), vines are grown on slopes covered with volcanic stones or fine gravel, between 200 and 700 m in elevation [15]. Fuencaliente, the island’s hottest and driest region, features vineyards grown on deep volcanic ash layers (often more than two meters thick), using dry-stone windbreaks and terraces at 200–1900 m [16]. It remains a key zone for cultivating Malvasia grapes.
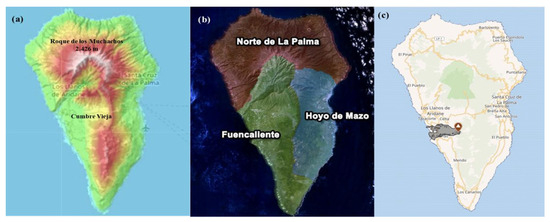
Figure 2.
(a) Topographic map of La Palma Island [17]; (b) viticultural production zones of La Palma [18]; (c) mapped map of lava flow and extent [19].
LP’s geographic isolation, traditional farming methods, and minimal introduction of external plant material have preserved a rich diversity of local grapevine varieties, many of which are not cataloged in international databases [20]. Among red varieties, Negramoll (Principal name (PN): Mollar cano) is the most widespread, followed by Listan negro (a local Canary variety), Bastardo negro (PN: Trousseau noir), and minor cultivars like Tintilla. For white grapes, prominent varieties include Gual (PN: Malvasia fina), Malvasia (PN: Malvasia Dubrovacka), Verdello (PN: Verdelho branco), and the local Albillo criollo. Other cultivated white varieties include Bastardo blanco (PN: Samarrinho), Bermejuela, Bujariego (or Vijariego blanco), Listan blanco de Canarias (PN: Palomino Fino), and Sabro, a local LP variety. Rarely found cultivars on the island include several pre-phylloxera European and Spanish varieties: red grapes like Alfrocheiro, Flot rouge, Molar, Muscat Hamburg, De Rey, Ferral, and Listan prieto; and white grapes such as Chasselas blanc, Morskoi 94, Muscat of Alexandria, and Beba [21]. These vines, many of them ancient and adapted to LP’s microclimates, produce wines with pronounced typicity, strongly influenced by the island’s volcanic soil. This soil imparts unique mineral and saline characteristics to the wine, contributing to its distinct sensory profile. Although highly valuable, the genetic documentation of these cultivars remains limited, hindering conservation and adaptation strategies [22].
Volcanic activity has also influenced LP’s agricultural and genetic biodiversity. Among the IC, LP has experienced the highest frequency of historical eruptions, all along the Cumbre Vieja Ridge, an active volcanic zone in the island’s south [23,24]. The most recent eruption—the Tajogaite volcano in 2021 (Figure 2c)—lasted 85 days and affected over 1200 hectares, including 370 hectares of crops and many vineyards [25,26,27]. This and other eruptions have likely reduced LP’s grapevine genetic diversity, with the island now believed to host fewer genetically distinct varieties compared to its neighbors [21].
Despite these challenges, viticulture remains central to the island’s agriculture. The AOC/AOP La Palma unites producers and regulates grape growing and winemaking [28,29,30,31]. Wines produced include reds, whites, rosés, and sweet wines. Especially noteworthy are naturally sweet wines from Malvasia aromatica, primarily from Fuencaliente and Villa de Mazo. These are made from overripe grapes with high sugar and alcohol content [32].
In this context, the current study aims to genetically characterize LP’s cultivated grapevine population, with particular emphasis on areas such as Fuencaliente, where extreme conditions prevail. These areas may be key to identifying genotypes with high tolerance to heat and drought—traits vital under climate change. The study sets out three main objectives: (1) To document the island’s genetic diversity, both inter-varietal and, where possible, intra-varietal. (2) To clarify varietal identities, correcting mislabeling and identifying synonymies or unique genotypes. To analyze genetic structure, evaluating how LP’s grapevine population behaves and differentiates from others. Furthermore, this research includes an essential conservation component: it examines plant material collected before the 2021 eruption, especially from areas affected by volcanic ash. This offers a rare opportunity to preserve potentially valuable genotypes that might otherwise be lost or obscured beneath volcanic deposits. The findings will not only help clarify LP’s unique genetic contributions to Vitis vinifera L. diversity but also provide valuable insights for climate adaptation and the development of distinct wines. In line with Wolkovich et al. [4], this work emphasizes the need to explore and protect grapevine diversity, both to buffer the effects of climate change through intra-varietal resilience and to counteract wine industry homogenization through inter-varietal innovation. Ultimately, the discovery and preservation of unique LP varieties open the door to creating single-varietal wines with unmatched sensory profiles and ecological adaptation.
2. Materials and Methods
2.1. Plant Material Collection
A total of 96 grapevine (Vitis vinifera L.) cane samples were collected from different cultivated plants across LP Island. The material was selected using a mass selection strategy in collaboration with various local winegrowers and under the supervision of technicians from the AOC/AOP “Vinos de La Palma” and the Consejería de Agricultura, Ganadería, Pesca y Soberanía Alimentaria del Cabildo Insular de la Isla de La Palma.
Sampling was conducted during vine dormancy, at the time of winter pruning, ensuring that the vines were physiologically mature and free of visible disease symptoms. After collection, the samples were transported under refrigerated conditions to Rovira i Virgili University and subsequently stored at −20 °C until laboratory processing. Specific information for each accession, including geographical location, local name, and preliminary phenotypic observations, is provided in Table S1.
2.2. Sample Preparation and DNA Extraction
The cane samples were processed through selective plant tissue preparation, carefully removing the outer woody bark (rhytidome) and the central pith to minimize the presence of contaminants that interfere with DNA extraction, such as lignin, tannins, polyphenols, and polysaccharides. Only the green cortical zone corresponding to the phloem and young vascular tissues were retained. The selected tissue fragments were immediately frozen and ground into a fine, homogeneous powder in the presence of liquid nitrogen (−196 °C). Aliquots of 0.2 g of powdered tissue were weighed and stored in Eppendorf tubes at −20 °C until use.
Genomic DNA was extracted following a modified protocol based on Fort et al. [33], and later optimized by Marsal et al. [34,35] for woody tissues. The procedure included two chloroform–isoamyl alcohol washes to enhance the removal of protein contaminants. DNA quality and concentration were assessed using spectrophotometry (NanoDrop™ 1000, Thermo Fisher Scientific, Wilmington, DE, USA). Extracts with an A260/A280 ratio between 1.8 and 2.0 and an A260/A230 ratio above 1.8 were considered optimal. Samples not meeting these criteria were either discarded or re-purified.
2.3. Selection of Microsatellite Markers (SSR)
A total of 20 highly polymorphic nuclear SSR markers were employed, selected for their proven efficacy and discriminating power in varietal identification and genetic diversity studies in Vitis vinifera L. These markers were chosen based on their high heterozygosity, reproducibility, and wide distribution across the grapevine genome: VVS2, VVS3, and VVS29 [36]; VVMD5, VVMD6, and VVMD7 [37]; VVMD27, VVMD28, and VVMD36 [38]; VrZAG21, VrZAG47, VrZAG62, VrZAG64, VrZAG79, and VrZAG83 [39]; SCU06vv [40]; VvUCH11, VvUCH12, and VvUCH19 [41]; and VChr19a [42].
Among these, VrZAG47 and VVMD27 are not independent markers, as they target the same genomic region using different primer pairs [43]. Additionally, 7 of the 9 SSR reference markers internationally accepted for grapevine varietal identification were included in this study [44]. Detailed information on primer sequences, genomic loci, fluorophores, and technical specifications is provided in Table S2, while their consensus location on the genetic map is illustrated in Figure S1.
2.4. DNA Amplification by Polymerase Chain Reaction (PCR)
The amplification of specific DNA regions was performed using the polymerase chain reaction (PCR) technique, employing the set of 20 previously described nuclear microsatellite (SSR) markers. Reactions were performed using the AmpliTaq DNA polymerase kit (Applied Biosystems, Foster City, CA, USA) in a final volume of 12.5 µL, containing 4 ng of genomic DNA, 1 µM of each primer (Fw and Rv), 1.25 µL of buffer, 2 µL of dNTPs, 0.125 µL of deionized formamide, and 0.0625 µL of Taq polymerase.
Forward primers were labeled with specific fluorochromes for subsequent detection via capillary electrophoresis (6-FAM: VVS3, VVMD7, VVMD28, VVMD36, VrZAG47, VrZAG62, VrZAG83, VvUCH11, and VvUCH19; HEX: VVS2, VVS29, VVMD6, VVMD27, VrZAG21, VrZAG79, and VChr19a; NED: VVMD5, VrZAG64, SCU06vv, and VvUCH12). SSRs were grouped according to their annealing temperature (Ta), which ranged between 50 and 58 °C (Table S2). PCRs were performed using an Applied Biosystems™ 2720 thermal cycler (Foster City, CA, USA) with the following cycling conditions: initial denaturation at 95 °C for 5 min; followed by 40 cycles of 95 °C for 45 s, specific annealing temperature for 30 s, and 72 °C for 90 s; with a final extension step at 72 °C for 7 min.
2.5. Capillary Electrophoresis and Fragment Analysis
PCR products were prepared for capillary electrophoresis by adding 20.5 µL of deionized formamide and 0.25 µL of the internal size standard GeneScan™ ROX 500 (Applied Biosystems, Foster City, CA, USA) to each sample. Different volumes of amplified product were added depending on the fluorophore used: 2 µL for HEX, 4 µL for 6-FAM, and 6 µL for NED. The plates were briefly denatured at 95 °C for 3 min before loading onto the ABI PRISM 3730® Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Fragment separation by size and fluorescent signal detection enabled the generation of electropherograms, which were analyzed using Peak Scanner™ Software v.1.0 (Applied Biosystems, Sparta, NJ, USA) to determine precise fragment lengths in base pairs (bp), allowing allele calling at each locus. Each sample was analyzed at least twice from independent DNA extractions to minimize genotyping errors.
2.6. Genotyping and Varietal Identification
Allelic profiles were compared with a local database developed by the TECNENOL Research Group [20,45,46,47,48,49,50]. Genotypes that did not match any component of our database were compared with entries in a much larger database, the Vitis International Variety Catalogue (VIVC) [51]. This approach also allows the detection of synonymous cultivar names and possible allelic mutations.
