Towards Understanding the Promotion of Plant Growth Under an Experimental Red-Fluorescent Plastic Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Growth Culture
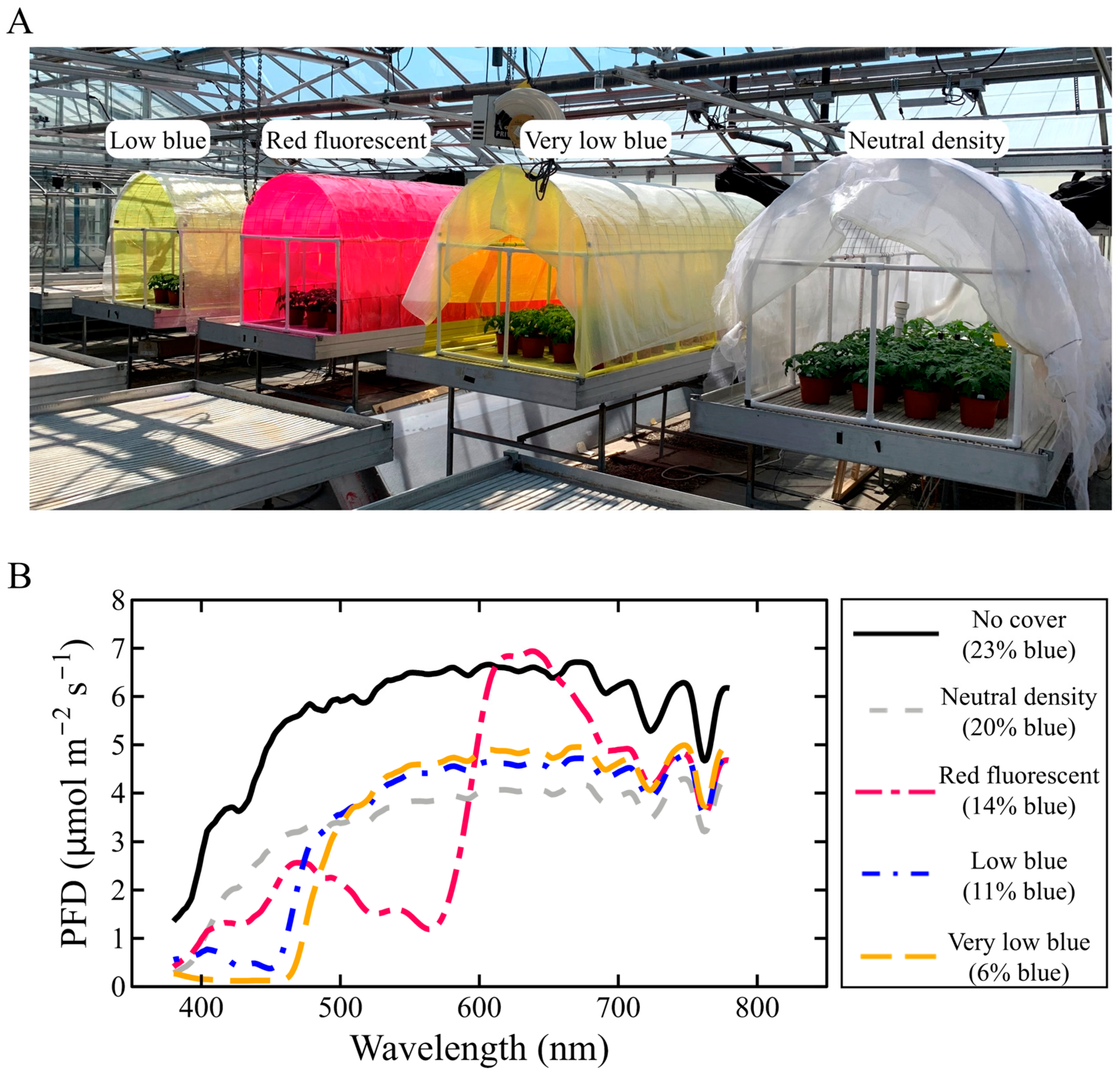
2.2. Chamber Design
2.3. Treatment Transmission
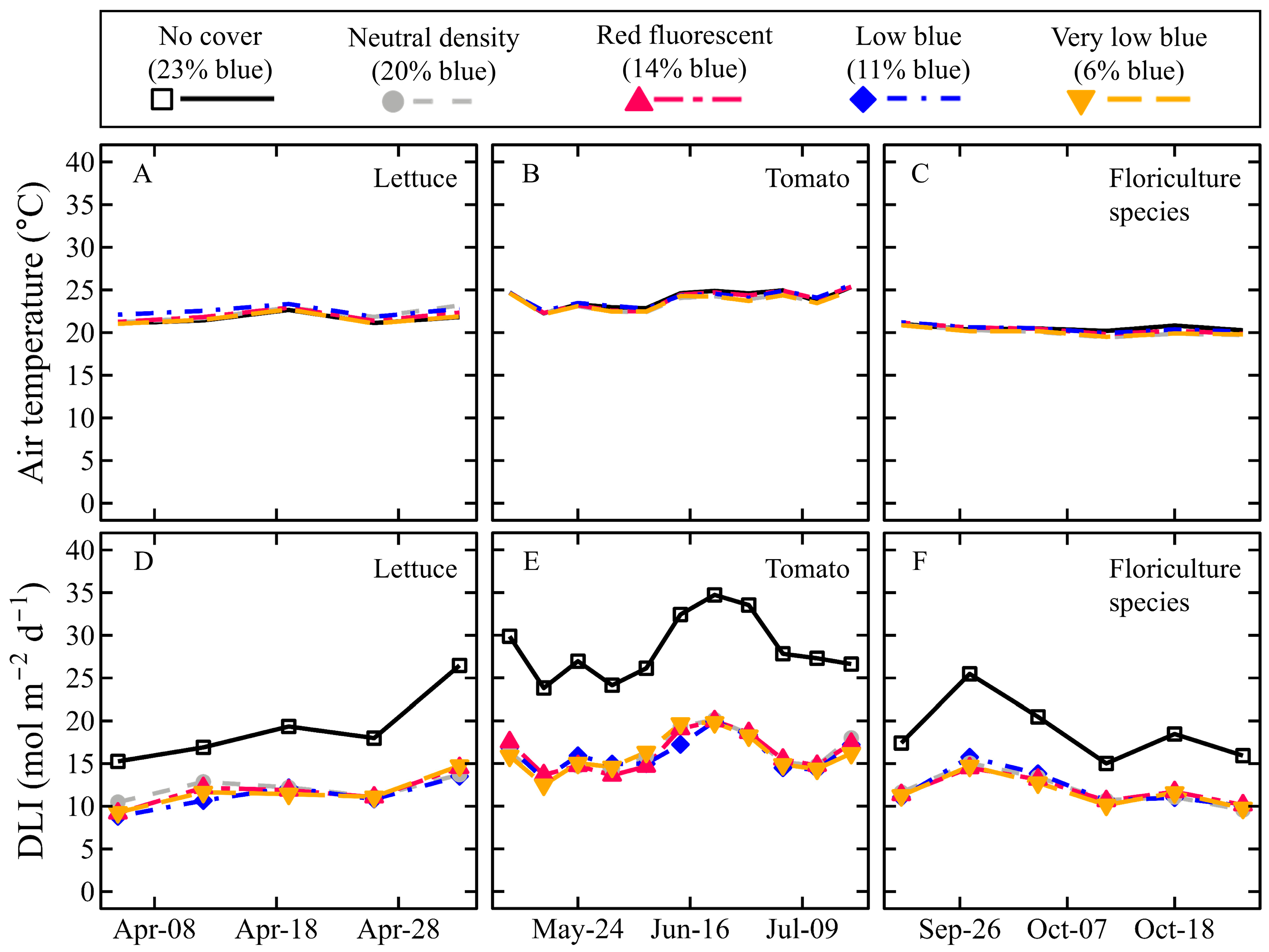