2.7. Analysis of Genetic Data
The genetic profiles obtained from the SSR markers were analyzed to characterize both intra- and inter-varietal genetic diversity among the Vitis vinifera L. accessions, and to infer phylogenetic relationships and population structure. For this purpose, a suite of well-established statistical and bioinformatics tools commonly used in population genetics studies was employed.
First, a multilocus genotype matrix was constructed and formatted for analysis in GenAlEx v6.5 [52,53]. Several genetic diversity parameters were calculated to assess the efficiency and reliability of the 20 SSR markers, including the number of alleles per locus (Na), effective number of alleles (Ne), observed heterozygosity (Ho), expected heterozygosity (He), fixation index (F), and probability of identity (PI). The software also facilitated the identification of genetically identical individuals, permitting the removal of redundant accessions and the detection of somatic mutations.
To investigate the underlying genetic structure of the collection, STRUCTURE 2.3 [54,55] was used. This Bayesian clustering method estimates the probability of individual membership in one or more genetic clusters (K), defined by allele frequencies at each locus under Hardy–Weinberg equilibrium. Simulations were conducted for K values ranging from 1 to 7, using an admixture model with correlated allele frequencies. Each run consisted of a burn-in period of 100,000 iterations followed by 1,000,000 additional MCMC iterations. The optimal number of genetic clusters (K) was determined using the ΔK method described by Evanno et al. [56], which calculates the second-order rate of change in the likelihood function across successive K values. Individuals were assigned to a genetic cluster if their coefficient (q) was ≥85%; individuals with lower values were considered admixed.
Assignment tests were subsequently performed in GenAlEx v6.5 to verify the distribution of individuals across structure-derived populations based on observed allele frequencies [57]. The log-likelihood probability of each individual’s membership to each subpopulation was calculated, and individuals were assigned to the group with the highest log-likelihood value. GenAlEx was also used to calculate the genetic differentiation coefficient (Fst) between populations through an Analysis of Molecular Variance (AMOVA), using 999 permutations and assuming the infinite allele model for SSR genotypes. Additionally, a Principal Coordinates Analysis (PCoA) was conducted based on standardized covariance of genetic distances derived from codominant markers, enabling graphical two-dimensional representations at both the individual and population levels. For three-dimensional visualization of the PCoA, the Python 3.5 programming [58] and the Matplotlib 2.0 library were used to generate spatial representations that enhanced the interpretation of clustering patterns and the identification of relationships among individuals and populations.
Furthermore, phylogenetic relationships and clustering designs were inferred using MEGA v7.0 software [59], applying the Neighbor-Joining algorithm [60], which groups genetic samples based on profile similarity. This approach enabled the construction of phylogenetic trees and circular dendrograms to detect consistent groupings and potential kinship relationships among the accessions, thereby providing deeper insights into their genetic structure and interrelationships.
3. Results
A total of 96 grapevine accessions from LP Island were genotyped (Table S1) using the set of 20 SSR markers that the TECNENOL Research Group has been applying [20,45,46,47,48,49,50] (Table S2 and Figure S1). This study evaluated the performance of the SSR kit for this population and investigated the presence of synonyms, homonyms, and identification errors among the samples. Understanding the extent of inter-varietal variability was one of the primary objectives, with the goal of assessing the grapevine biodiversity on this island. Finally, studying the singularity of the local variety population would highlight the significance of the viticultural heritage of LP Island.
3.1. SSR Polymorphism
The molecular profile (MP-SSR) found for each of the 96 individuals analyzed allowed the detection of identical accessions. Thus, the first normalization of the data obtained for this grapevine population consisted of eliminating redundant MP-SSRs. Consequently, 52 accessions were removed. Table S3 presents the 44 unique profiles found in this population, as well as two sport samples (same MP-SSR but with a change in berry color) that are also exceptionally included in this table. With the population of the 44 unique MP-SSRs, the study of the statistical parameters began, which would demonstrate the efficacy of the selected SSR kit for carrying out the present work.
Table S4 shows the main evaluated statistical parameters. The average number of alleles (Na) present in this grapevine population from LP Island was 11.2 alleles. The SSR markers that exhibited the highest number of alleles (Na) and the greatest capacity to discriminate among genotypes were VVMD36 (18 alleles), ZAG79 (17 alleles), and VVMD27 (16 alleles), while the SSRs with the lowest Na were VVS3 and VVS29 (5 alleles), ZAG62 (7 alleles), and UCH19 (8 alleles). The average effective number of alleles (Ne) for this population was 5.2, with the best-performing SSRs being VVMD27 (9.410) and ZAG79 (8.620), while the poorest performers were VVS29 (1.371) and VVS3 (1.739). The averages for observed heterozygosity (Ho) and expected heterozygosity (gene diversity index) (He) were 0.783 and 0.746, respectively. The SSR with the highest Ho was ZAG79 (0.953, followed by VChr19a (0.930), and VVS2, VVMD7, and VVMD27, all with values of 0.907. At the opposite extreme, the lowest values were observed in VVS29 (0.256), VVS3 (0.442), and UCH19 (0.488). The SSR with the highest He was VVMD27 (0.894) followed by ZAG79 (0.884) and VVMD36 (0.874), while the lowest results were observed for VVS29 (0.270), VVS3 (0.425), and UCH19 (0.555). The SSRs with the worst fixation index (or probability of null alleles) were UCH19 (0.120), ZAG83 (0.106), and VVS29 (0.054), while the others showed very acceptable performance. Overall, the SSR kit showed a cumulative probability of identity (PI) of 2.0 × 10−23 for this LP population. The SSRs with the highest PI values were VVMD27 (2.0 × 10−2), ZAG79 (2.3 × 10−2), and VVMD36 (2.6 × 10−2), while the worst values corresponded to VVS29 (5.5 × 10−1), VVS3 (3.7 × 10−1), and UCH19 (2.3 × 10−1).
3.2. Grapevine Variety Analysis
Table S1 presents all the original information as well as the data obtained in this study for each of the 96 genotyped accessions. A total of 31 varieties and 2 color sports were identified using the TECNENOL database [20,45,46,47,48,49,50] and the VIVC database [51]. Table S3 details the 44 individuals with unique MP-SSR profiles, including their genetic profile based on the seven international SSR markers. Exceptionally, MP-SSR profiles for the two sports in this collection were also included.
The population of local peninsular varieties from Spain comprised 34 accessions cor-responding to seven varieties registered in the VIVC. Of these, De Rey [2] and Ferral were each represented by a single accession with no observed allelic differences. The variety Beba included three samples: two consistent with known profiles and one displaying a novel mutation at the second allele of SSR marker VVS3 (designated VVS3-2). All five Listan prieto accessions exhibited known variations, VVS3-2 and VVMD28-1 [49,61], while all eight Mollar cano samples shared the VVS3-2 mutation, previously reported in other IC [47,48,49]. Among the 12 Palomino Fino accessions, the majority matched the common TECNENOL profile, while three exhibited a novel VVMD27-2 variation. Notably, accession P-69 showed four variations: VVS3-2, VVMD27-2, and triallelic profiles at markers ZAG64 and VChr19a. The variety Vijariego blanco, represented by four accessions, showed considerable genetic variation. Individual P-08 aligned with a previously reported LZ profile (VVS3-2 and ZAG64-2) [47,61], whereas P-60 exhibited a novel combination (VVMD28-1 and ZAG64-2). Accessions P-57 and P-68 shared a new composite profile (VVS3-2, VVMD28-1, and ZAG64-2).
The Portuguese group included 13 individuals from the varieties Alfrocheiro, Malvasia fina, Molar, Samarrinho, and Verdelho branco. The single Alfrocheiro accession exhibited novel variations at VVS3-2 and VVMD7-1. Malvasia fina had two individuals with variations (P-12 at VVS3-2, and P-13 at VVS3-2 and VVMD36-2), and its rosé sport (Malvasia fina roxa) included one individual with a variation at VVS3-2. Among the four Molar accessions, one (P-75) matched the reference profile, while three carried a novel mutation at VVS3-2. The three Samarrinho samples showed the most common profile, and therefore no variations. Lastly, Verdelho branco had one non-mutated individual and another with a newly described variation at VVS3-2.
Among non-Iberian varieties, French cultivars Flot rouge and Trousseau noir, both with two accessions, exhibited no allelic variation. Similarly, Muscat of Alexandria (Greece), Muscat d’Hamburg (UK), and Morskoi 94 (Ukraine) displayed no mutations.
Two varieties of uncertain origin were also studied. Chasselas blanc showed a triallelic pattern at VVMD36, suggesting somatic variation or hybrid ancestry. Malvasia Dubrovacka, erroneously listed as Spanish in the VIVC [51], was represented by four accessions with three MP-SSR profiles. P-81 matched the reference, while P-16 and P-35 shared the VVS3-2 mutation known from LG [50]. A fourth accession, P-48, exhibited a new combination of three mutations (VVS3-2, UCH12, SCU06-2).
The collection also included five local varieties to another IC: Bermejuela, Forastera gomerae, Listan negro, Malvasia volcanica, and Torrontes volcanico. While Bermejuela, Forastera gomerae, and Torrontes volcanico showed no variation, Listan negro had three accessions, two of which carried the VVS3-2 mutation, consistent with previous findings [47,49,50,61]. Among four Malvasia volcanica accessions, three exhibited the VVS3-2 mutation noted in LZ [46,61]; one aligned with the reference. Additionally, a sport variety, Malvasia di Sardegna rose, included two unmutated individuals and one with a novel dual-mutation profile (VVS3-2 and SCU06-2).
Seven local varieties were unique to LP Island. Three, Albillo criollo, Bienmesabe tinto, and Gual Mazo, were each represented by a single, no variation accession [20,62,63], while Sabro, with eight individuals, also showed no variation. Three newly described local varieties, Aromatica Eufrosina, Cagarruta de oveja, and Viñarda rosada, were each represented by two genetically distinct individuals, constituting new varietal entities.
At the lexicographic level, the registration status of accession names (Table S1) was as follows: 12 accessions matched the PN in VIVC (in black); 31 were registered under synonyms recorded in VIVC (black and bold); 14 samples with first-time described variations were assigned new names proposed by the authors (green and bold); 14 new synonyms for varieties already registered in VIVC (brown and bold); 8 had erroneous names (red); 1 accession was registered under a synonym belonging to another variety but was considered to also describe the variety it was registered under (fuchsia); 14 were labeled as “unknown” or with generic names indicating lack of knowledge (light green), such as Tintilla; and 1 individual, corresponding to one of the new varieties, presented a new registration name, proposed as a new PN (turquoise blue). Therefore, this study contributes at the lexicographic level: 3 new PN for the new local varieties (turquoise blue), 14 new names defining individuals with first-time described variations (green and bold), 9 new synonyms for varieties already registered in VIVC (brown and bold), 1 existing synonym name for a given variety proposed also for another variety by the authors (fuchsia), 8 MP-SSR profiles registered erroneously, and 4 accessions with names indicating lack of knowledge about the corresponding variety. Table S3 summarizes this information exclusively for the unique MP-SSR profiles.
3.3. La Palma Grapevine Population Genetic Structure
Before proceeding with the study of the genetic structure of the samples that would define the final population of LP Island, a new data normalization step was performed. From the 44 unique MP-SSR profiles, accessions corresponding to sport mutations and to variations relative to the principal or most widespread MP-SSR profiles in the databases were removed. Thus, the final collection of cultivated grapevines on LP Island consisted of representatives from 31 varieties, 28 previously known and 3 described here for the first time. It should be noted that among the representatives of these 31 varieties, 5 corresponded to individuals with variations. Specifically, the varieties Mollar cano and Malvasia fina were represented by individuals who presented a variability of 97.5% in our MP-SSR, while Vijariego blanco, Listan prieto, and Alfrocheiro were represented by individuals exhibiting a 95% variation.
The program STRUCTURE 2.3 allowed us to classify the 31 varieties into 3 populations (K = 3) after testing combinations from K = 1 to K = 7, as shown in Figure S2. Each population was assigned a representative color, and its members were classified based on the q parameter. In our study, we adopted the arbitrary threshold of 85% [64], so that q values ≥ 85% corresponded to pure individuals of a given population, while q values < 85% indicated admixed individuals within the same population. Figure 3 shows the population structure diagram for LP Island, with an assignment accuracy of 89%, and Figure S3 presents all individuals classified according to their q membership values.
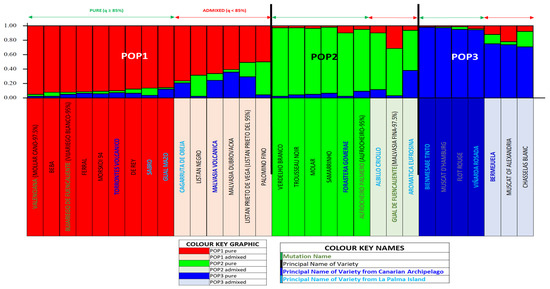
Figure 3.
La Palma grapevine varieties population (unique molecular profiles). Structure 3.2. diagram: K = 3 distribution for pure and admixed individuals.
POP1 was composed of 15 varieties (48%), of which 9 (60%) were considered pure (Mollar cano-97.5%, Beba, Vijariego blanco-95%, Ferral, Morskoi 94, Torrontes volcanico, De Rey, Sabro, and Gual Mazo), while 6 (40%) were considered admixed (Cagarruta de oveja, Listan negro, Malvasia volcanica, Malvasia Dubrovacka, Listan prieto-95%, and Palomino fino). Among pure varieties, 55.5% were Spanish, 22.2% local to LP, 11.1% from the IC, and 11.1% Ukrainian. Most pure varieties (77.8%) were white, with 55.6% used for dual purposes (table and winemaking) and 44.4% exclusively for winemaking. Among admixed varieties, 33.3% were Spanish, 33.3% from IC, 16.7% from LP, and 16.7% of unknown origin. White varieties constituted 66.6% of this group, used for wine (50%), dual-purpose (33.3%), and as table grapes (16.7%).
POP2 comprised nine varieties (29%), of which six (67%) were classified as pure (Verdelho branco, Trousseau noir, Molar, Samarrinho, Forastera gomerae, and Alfrocheiro-95%) and three (33%) as admixed (Albillo criollo, Malvasia fina-97.5%, and Aromatica Eufrosina). Among pure varieties, 66.7% were Portuguese, 16.7% French, and 16.7% from IC. Half were white, primarily used for winemaking (66.7%) and dual-purpose (33.3%). The admixed varieties in this population originated from LP (66.7%, including a new variety) and Portugal (33.3%). All were white and used for winemaking.
POP3 included seven varieties (23%), with four (57%) pure (Bienmesabe tinto, Muscat d’Hamburg, Flot rouge, and Viñarda rosada) and three (43%) admixed (Bermejuela, Muscat of Alexandria, and Chasselas blanc). Among pure varieties, 75% were red and 25% rosé, and were used for winemaking (50%), one for dual-purpose (25%), and one with an undetermined use (25%). All admixed varieties were white; 66.7% were used for winemaking, and 33.3% for triple use (table, raisin, and wine production).
Figure 4 shows the population structure analysis of the three cultivated grapevine populations in LP based on their Nei’s Genetic Distances. For this analysis, a third normalization step was applied, excluding admixed individuals, reducing the dataset to 19 varieties (Figure 3 and Figure S3). Figure 4a (bidimensional PCoA by populations) displays the distribution of these three populations. Coordinate 1, which accounts for 56.40% of the variation, clearly separates POP1 (right quadrants) from POP2 and POP3 (both in the left quadrants). Coordinate 2 (43.60% goodness), distinguishes POP1 and POP3 (upper quadrants) from POP2 (lower quadrant). According to the Fst parameter, POP1 was the most genetically distinct population. The distribution of individuals shown in Figure 4b (two-dimensional) and Figure 4c (three-dimensional) show the same pattern observed in Figure 4a.
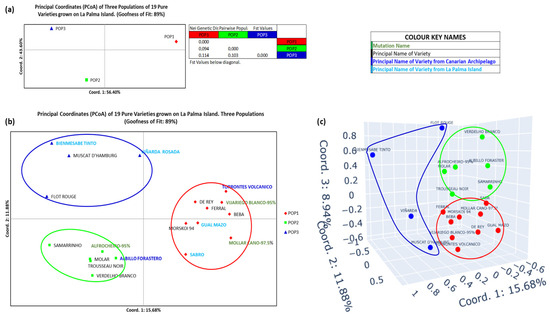
Figure 4.
PCoA representation of the grapevine varieties population from La Palma Island normalized for K = 3. (a) Two-dimensional representation of the 3 populations. Values of the Fst statistic for each population. (b) Two-dimensional representation of the 3 populations by individuals. (c) Three-dimensional representation of the 3 populations by individuals.
3.4. Relation of La Palma Grapevine Population to the Canary Archipelago Population
To explore the genetic relationship between the grapevine population of LP and the rest of the IC, the seven local LP varieties identified in this study were analyzed alongside 33 varieties previously described as local to the archipelago [20,47,48,49,50,62,63], obtaining a total of 40 varieties. These were grouped into six insular populations. The IC group comprised four varieties (10%): Vallera (Tenerife), Albillo Monte Lentiscal (Gran Canaria), and two widespread varieties across the archipelago (Listan negro and Bermejuela). The LZ group included 12 varieties (30%): Blanca de la granja del Cabildo, Burra chinija, Diego chinija, Lemes de El Cabezo, Malvasia alistanada fina, Sinforiano chano, Uvillón negro, Vijariego blanco de la granja, Uva de año, Malvasia volcanica, Torrontes volcanico, and Breval negro. HI included 11 varieties (27%): Burra volcanica, Uvalero volcanico, Huevo de gallo, Verijadiego, Verijadiego negro, Pinar negro, Uval negro, Seis de Carlos, Verdello de El Hierro, Uval piñero, and Tesoro blanco. The sole representative of FT was Majorera (3%). LG was represented by five varieties (13%): Forastera gomerae, Barrerita negra, Coello blanca, Malvasia periquin gomerae, and Verdello gomerae. Lastly, the LP population (17%) included four previously described varieties (Bienmesabe tinto, Albillo criollo, Sabro, and Gual Mazo) and three newly identified ones (Cagarruta de oveja, Aromatica Eufrosina, and Viñarda rosada).
Population structure was assessed using STRUCTURE 2.3, testing K values from 1 to 7. Based on the Evanno method [56], the optimal structure was achieved with K = 5, corresponding to 5 ancestral populations for the 40 individuals studied (Figure S4). As in the previous case, each individual was assigned to a specific population and position within that population based on the q parameter. This parameter allowed us to identify pure individuals (q ≥ 85%) and admixed individuals (q < 85%).
Figure 5 presents the graphical representation of the optimal distribution for the 40 varieties of the Canary Archipelago, corresponding to five ancestral populations. In addition, Figure S5 shows the distribution of these 40 varieties among the five populations according to their q values.
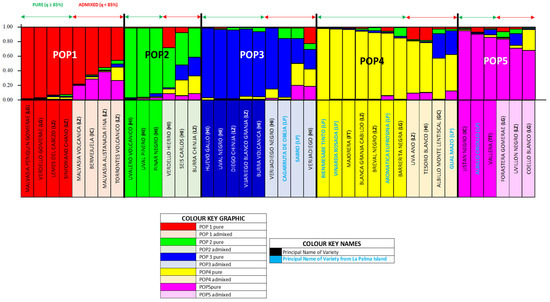
Figure 5.
Canary grapevine varieties population (unique molecular profiles). Structure 2.3 Diagram: K = 5 distribution for pure and admixed individuals.
In this analysis, POP1 comprised eight varieties (20%), equally split between pure and admixed. Pure varieties included Malvasia periquin gomerae and Verdello gomerae (LG), and Lemes de El Cabezo and Sinforiano Chano (LZ). The admixed group were Malvasia volcanica, Malvasia alistanada fina, and Torrontes volcanico (LZ), and Bermejuela (IC). POP2 included six varieties (15%), also evenly split between pure and admixed. Pure individuals were Uvalero volcanico, Uval piñero, and Pinar negro (HI), while admixed ones included Verdello de El Hierro and Seis de Carlos (HI), and Burra chinija (LZ). POP3 contained nine varieties (22%), with 56% pure individuals (Huevo de gallo, Uval negro, and Burra volcanica (HI), and Diego chinija and Vijariego blanco de la granja (LZ)) and 44% admixed (Verijadiego and Verijadiego Negro (HI), and Cagarruta de oveja and Sabro (LP)). POP4 was the largest group, with 11 varieties (28%). Pure individuals (64%) included Bienmesabe tinto, Viñarda rosada, and Aromatica Eufrosina (LP), Blanca de la granja del Cabildo and Breval negro (LZ), Majorera (FT), and Barrerita negra (LG). The admixed varieties (36%) were Uva de año (LZ), Tesoro blanco (HI), Albillo monte Lentiscal (IC), and Gual Mazo (LP). POP5 included six varieties (15%), again split evenly. The pure group included Listan negro and Vallera (IC), and Albillo criollo (LP). Admixed individuals were Forastera gomerae and Coello blanco (LG), and Uvillón negro (LZ).