2.4. Greenhouse Environment and Sensing
2.5. Mature Growth Culture
2.6. Data Collection
2.7. Light Response Curves
2.8. Stomatal Conductance and Quantum Yield Measurements
2.9. Experimental Design and Statistical Analysis
3. Results
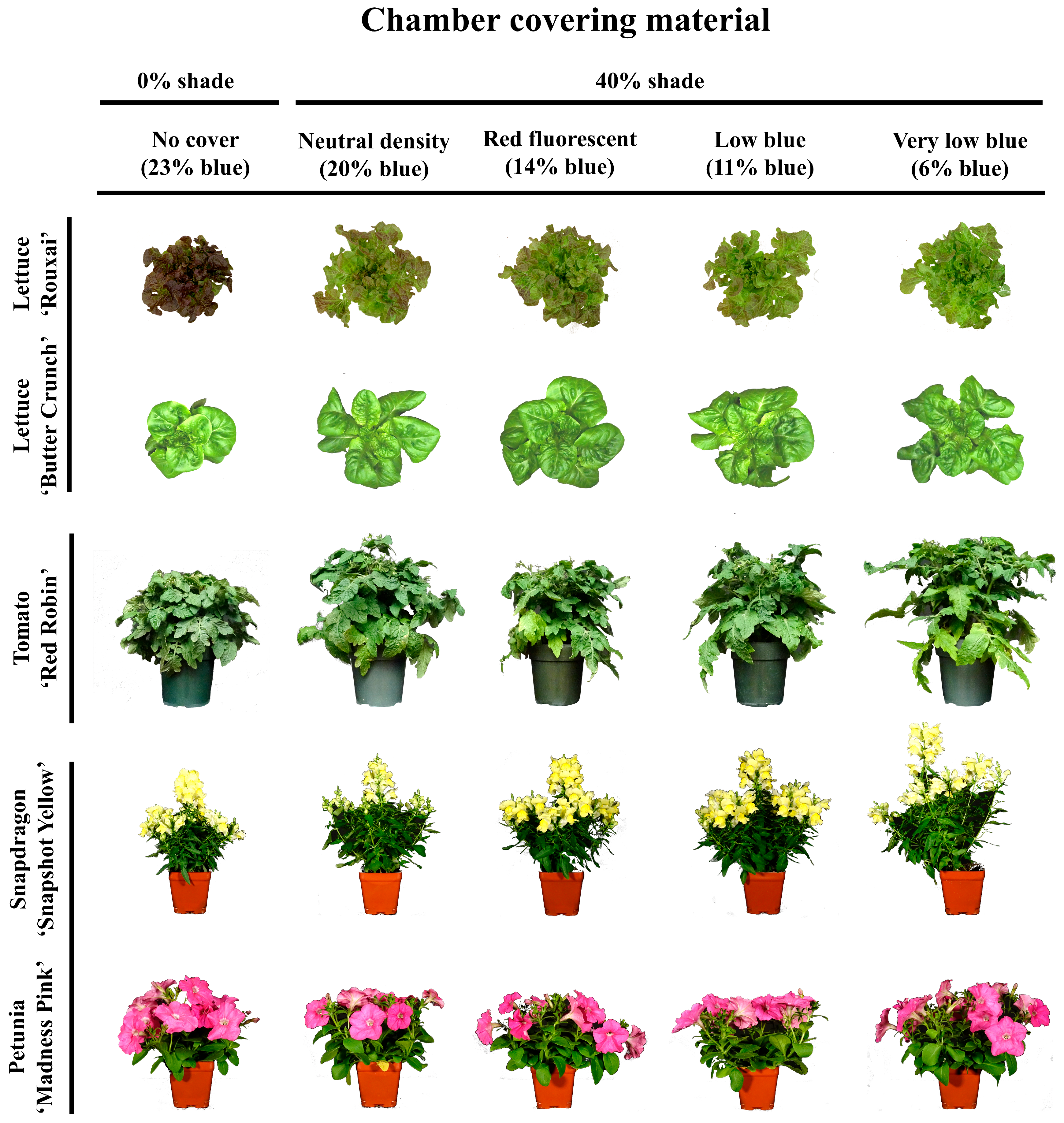
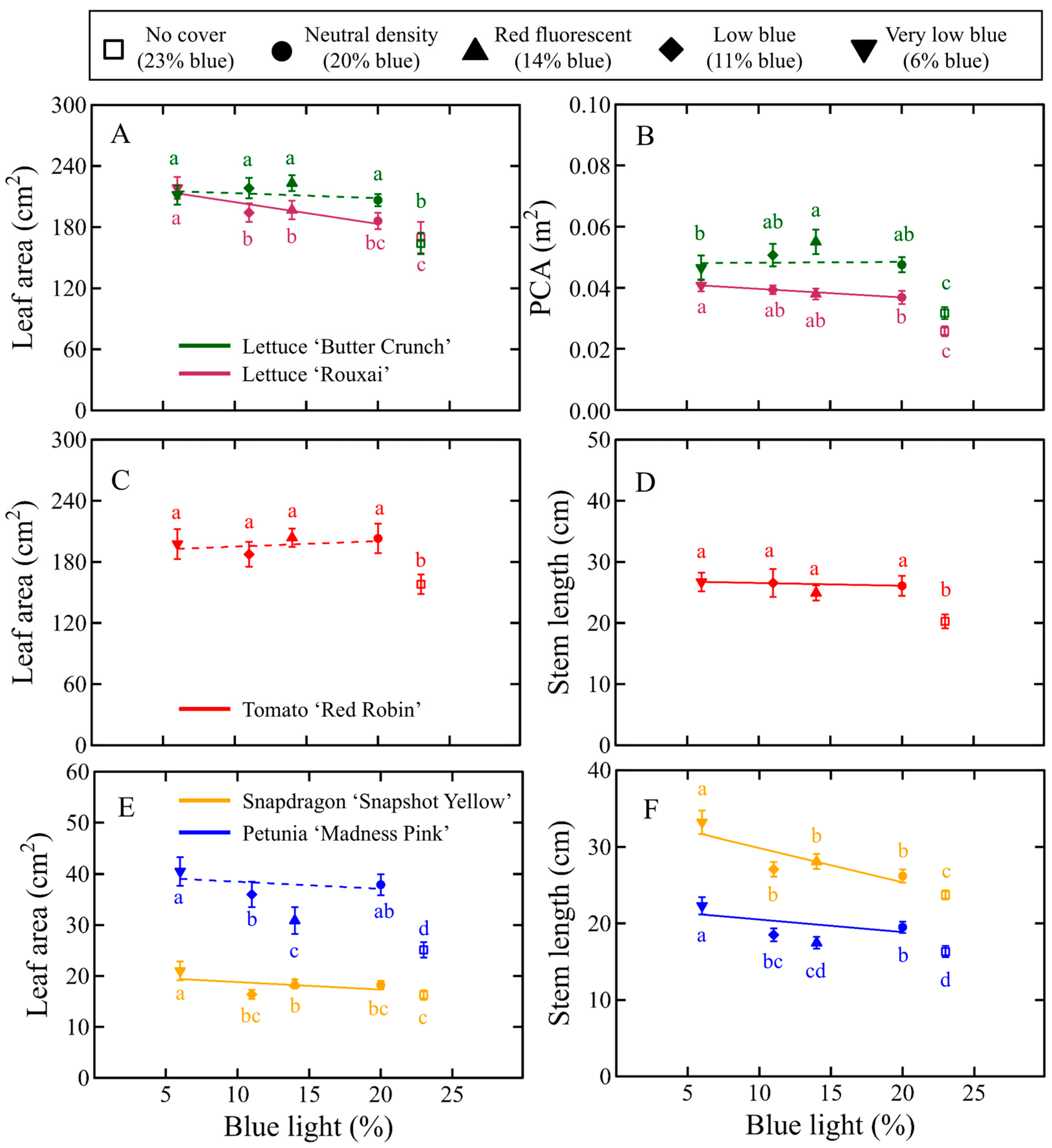
3.1. Morphology