For the population structure analysis, 18 admixed individuals were removed, resulting in a set of 22 pure varieties (Figure 5 and Figure S5). The initial assignment quality (86%) was improved to 100% by reassigning three individuals from POP4 to more appropriate populations (POP1, POP2, and POP3). In the two-dimensional population PCoA (Figure 6a), Coordinate 1 (32.12% variance) clearly separated POP5 (upper right quadrant) from POP1 (upper left quadrant). The remaining populations were more closely clustered: POP3 and POP4 appeared near the origin in the lower right quadrant, with POP2 slightly apart in the lower left quadrant. Fst values confirmed that POP1 and POP5 were the most genetically distant, while POP2, POP3, and POP4 were more closely related. The individual level PCoA (Figure 6b,c) showed lower resolution and was less conclusive. Only POP1 remained clearly distinct and consistently separated. POP2, POP3, POP4, and to a lesser extent POP5, showed some degree of overlap, with several outliers. Notably, Bienmesabe tinto from LP appeared clearly differentiated from all other individuals.
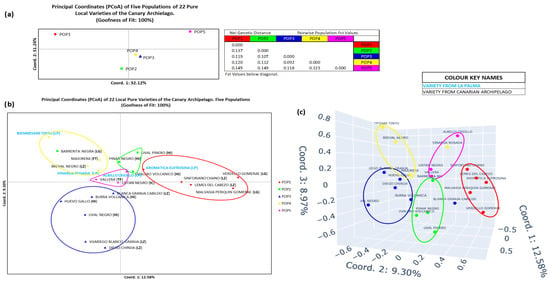
Figure 6.
Population of varieties of the Canary Islands (22 pure varieties). (a) Two-dimensional representation of the 5 populations of the Canary Islands by population. Values of the Fst statistic for each population. (b) Two-dimensional representation of the 5 populations by individuals. (c) Three-dimensional representation of the 5 populations by individuals.
3.5. Relation of La Palma Grapevine Population to the World Population
To study the seven grapevine genotypes from LP in the context of global diversity, two complementary approaches were employed: a genetic and a geographic strategy.
3.5.1. Genetic Strategy
This approach involved compiling a worldwide dataset of Vitis vinifera varieties from natural crosses (from the TECNENOL database [20,45,46,47,48,49,50]) into a single dataset, regardless of known parentage, totaling 319 genotypes from approximately 23 countries, including those from LP. Using STRUCTURE 2.3, we tested K values from 1 to 7, with the optimal solution revealing two ancestral groups (K = 2) (Figure 7a and Figure S6), based on the q coefficient (Figure S7), differentiating between pure (q ≥ 85%) and admixed (q < 85%).
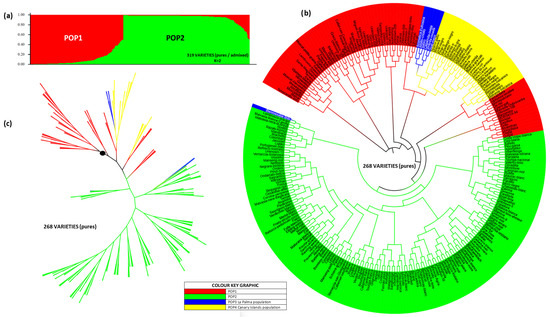
Figure 7.
World population (319 individuals) distributed in 2 populations. (a) Graphical representation of K = 2 according to Structure 2.3 (with pure and admixed individuals) (b) Circular Neighbor-Joining dendrogram of the world population 268 pure individuals, (c) Pure individuals world population phylogenetic tree.
POP1 contained 135 varieties (42%), and POP2 had 184 (58%). All IC varieties clustered in POP1. A total of 53 admixed varieties (16.6%) were removed to normalize the dataset: 31 from POP1 and 22 from POP2. Among these, three were from IC (Malvasia periquin gomerae (LG), Malvasia alistanada fina (LZ), and Coello blanco (LG)) and two from LP (Albillo criollo and Aromatica Eufrosina). After normalization, POP1 comprised 104 varieties: 71 Spanish, 10 of unknown origin (including Malvasia Dubrovacka [65]), 9 Italian, 4 French, 3 Portuguese, 3 Greek, and 1 each from Bulgaria, Croatia, Lebanon, and Serbia. POP2 included 162 varieties: 51 Italian, 39 French, 24 Spanish, 12 Portuguese, 11 Greek, 4 Croatian, 4 unknown, 2 each from Slovenia, Germany, Bulgaria, Hungary, and Bosnia and Herzegovina, and 1 each from Turkey, Montenegro, Switzerland, Israel, Ukraine, Georgia, and Algeria. Assignment precision reached 99%, and after reassigning one misclassified variety, accuracy was 100% with a final normalized dataset of 266 pure varieties.
To evaluate the LP and IC populations more specifically, these individuals were extracted from POP1 and reclassified into two new populations: LP (POP3) and IC (POP4). IC remained normalized, while the two previously excluded admixed LP varieties (Albillo criollo and Aromatica Eufrosina) were reinstated. This slightly reduced the overall assignment accuracy to 88% (compared to 89% if LP had remained fully normalized).
The circular dendrogram (Figure 7b) illustrates the distribution of the 268 pure varieties (including the two admixed LP varieties) across four clusters: POP1 (70), POP2 (161), IC (30), and LP (seven: five pure and two admixed). The IC population, nested within POP1, formed two sub-branches (in yellow). Within one of these, a further differentiation emerges, forming two smaller sub-branches, one of which comprised six LP varieties (in blue). The exception was Bienmesabe tinto, which clustered separately with POP2. This pattern also appeared in the phylogenetic tree (Figure 7c).
Figure 8 presents the PCoA analysis. In the population-level plot (Figure 8a), Coordinate 1 (76.79% of variance) separated POP2 (right quadrants) from all others (left), while Coordinate 2 (21.06%) distinguished POP1 (upper quadrants) from IC and LP (lower quadrants). Thus, POP2 appeared isolated (upper right), POP1 in the upper left, and IC and LP in the lower left. Fst values supported these patterns. At the individual level (Figure 8b), most POP2 varieties grouped on the left, while other populations clustered on the right. Coordinate 1 explained 5.64% of variance and Coordinate 2 explained 3.57%, providing limited resolution. However, LP varieties consistently appeared in the lower right quadrant, close to the Coordinate 1 axis. The three-dimensional PCoA (Figure 8c) did not provide additional insights beyond those revealed in the two-dimensional representation, though Bienmesabe tinto from LP remained clearly separated from all others, and Aromatica Eufrosina appeared in the upper region of POP2.
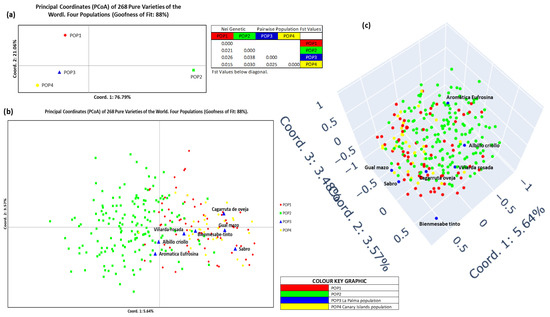
Figure 8.
PCoA representation of La Palma Island, Canary Islands, and worldwide population, normalized for K = 2. (a) Two-dimensional representation of the 4 populations per population, and values of the Fst statistic for each population, (b) two-dimensional representation of the 4 populations per individual. (c) three-dimensional representation of the 4 populations per individual.
3.5.2. Geographic Strategy
To validate the previous results, a geographic criterion was applied to the global grapevine dataset (319 varieties), grouping varieties into seven regions based on their countries of origin according to the VIVC database [51]. The decision to group by areas rather than individual countries was made because some countries were represented by only a single variety. The regions defined (Figure S8) were EASTMED-CAU (Algeria, Cyprus, Georgia, Israel, Lebanon, Tunisia, and Turkey), BP (Bosnia and Herzegovina, Bulgaria, Croatia, Greece, Serbia, Slovenia, and Montenegro), ITA (Italy), FRA-CE (Austria, France, Germany, Hungary, Ukraine, and Switzerland), IP (Spain and Portugal), IC, and LP.
An assignment test in GenAlEx 6.5 yielded a 60% accuracy rate, allowing for the identification of misassigned and admixed individuals. The group compositions were as follows: EASTMED-CAU (12 varieties, 4%) contained 42% pure (5) and 58% admixed (7); BP (28 varieties, 9%) contained 50% pure (14) and 50% admixed (14); ITA (73 varieties, 23%) contained 53% pure (39) and 47% admixed (34); FRA-CE (61 varieties, 19%) contained 69% pure (42) and 31% admixed (19); IP (105 varieties, 33%) contained 65% pure (68) and 35% admixed (37); IC (33 varieties, 10%) contained 70% pure (23) and 30% admixed (10); and LP (7 varieties, 2%), where all individuals were retained including admixed varieties.
After removing 122 admixed or misassigned individuals (except from LP), the normalized dataset comprised 197 well-assigned or pure varieties. A second assignment test improved accuracy to 87%.
Figure 9 shows a circular dendrogram (Figure 9a) and phylogenetic tree (Figure 9b) based on this geographic grouping. In both representations, IC and LP stood out clearly, always clustering within the IP domain and forming a major sub-branch. Notably, LP also formed a distinct sub-branch within IC. Under these conditions, Bienmesabe tinto was fully integrated into the LP group.
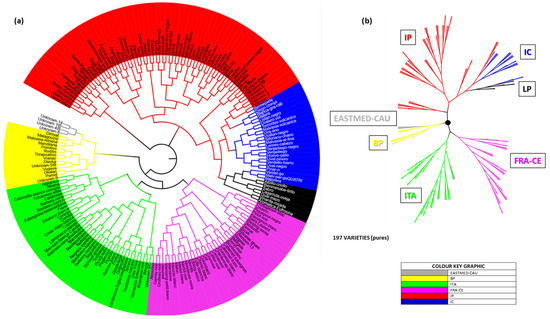
Figure 9.
World population (197 individuals) distributed in populations corresponding to 7 geographical areas. (a) Circular Neighbor-Joining dendrogram of the 197 pure individuals of the world population and La Palma, (b) phylogenetic tree of 7 populations distribution with all their individuals.
Figure 10 presents the graphical representation of the PCoA applied to the global population (197 individuals), distributed across seven arbitrary regions based on geographic criteria.
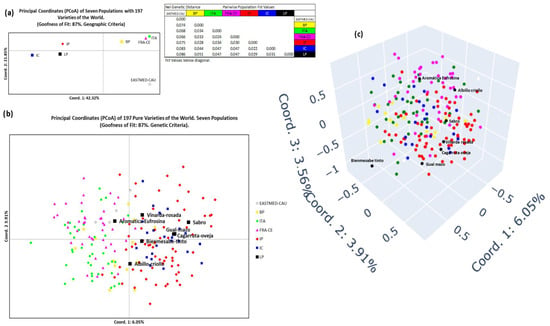
Figure 10.
PCoA representation of La Palma Island, Canary Islands, and world population for the geographical criterion. (a) Two-dimensional representation of the 7 populations per population. Values of the Fst statistic for each population, (b) two-dimensional representation of the 7 populations per individual. (c) Three-dimensional representation of the 7 populations per individual.
In the two-dimensional population-level plot (Figure 10a), Coordinate 1 (42.32% of variance) separated IP-related groups (IP, IC, LP) into the left quadrants. Coordinate 2 (21.85%) slightly differentiated IP and separated BP, ITA, and FRA-CE from IC, LP, and EASTMED-CAU. The most distant cluster was EASTMED-CAU (lower right quadrant). Furthermore, two main clusters can be identified: (1) BP, ITA, FRA-CE (upper right), and (2) IP, LP, and, to a lesser extent, IC (central left). These groupings were supported by Fst values.
The two-dimensional individual-level plot (Figure 10b) reflected the same structure, with a rotation of the first coordinate axis. Coordinate 1 (6.05% of variance) separated EASTMED-CAU, BP, ITA, and FRA-CE (left) from IP, IC, and LP (right). Coordinate 2 (3.91%) does not reveal any discernible structure. Within LP, Albillo criollo and Aromatica Eufrosina were slightly divergent, while Bienmesabe tinto clustered with the rest.
The three-dimensional PCoA plot (Figure 10c) supports these observations. EASTMED-CAU, BP, ITA, and FRA-CE occupied the lower/internal space, while IP, IC, and LP appeared toward the front/upper space. Within LP, Albillo criollo, Aromatica Eufrosina, and Bienmesabe tinto were clearly separated, with the latter showing the greatest degree of differentiation from the other varieties included in the overall study.
4. Discussion
This study represents the first exhaustive genetic characterization of Vitis vinifera ssp. vinifera in LP using SSR markers. The results confirm that, despite the island’s small size and a history marked by frequent volcanic disturbances, LP preserves a genetically diverse and unique grapevine germplasm. This genetic resource should be considered of high value for both the conservation of viticulture heritage and for its potential use in adaptation strategies against climate change.
4.1. Analysis of Grapevine Varieties
The study conducted on LP Island represents a comprehensive genetic survey of its cultivated grapevine population. A total of 96 vine accessions were collected across the island’s principal viticultural zones: Northern, Hoyo de Mazo, and Fuencaliente. After genotyping using SSR markers and comparing against the TECNENOL database, 52 redundant profiles were removed. This level of redundancy (54.2%) is consistent with that of another IC: similar to HI (52.9%), slightly below LG (56.67%) and LZ (55%), but notably higher than FT (37.5%). Ultimately, 44 unique multilocus profiles (MP-SSR) were identified (Table S3). Among these, 28 were known varieties referenced in the TECNENOL and/or VIVC databases, while three remained unidentified. Notably, several profiles exhibited minor allelic variations when compared to their standard references. To distinguish between mutants and new varieties, the study adopted a similarity threshold of 87.5%, i.e., up to five allele differences across 20 SSR loci were considered mutations, whereas greater divergence indicated a new variety. This follows strategies previously outlined by Ibáñez et al. [66], Vélez [67], and Cabezas et al. [68].
The grapevine varieties on LP Island were found to originate from nine distinct regions. Spanish varieties, most genetically related to Heben, included Beba, De Rey, Ferral, Listán prieto (95% similarity), Mollar cano (97.5%), Palomino fino, and Vijariego blanco. Portuguese varieties, primarily related to Savagnin blanc, included Alfrocheiro (95%), Malvasia fina (97.5%), Molar, Samarrinho, and Verdelho branco. Other international varieties found included Trousseau noir and Flot rouge (France), Chasselas blanc (possibly Central European [69]), Malvasia Dubrovacka (likely Balkan [65]), Muscat of Alexandria (Greece), Muscat d’Hamburg (England), and Morskoi 94 (Ukraine), which corresponds to “Unknown No. 5” from Rodríguez-Torres [62]. The local IC varieties included Bermejuela, Forastera gomerae, Listan negro, Malvasia volcanica, and Torrontes volcanico. In addition, LP is home to local varieties such as Albillo criollo, Bienmesabe tinto (likely an interspecific hybrid [20]), Gual Mazo (erroneously listed as Italian in VIVC [51,63]), and Sabro. Two previously known color sports were also recorded: Malvasia di Sardegna rosada (Canary Island) and Malvasia fina roxa (Portugal). Three entirely new varieties were identified—Aromatica Eufrosina, Cagarruta de oveja, and Viñarda Rosada—bringing the total to 31 distinct varieties cultivated on LP.
Significant intra-varietal diversity was detected (Tables S1 and S3). Forty accessions showed such variation, leading to 20 unique MP-SSR profiles. Several variants corresponded to those found on other islands: Listán negro santanero (variation in VVS3-2, first described in LZ [47,49,50,61]), Listán prieto de Vega (VVS3-2 and VVMD28-1, from FT [49,61]), Malvasia blanca de Agulo (LG [50]), Malvasia volcánica cabezuda (LZ [47,61]), Mollar Bonilla (LZ [47,49]), and Diego de El Raso (VVS3-2 and ZAG64-2, LZ [47,61]). The study adheres to the VIVC [51] practice of naming somatic mutations (e.g., Pinot meunier from Pinot noir). Thus, 14 new names were proposed for detected mutants: Alfrocheiro palmero, Beba de Bienes, Malvasia de Mazo, Malvasia rosada de Breña, Gual de Fuencaliente, Gual Jeremias, Gual rosado de Armas, Molar de Bienes, Listan blanco menudo, Verdello palmero, Bujariego de Fuencaliente, Bujariego palmero, Chasselas palmero (a triallelic variant), and Albillo baboso palmero (which showed two SSR mutations and two cases of triallelism). Additionally, 8 misidentified accessions (marked in red) and 14 unknown to local growers (light green) were identified. One notable case was P-35, locally called Malvasia blanca (fuchsia in Table S1), a VIVC synonym for Alarije, commonly mistaken for “Malvasia”, although it matched Malvasia Dubrovacka genetically. The authors argue the synonym is better reserved for a true “Malvasia” [65].
The initial sampling was conducted shortly before the 2021 eruption of the Tajogaite volcano. Fourteen accessions were buried by lava or ash (Table S1), including representatives of De Rey, Flot rouge, Forastera gomerae, Muscat of Alexandria, Samarrinho, Trousseau noir, and Sabro, with no variants among them. Of the four variants affected, Malvasia volcanica cabezuda and Mollar Bonilla were collected and preserved previously on the LZ island. Only the sport known as Malvasia fina roxa was lost. Therefore, the impact of the eruption on grapevine biodiversity was much smaller than anticipated.
The authors propose several additions to the VIVC ampelographic catalog: (1) the three new variety names (in turquoise): Aromatica Eufrosina, Cagarruta de oveja, and Viñarda rosada; (2) the 14 newly named somatic variants (dark green); and (3) twelve new synonyms (brown), including Gallo (PN: Beba), Tintilla palmera (PN: Flot rouge), Almuñeco blanco (PN: Forastera gomera), Almuñeco negro and Muñeco (PN: Listan negro/mutation: Listán negro santanero), Tinta milrera (PN: Listan prieto/mutation: Listan prieto de Vega), Valenciana (PN: Mollar cano/mutation: Mollar Bonilla), Dulzal (PN: Morskoi 94), Moscatel antiguo (PN: Muscat of Alexandria), Listan blanco alto (PN: Palomino fino), Verdello grande (PN: Sabro), and Baboso blanco (PN: Samarrinho). These names were submitted to VIVC for inclusion in 2023 [48]. Hence LP Island’s grapevine population demonstrates remarkable genetic diversity, with 31 distinct varieties and numerous somatic variants. This richness is of great relevance for both conservation and viticultural innovation, particularly considering climate stress and volcanic challenges. The authors emphasize the need to preserve and study these genotypes further, recognizing LP as a valuable genetic reservoir within the global Vitis vinifera L. landscape.
4.2. Genetic Structure of the Grapevine Population in La Palma
Before delving into the analysis of population genetic structure, three methodological clarifications are necessary. First, the program Structure 2.3 was used to assess population structure. This involved determining the most accurate distribution of grapevine individuals, with membership evaluated via the q statistic, which estimates the degree of genetic affiliation to each population. Based on q, individuals were classified as “pure” or “admixed.” To ensure consistency and reduce analytical noise, admixed were excluded from downstream analyses (Principal Coordinates Analysis (PCoA), dendrograms, and phylogenetic trees) so as to better understand the genetic identity of LP’s cultivated grapevines. Second, the use of 20 SSR markers generated up to 40 allelic data points per diploid sample. Although this allows for highly accurate classification, full precision in PCoA would require 40 dimensions, which is not feasible. Since graphical representations are limited to two or three dimensions, some data distortion is inevitable, particularly as sample size increases. This limitation, however, is widely recognized in the literature, and PCoA is typically used to interpret overall patterns rather than precise placement [70,71]. Third, pedigree and origin data for known varieties and their crosses were sourced primarily from the VIVC database [51].