3.1.1. Lettuce Morphology
3.1.2. Tomato Morphology
3.1.3. Floriculture Crops Morphology
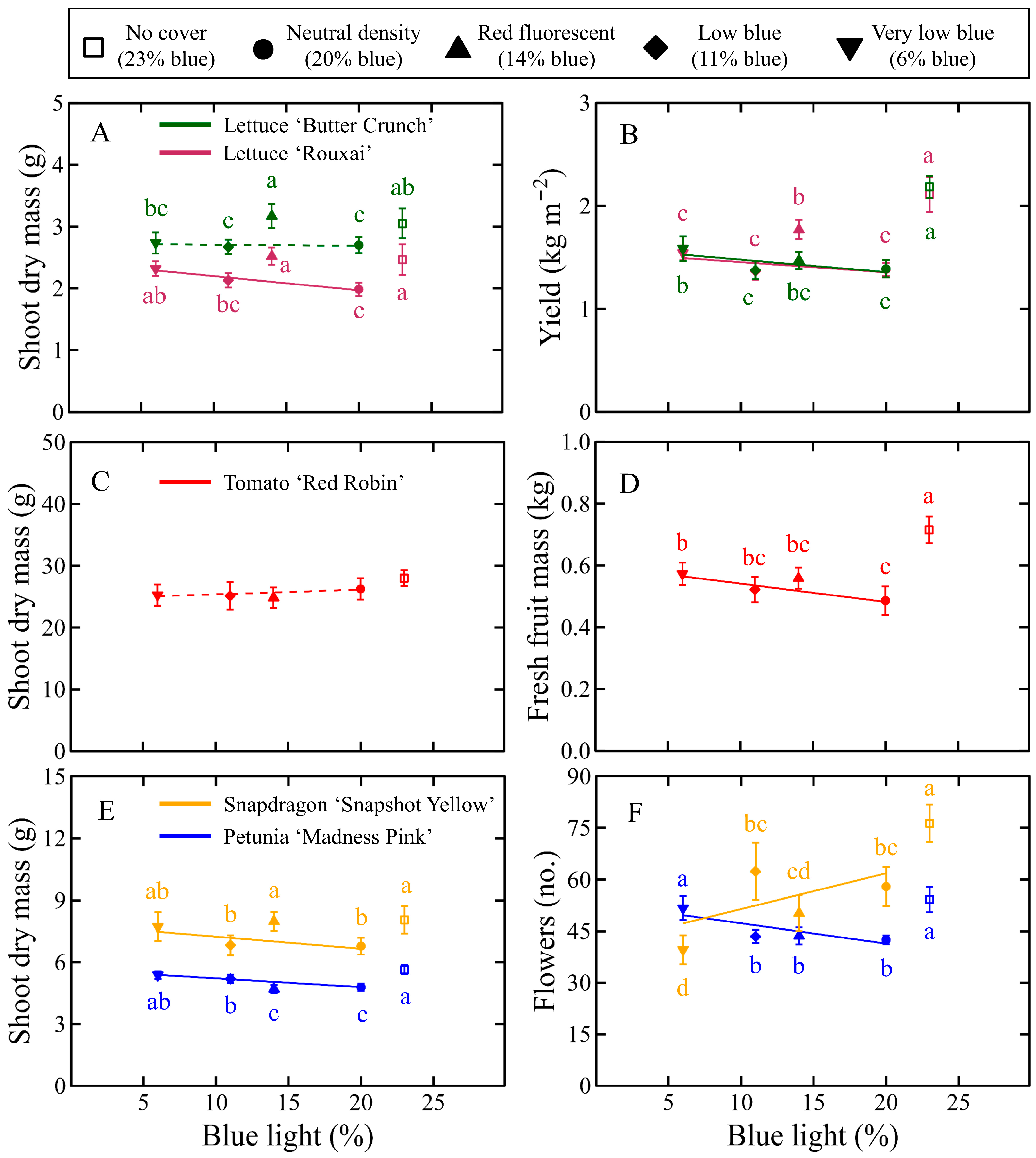
3.2. Biomass Accumulation