Analysis using Structure 2.3 (Figure 3, Figures S2 and S3) on 31 non-redundant Vitis vinifera ssp. vinifera varieties revealed three ancestral populations with 89% confidence. Population 1 (POP1) included 15 varieties, predominantly of Spanish origin. Notable members include Beba, Mollar cano (97.5%), Vijariego blanco (95%), Ferral, and Sabro, all presumed descendants of the Spanish variety Heben [69]. Also grouped here were De Rey, Torrontes volcanico (IC), Gual Mazo (LP), Malvasia Dubrovacka (likely Balkan [65]), and the Ukrainian Morskoi 94. Several admixed with Spanish heritage also clustered here: Listan negro, Listan prieto (95%), Malvasia volcanica, and local LP varieties like Cagarruta de oveja. This distribution underscores a dominant Iberian lineage in LP’s grapevines. Population 2 (POP2) encompassed mostly Portuguese varieties or those with a strong Savagnin blanc ancestry. Pure accessions included Verdelho branco, Trousseau noir, Samarrinho, Molar, Alfrocheiro (95%), and the Canary Island Forastera gomerae (a Palomino fino × Verdelho branco cross). LP’s Albillo criollo, Malvasia fina (97.5%), and the new variety Aromatica Eufrosina were also grouped here, suggesting Portuguese or broader European ancestry. The influence of Savagnin blanc, believed to have been introduced to northern Iberia via the Camino de Santiago [72], is evident throughout this cluster. Population 3 (POP3) brought together genetically divergent or ambiguous varieties. These included Flot rouge, a complex interspecific cross, Muscat Hamburg (a breeder-developed cross between Schiava grossa and Muscat of Alexandria), and LP’s Bienmesabe tinto, which likely has interspecific origins. New variety Viñarda rosada also clustered here, lacking a known pedigree. Admixed varieties in this group include Chasselas blanc (likely Central European), Bermejuela (parent of Malvasia volcanica), and Muscat of Alexandria. These population assignments suggest a nuanced picture of LP’s grapevine heritage. Varieties like Sabro, Cagarruta de oveja, and Gual Mazo are more strongly tied to Spanish ancestry. In contrast, Albillo criollo and Aromatica Eufrosina reflect connections to Portuguese or continental lineages. The positions of Bienmesabe tinto and Viñarda rosada remain speculative, pending further genetic and historical investigation.
The PCoA results, shown in Figure 4, further support this structure. Figure 4a, based on Nei’s Genetic Distance, displays the clear separation of the three populations, each occupying distinct quadrants with 100% confidence. POP3, the most genetically distinct, showed higher internal diversity. This trend continues in Figure 4b (27.56% confidence) and Figure 4c (36.5%), where POP3 individuals are more widely dispersed. The greatest outliers included Flot rouge, Bienmesabe tinto, Muscat d’Hamburg, and Viñarda rosada, all of which exhibit complex or interspecific backgrounds.
In this way it can be hypothesized that LP’s cultivated grapevine population appears to derive from two primary historical sources. The first is linked to the Iberian Peninsula (mainly Spain), likely introduced during the island’s colonization under the Castilian Crown. The second reflects Portuguese influence, possibly through settlers from Madeira [73] or the historical introduction of Savagnin blanc-related cultivars. A third, less defined genetic input may stem from the introduction of Direct Producer Hybrids (DPH) during the phylloxera crisis or more recent introductions by professional breeders. Together, these findings highlight the unique and diverse nature of LP’s grapevines and support their relevance for conservation, breeding, and further genetic study.
4.3. Relationship of the La Palma Grapevine Population with the Canary Islands
To explore the genetic relationship between the seven local grapevine varieties from LP and those from the rest of the IC, a reference group of 33 individuals from across the archipelago was analyzed. This included 12 from LZ, 11 from HI, 5 from LG, 4 generic IC types, and 1 Majorera variety from FT, along with the seven LP varieties under investigation. All individuals were assigned to specific population groups using a consistent methodology (Figures S4 and S5).
The genetic structure revealed five main populations (POP1–POP5), with Figure 5 displaying the distribution of 40 varieties. However, limited historical and genetic data on many of these cultivars, some recently described, restricted the depth of analysis. POP1 includes eight varieties largely associated with the “Malvasia” group. This cluster is mainly composed of individuals from LZ, including Malvasia volcanica, a known cross of Malvasia Dubrovacka and Bermejuela. Two other varieties in this group also contain “Malvasia” in their names, reflecting their genetic closeness [47,50]. Some accessions from LG were also present. POP2 is composed almost exclusively of HI varieties, with Verdello de El Hierro being the only one with documented parentage (Verijadiego × Alfrocheiro). The genetic data suggests a potential Portuguese or Central European connection in this group. POP3 holds nine varieties, most of Spanish origin. Many in this group are closely related to Vijariego blanco (also known as Diego in IC), which has Heben as a parent. Sabro, another variety with Heben lineage, appears as an admixed member. Two LP varieties, Cagarruta de oveja and Sabro, are also included here as admixed individuals indicating shared ancestry. POP4, the largest group with 11 accessions, includes varieties derived from unusual parentages or interspecific hybridizations. Notably, three pure LP varieties, Bienmesabe tinto, Viñarda rosada, and Aromatica Eufrosina, are included here. Other members such as Gual Mazo, Majorera (FT), Blanca de la granja del Cabildo (LZ), and Tesoro blanco (HI) have been characterized as genetic outliers in earlier studies [48,49,50]. POP5, comprising six members, cluster varieties are genetically related to Palomino fino, a Spanish cultivar. This group includes Listan negro (IC), Albillo criollo (LP), and Forastera gomerae (LG), all genetically linked through shared parentage. The study confirms a genetic connection between the Spanish variety Heben and the LP cultivars Cagarruta de oveja and Sabro. It also reinforces the close relationship among Bienmesabe tinto, Viñarda rosada, and Aromatica Eufrosina.
To enhance structural analysis, admixed individuals were excluded, yielding a refined dataset of 22 IC varieties. This initially resulted in an 86% assignment accuracy, which was improved to 100% following reassignment of misclassified varieties. Figure 6 presents Principal Coordinate Analysis (PCoA) outcomes. The 2D distribution (Figure 6a) reveals POP1 and POP5 as the most genetically distinct, supported by Fst statistics and a 63.36% explained variance. POP2 remains distinct, while POP3 and POP4 form a more central, overlapping cluster. Figure 6b,c, illustrating two- and three-dimensional individual-level PCoA plots (21.88% and 30.78% goodness, respectively), confirm these trends. POP1, POP2, and POP5 appear more genetically cohesive, while POP3 and particularly POP4 show greater diversity. Bienmesabe tinto and Viñarda rosada stand out for their unique genetic profiles, likely due to distinct hybridization events.
4.4. Relationship of La Palma Population with the Global Diversity
This final section evaluates the distinctiveness of the LP grapevine population in relation to globally distributed varieties. The primary objective was to clarify the genetic identity and potential affinities of the seven local LP varieties. Two complementary strategies were employed: one based purely on genetic analysis and another incorporating a geographic component. In both approaches, the LP population was kept intact (unnormalized) to preserve individual variety behaviors, despite a slight decrease in assignment accuracy. The dataset used included 319 MP-SSR profiles from varieties originating in 23 countries, all resulting from natural crosses, regardless of known parentage.
In the genetic approach, the varieties were initially grouped into two ancestral populations (Figures S6, S7 and Figure 7a). Population 1 (POP1), largely composed of Iberian Peninsula (IP) varieties, mostly Spanish, contained all LP accessions and members of the IC group. After removing admixed individuals (except Albillo criollo and Aromatica Eufrosina), POP1 had 106 accessions, while POP2 had 162, for a total of 268 varieties with 99% assignment accuracy. Reclassifying one misassigned variety raised accuracy to 100%. For more precise analysis, POP1 was subdivided into IC and LP clusters, which slightly lowered assignment accuracy to 88%. The geo-genetic approach normalized the dataset by removing misallocated varieties in the VIVC database, resulting in 197 total profiles: 190 from global sources and the 7 LP accessions. These were divided into seven populations: EASTMED-CAU, BP, ITA, FRA-CE, IP, IC, and LP. Assignment accuracy improved significantly from 60% to 87% following this normalization.
Circular dendrograms (Figure 7b and Figure 9a) illustrated that LP varieties formed a distinct group within the broader IC and IP populations. One notable exception was Bienmesabe tinto, which appeared as an outlier in the genetic dendrogram (Figure 7b), but clustered with other LP accessions in the geographic dendrogram (Figure 9a). These trends were further supported by phylogenetic trees (Figure 7c and Figure 9b), where LP accessions consistently retained a distinct genetic position. Principal Coordinate Analysis (PCoA) plots by population (Figure 8a and Figure 10a) showed coherent clustering. In the genetic approach, 97.85% of goodness was explained, while in the geographic method, the explained goodness was lower at 64.17%, likely due to the presence of more population groups. Spanish origin populations clustered on the left of the PCoA space, while non-Spanish groups (POP2) appeared on the right. The geographic analysis further split POP2 into four subpopulations, with EASTMED-CAU emerging as the most genetically distant. At the individual level (Figure 8b,c and Figure 10b,c), clustering patterns were similar. While Bienmesabe tinto grouped with LP accessions in 2D space, its separation in 3D suggested a unique origin. The remaining six LP varieties followed two trends: Cagarruta de oveja, Gual Mazo, and Sabro clustered with Spanish varieties (implying Iberian ancestry), whereas Viñarda rosada, Albillo criollo, and Aromatica Eufrosina aligned more closely with non-Iberian groups, and showing Aromatica Eufrosina a strong divergence.
In conclusion, (1) LP varieties are genetically distinct from IC, IP, and global varieties; (2) Bienmesabe tinto is likely an interspecific hybrid; (3) some LP varieties show connections to Central European lineages; and (4) others have probable Spanish ancestry, which could be from the progenitor Heben or not.
5. Conclusions
This study represents the first comprehensive genetic characterization of the cultivated grapevine population on LP island, using a robust SSR-based methodology. The SSR kit previously used on other IC has again proven efficient and effective.
Among the 96 accessions collected across the island, 44 unique MP-SSRs were identified, including 3 entirely new varieties previously unknown to science: Cagarruta de oveja, Viñarda rosada, and Aromatica Eufrosina. In addition, 14 new mutations exclusive to this island were identified, including 3 cases of triallelism. Additionally, 8 accessions were determined to be labeling or sampling errors, and 14 accessions unknown by either growers or technicians could be identified.
LP’s contribution also holds lexicographic value. Thus, inclusion in the VIVC is proposed for (1) the three new varieties PN (Aromatica Eufrosina, Cagarru-ta de oveja, Viñarda rosada), along with their SSR profiles; (2) the 14 novel mutant names listed above; and (3) 12 new synonyms drawn from local viticultural tradition, referring either to existing varieties or specific mutations.
Of the 14 accessions affected by lava or ash during the Tajogaite volcanic eruption, only 1 was permanently lost, a mutated individual of Malvasia fina roxa, making the overall biodiversity loss far lower than initially feared.
Regarding LP’s genetic structure, results support the existence of seven representative varieties. Four had already been described while three are new, representing a major finding. These varieties likely originate from Spanish or broader European lineages, with genetic traits suggesting unique evolutionary paths. These genotypes represent an irreplaceable component of LP’s viticultural heritage and should be prioritized for conservation, morphological and oenological characterization, and integration into principal germplasm collections. Their genetic and adaptive uniqueness highlights the need to incorporate them into regional and national strategies for plant genetic resource conservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11080983/s1, Table S1. Information on 96 accessions from La Palma (Original and Conclusive); Table S2. List of 20 primers used for the amplification of the selected microsatellite regions. Characteristics; Figure S1. Approximation of the genomic SSR map used in this study. Consensus location of each of the regions selected for molecular characterization; Table S3. Unique molecular profile of 44 accessions (and 2 sport) corresponding to 31 varieties and 2 sport (color mutations) collected during the La Palma Island prospection. International SSR coincides with the SSR of TECNENOL; Table S4. Statistical characterization of the twenty microsatellite markers used in this study; Figure S2. The four steps of the graphical method of Evanno et al. [56], allowing the estimation of the true number of ancestral K groups for a population with 31 individuals from La Palma Island; Figure S3. Genetic structure of La Palma Island population. Distribution K = 3 (Individuals belonging to each group or population). Details of the ratio of pure and admixed individuals according to the value of q (pure (q ≥ 85%) and admixed (q < 85%)); Figure S4. The four steps of the graphical method of Evanno et al. [56], allowing the estimation of the true number of ancestral K groups for a population with 40 individuals from Canary Islands collection (IC including La Palma Island); Figure S5. Genetic structure of the Canary Islands population (40 varieties). Distribution K = 5 (Individuals belonging to each group or population). Details of the ratio of pure and admixed individuals according to the value of q (pure (q ≥ 85%) and admixed (q < 85%)). Population structure; Figure S6. The four steps of the graphical method of Evanno et al. [56], allowing the estimation of the true number of ancestral K groups for a population with 319 individuals from the TECNENOL database (including La Palma Island); Figure S7. Genetic structure of the world population. Distribution K = 2 (Individuals belonging to each group or population). Detail the proportion of pure and admixed individuals as a function of q value. Nationalities that make up pure and admixed groups; Figure S8. Genetic structure of the world population (319 individuals). Distribution in 7 geographical areas. Detail of the ratio of well-assigned (pure) and misassigned (admixed) individuals. Nationalities that make up each of the groups: EASTMED-CAU (Algeria, Cyprus, Georgia, Israel, Lebanon, Tunisia and Turkey), BP (Bosnia and Herzegovina, Bulgaria, Croatia, Greece, Serbia, Slovenia and Montenegro), ITA (Italy), FRA-CEU (Austria, France, Germany, Hungary, Switzerland and Ukraine), IP (Spain and Portugal), IC (Canary archipelago) and LP (La Palma island).
Author Contributions
Contributions: Conceptualization, Q.L.-Y., F.F. and L.D. methodology, Q.L.-Y., L.D. and F.F.; software, Q.L.-Y., L.D. and F.F.; validation, Q.L.-Y. and L.D.; formal analysis, Q.L.-Y. investigation, Q.L.-Y.; resources, Q.L.-Y. and L.D.; data curation, Q.L.-Y. and L.D.; writing—original draft preparation, Q.L.-Y. and F.F.; writing—review and editing, L.D. and Q.L.-Y.; visualization, J.M.C. and F.Z.; supervision, F.F., Q.L.-Y., L.D., J.M.C. and F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejería de Agricultura, Ganadería, Pesca y Soberanía Alimentaria del Cabildo Insular de la Isla de La Palma at the request of Consejo Regulador de la Denominación de Origen Vinos de La Palma.
Data Availability Statement
Data not available because it is confidential.
Acknowledgments
The authors wish to express their gratitude to the Consejería de Agricultura, Ganadería, Pesca y Soberanía Alimentaria del Cabildo Insular de la Isla de La Palma for their interest in supporting a study of this nature. In particular, they thank Elías Bienes, Eva Hernández, José Adrían Hernández and Elías Bienes for being the driving force behind the project. They also appreciate the contributions of Adalberto Martín representative of the Regulatory Council of the Protected Designation of Origin “Vinos de La Palma.” Additionally, the authors thank Carlos Lozano, Asociación Técnica Canaria de Enología president. Finally, sincere thanks are extended to Rosa Pastor, Braulio Esteve-Zarzoso, Laia Fañanás, Cristina Domènech, and Iris Ginés for their invaluable logistical support in the completion of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Preliminary Study of Somatic Variants of Palomino Fino (Vitis vinifera L.) Grown in a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 654. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Genetical, Morphological and Physicochemical Characterization of the Autochthonous Cultivar ‘Uva Rey’ (Vitis vinifera L.). Agronomy 2019, 9, 563. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Change 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Identification and characterization of white grape varieties autochthonous of a warm climate region (Andalusia, Spain). Agronomy 2020, 10, 205. [Google Scholar] [CrossRef]
- Arco, M.J.; Atiénzar, E.; Rosario, M.C.; Arco, M.M.; González, C.; Arco, M.C. El Menceyato de Icod en el poblamiento de Tenerife: D.; Gaspar, Las Palomas y Los Guanches. Sobre el poblamiento y las estrategias de alimentación vegetal entre los Guanches. Eres 2000, 9, 67–129. [Google Scholar]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wikimedia Commons. Macaronesia Location. Available online: https://commons.wikimedia.org/w/index.php?curid=33566121 (accessed on 6 May 2025).
- NASA Earth Observatory. Canary Islands. Available online: https://www.flickr.com/photos/gsfc/6630087415/ (accessed on 9 May 2025).
- GEVIC (Gran Enciclopedia Virtual Islas Canarias). Fisiografía de Canarias. Relieve. Available online: http://www.gevic.net/info/contenidos/mostrar_contenidos.php?idcat=22&idcap=91&idcon=529 (accessed on 5 May 2025).
- Canary Wine. Isla de La Palma. Available online: https://www.canarywine.com/isla-de-la-palma/ (accessed on 5 May 2025).
- Climate Data. Clima La Palma (España). Available online: https://es.climate-data.org/europe/espana/la-palma-10272/ (accessed on 6 May 2025).
- Boletín Oficial del Estado (BOE). Ley 25/1994, de 12 de Julio, Por la Que Se Incorpora al Derecho Español la Directiva 91/680/CEE, Sobre el Sistema Común Del Impuesto Sobre el Valor Añadido. BOE núm. 161, pp. 21568–21576. Available online: https://www.boe.es/boe/dias/1994/07/05/pdfs/A21568-21576.pdf (accessed on 5 May 2025).
- Vinos La Palma. Subzona Norte. Available online: https://www.vinoslapalma.com/viticultura/zonas-de-produccion/subzona-norte.html (accessed on 5 May 2025).
- Vinos La Palma. Subzona Hoyo de Mazo. Available online: https://www.vinoslapalma.com/viticultura/zonas-de-produccion/subzona-hoyo-de-mazo.html (accessed on 5 May 2025).
- Vinos La Palma. Subzona Fuencaliente. Available online: https://www.vinoslapalma.com/viticultura/zonas-de-produccion/subzona-fuencaliente.html (accessed on 5 May 2025).
- Topographic-map.com. Mapa topográfico de La Palma. Available online: https://es-ar.topographic-map.com/map-c3c3cz/La-Palma/ (accessed on 12 May 2025).
- Vinos La Palma. Zonas de Producción Vitícola. Available online: https://www.vinoslapalma.com/viticultura/zonas-de-produccion.html (accessed on 12 May 2025).
- Wikipedia. Erupción Volcánica de La Palma de. 2021. Available online: https://es.wikipedia.org/wiki/Erupción_volcánica_de_La_Palma_de_2021 (accessed on 12 May 2025).
- Marsal, G.; Mendez, J.J.; Mateo-Sanz, J.M.; Ferrer, S.; Canals, J.M.; Zamora, F.; Fort, F. Molecular characterization of Vitis vinifera L. local cultivars from volcanic areas (the Canary Islands and Madeira) using SSR markers. OENO One 2019, 53, 667–680. [Google Scholar] [CrossRef]
- Vinos La Palma. Variedades de Uva. Available online: https://www.vinoslapalma.com/viticultura/variedades-de-uva.html (accessed on 24 May 2025).
- Mendez, J.J. Acerca del Canary Wine. In Compendio de la Vitivinicultura del Archipiélago Canario, 2nd ed.; Asociación de Viticultores y Bodegueros de Canarias AVIBO, Ed.; Centro de la Cultura Popular Canaria (CCPC): Canary Islands, Spain, 2024. [Google Scholar]
- GEVIC (Gran Enciclopedia Virtual Islas Canarias). Fisiografía de Canarias. Erupciones Históricas en Canarias. Available online: https://www.gevic.net/info/contenidos/mostrar_contenidos.php?idcomarca=-1&idcon=716&idcap=91&idcat=22 (accessed on 12 May 2025).
- Instituto Geográfico Nacional (IGN). Descripción Geológica de La Palma. Available online: https://www.ign.es/web/resources/sismologia/tproximos/sismotectonica/pag_sismotectonicas/can_la_palma_en.html (accessed on 12 May 2025).
- Carracedo, J.C.; Troll, V.R.; Day, J.M.; Junca, M.A.; Soler, V.; Deegan, F.M.; Pérez-Torrado, F.J.; Gisbert, G.; Gazel, E.; Rodríguez-González, A.; et al. The 2021 eruption of the Cumbre Vieja volcanic ridge on La Palma, Canary Islands. Geol. Today 2022, 38, 94–107. [Google Scholar] [CrossRef]
- Marca Canaria. Erupciones Históricas en Canarias. Available online: https://marcacanaria.com/erupciones-historicas-en-canarias/ (accessed on 12 May 2025).
- RTVE. Erupción Volcánica en La Palma. Finaliza La Erupción del Volcán de La Palma tras 85 Días de Actividad. RTVE Noticias. Available online: https://www.rtve.es/noticias/20211225/finaliza-erupcion-volcan-palma-tras-85-dias-actividad/2244006.shtml (accessed on 12 May 2025).
- Vinos La Palma. Estadísticas. Available online: https://www.vinoslapalma.com/bodegas/estadisticas.html#:~:text=2019%20561%20937%2019%20645,982%20Buena (accessed on 24 May 2025).
- Vinos La Palma. La Vendimia 2024 en la Denominación de Origen Protegida Vinos La Palma Avanza Con Bajos Rendimientos y Marcada Por Condiciones Climáticas Adversas. Available online: https://vinoslapalma.com/noticias-vinos-la-palma/93-actualidad/420-la-vendimia-2024-en-la-denominacion-de-origen-protegida-vinos-la-palma-avanza-con-bajos-rendimientos-y-marcada-por-condiciones-climaticas-adversas.html#:~:text=En%20cuanto%20a%20la%20tipología,de%20uva%20tinta (accessed on 24 May 2025).
- Vinos La Palma. Informe de Daños Viñedos y Vendimia 2021. Available online: https://www.vinoslapalma.com/bodegas/vendimia/ano-2021.html (accessed on 24 May 2025).
- Vinos La Palma. Informe Valoración Daños Erupción Volcánica y Vendimia. Enero 2022. Available online: https://www.vinoslapalma.com/files/noticias/danos-vendimia-2021.pdf (accessed on 24 May 2025).
- Vinos La Palma. Tipos de Vino. Available online: https://www.vinoslapalma.com/bodegas/tipos-de-vinos.html#:~:text=Vinos%20de%20tea (accessed on 24 May 2025).
- Fort, F.; Hayoun, L.; Valls, J.; Canals, J.M.; Arola, L.; Zamora, F. A new and simple method for rapid extraction and isolation of high-quality RNA from grape (Vitis vinifera) berries. J. Sci. Food Agric. 2008, 88, 179–184. [Google Scholar] [CrossRef]
- Marsal, G.; Baiges, I.; Canals, J.M.; Zamora, F.; Fort, F. A fast, efficient method for extracting DNA from leaves, stems, and seeds of Vitis vinifera L. Am. J. Enol. Vitic. 2011, 62, 376–381. [Google Scholar] [CrossRef]
- Marsal, G.; Boronat, N.; Canals, J.M.; Zamora, F.; Fort, F. Comparison of the efficiency of some of the most usual DNA extraction methods for woody plants in different tissues of Vitis vinifera L. J. Int. Sci. Vigne Vin. 2013, 47, 227–237. [Google Scholar] [CrossRef]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analyzed as sequence-tagged sites (STSs). Theor. Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.D.; Eggler, P.; Seaton, G.; Rosseto, M.; Abblet, E.M.; Lee, L.S.; Henry, R.J. Analysis of SSRs derived from grape ESTs. Theor. Appl. Genet. 2000, 100, 723–726. [Google Scholar] [CrossRef]
- Lefort, F.; Kyvelos, C.; Zervou, M.; Edwards, K.; Roubelakis-Angelakis, K. Characterization of new microsatellite loci from Vitis vinifera and their conservation in some Vitis species and hybrids. Mol. Ecol. Resour. 2002, 2, 20–21. [Google Scholar] [CrossRef]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages and reveals a large admixture amongst varieties of different geographic origins. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef]
- Dalbó, M.A.; Ye, G.N.; Weeden, N.F.; Steinkellner, H.; Sefc, K.M.; Reisch, B.I. A gene-controlling sex in grapevines is placed on a molecular marker-based genetic map. Genome 2000, 43, 333–340. [Google Scholar] [CrossRef]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Costantini, L.; Crespan, M.; Dangl, G.S.; Eisenheld, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for the identification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef]
- Marsal, G.; Mateo, J.M.; Canals, J.M.; Zamora, F.; Fort, F. SSR analysis of 338 accessions planted in Penedès (Spain) reveals 28 unreported molecular profiles of Vitis vinifera L. Am. J. Enol. Vitic. 2016, 67, 466–470. [Google Scholar] [CrossRef]
- Marsal, G.; Bota, J.; Martorell, A.; Canals, J.M.; Zamora, F.; Fort, F. Local cultivars of Vitis vinifera L. in Spanish islands: Balearic Archipelago. Sci. Hortic. 2017, 226, 122–132. [Google Scholar] [CrossRef]
- Fort, F.; Marsal, G.; Mateo-Sanz, J.M.; Pena, V.; Canals, J.M.; Zamora, F. Molecular characterisation of the current cultivars of Vitis vinifera L. in Lanzarote (Canary Islands, Spain) reveals nine individuals which correspond to eight new varieties and two new sports. OENO One 2022, 56, 281–295. [Google Scholar] [CrossRef]
- Fort, F.; Lin-Yang, Q.; Suárez-Abreu, L.R.; Sancho-Galán, P.; Canals, J.M.; Zamora, F. Study of molecular biodiversity and population structure of Vitis vinifera L. ssp. vinifera on the volcanic island of El Hierro (Canary Islands, Spain) by using microsatellite markers. Horticulturae 2023, 9, 1297. [Google Scholar] [CrossRef]
- Fort, F.; Lin-Yang, Q.; Valls, C.; Sancho-Galán, P.; Canals, J.M.; Zamora, F. Characterisation and identification of vines from Fuerteventura (Canary Volcanic Archipelago (Spain)) using simple sequence repeat markers. Horticulturae 2023, 9, 1301. [Google Scholar] [CrossRef]
- Fort, F.; Lin-Yang, Q.; Valls, C.; Sancho-Galán, P.; Canals, J.M.; Zamora, F. Analysis of the diversity presented by Vitis vinifera L. in the volcanic island of La Gomera (Canary Archipelago, Spain) using simple sequence repeats (SSRs) as molecular markers. Horticulturae 2024, 10, 14. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue (VIVC). Available online: https://vivc.de (accessed on 22 May 2025).
- Paetkau, D.; Calvert, W.; Stirling, I.; Strobeck, C. Microsatellite analysis of population structure in Canadian polar bears. Mol. Ecol. 1995, 4, 347–354. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Paetkau, D.; Slade, R.; Burden, M.; Estoup, A. Genetic assignment methods for the direct, real-time estimation of migration rate: A simulation-based exploration of accuracy and power. Mol. Ecol. 2004, 13, 55–65. [Google Scholar] [CrossRef]
- Three-Dimensional Plotting in Matplotlib (Python Data Science Handbook). Available online: https://jakevdp.github.io/PythonDataScienceHandbook/04.12-three-dimensional-plotting.html (accessed on 20 May 2025).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic threes. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Fort, F.; Suárez-Abreu, L.R.; Lin-Yang, Q.; Deis, L.; Canals, J.M.; Zamora, F. Preliminary Clonal Characterization of Malvasia volcanica and Listan prieto by Simple Sequence Repeat (SSR) Markers in Free-Phylloxera Volcanic Vineyards (Lanzarote and Fuerteventura (Canary Island, Spain)). Horticulturae 2025, 11, 823. [Google Scholar] [CrossRef]
- Rodriguez-Torres, I. Variedades de vid Cultivadas en Canarias. Descriptores Morfológicos. Caracterización Morfológica, Molecular, Agronómica y Enológica, 2nd ed.; Instituto Canario de Investigaciones Agrarias. Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2018; Available online: https://www.icia.es/icia/download/Publicaciones/Variedades_Vid_Canarias.pdf (accessed on 4 March 2025).
- Zerolo, J.; Cabello, F.; Espino, A.; Borrego, J.; Ibañez, J.; Rodriguez-Torres, I.; Muñoz-Organero, G.; Rubio, C.; Hernández, M. Variedades de Vid de Cultivo Tradicional en Canarias, 1st ed.; Instituto Canario de Calidad Agroalimentaria. Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2006; ISBN 978-84-606-3977-0. [Google Scholar]
- Bacilieri, R.; Lacombe, T.; Cunff, L.L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Perós, J.P.; This, P.; Boursiquot, J.M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 25. [Google Scholar] [CrossRef]
- Fort, F.; Suárez-Abreu, L.R.; Lin-Yang, Q.; Deis, L.; Canals, J.M.; Zamora, F. Origin and Possible Members of the ‘Malvasia’ Family: The New Fuencaliente de La Palma Hypothesis on the True ‘Malvasia’. Horticulturae 2025, 11, 561. [Google Scholar] [CrossRef]
- Ibañez, J.; De Andrés, M.T.; Molino, A.; Borrego, J. Genetic study of key Spanish grapevine varieties using microsatellite analysis. Am. J. Enol. Vitic. 2003, 54, 22–30. [Google Scholar] [CrossRef]
- Vélez, M.D.; Ibáñez, J. Evaluation of the uniformity and stability of Microsatellite markers in grapevine. Acta Hortic. 2009, 827, 163–168. [Google Scholar] [CrossRef]
- Cabezas, A.; Ibañez, J.; Lijavetzky, D.; Vélez, D.; Bravo, G.; Rodríguez, V.; Carreño, I.; Jermakow, A.M.; Carreño, J.; Ruiz-García, L.; et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Galet, P. Dictionnaire Encyclopédique des Cépages, 1st ed.; Hachette: Paris, France, 2000. [Google Scholar]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.; Mozas, P.; Ortiz, J.M. Ampelography and microsatellite DNA analysis of autochthonous and endangered grapevine cultivars in the province of Huesca (Spain). Span. J. Agric. Res. 2011, 9, 790–800. [Google Scholar] [CrossRef]
- Otamendi, J.J. La Malvasía Atlántica. Dudas, Errores y algunas Certezas. Available online: https://dolanzarote.com/wp-content/uploads/2011/06/Malvasia-atlantica.pdf (accessed on 6 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).