3.2.1. Lettuce Biomass Accumulation
3.2.2. Tomato Biomass Accumulation
3.2.3. Floriculture Crops Biomass Accumulation
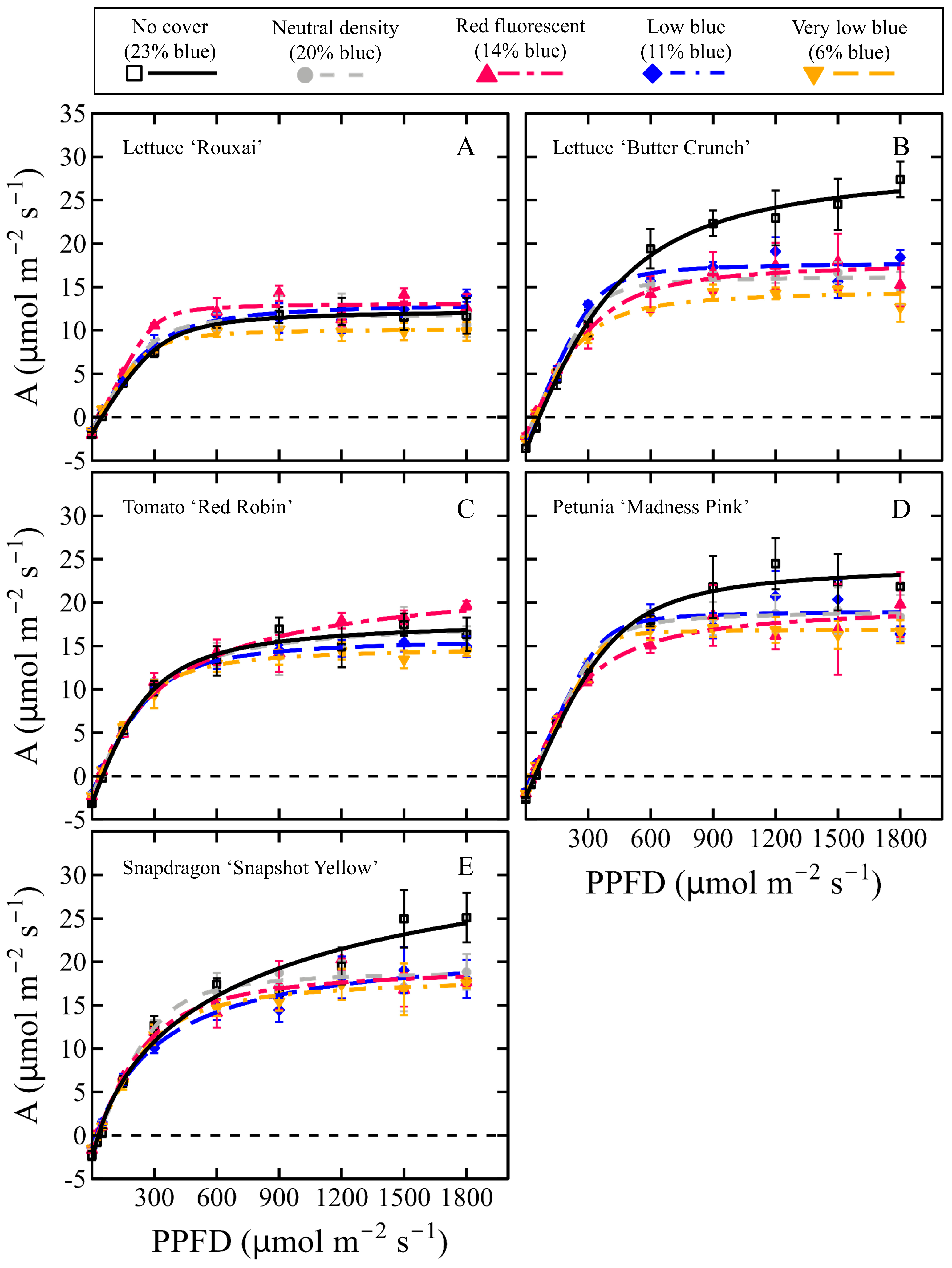
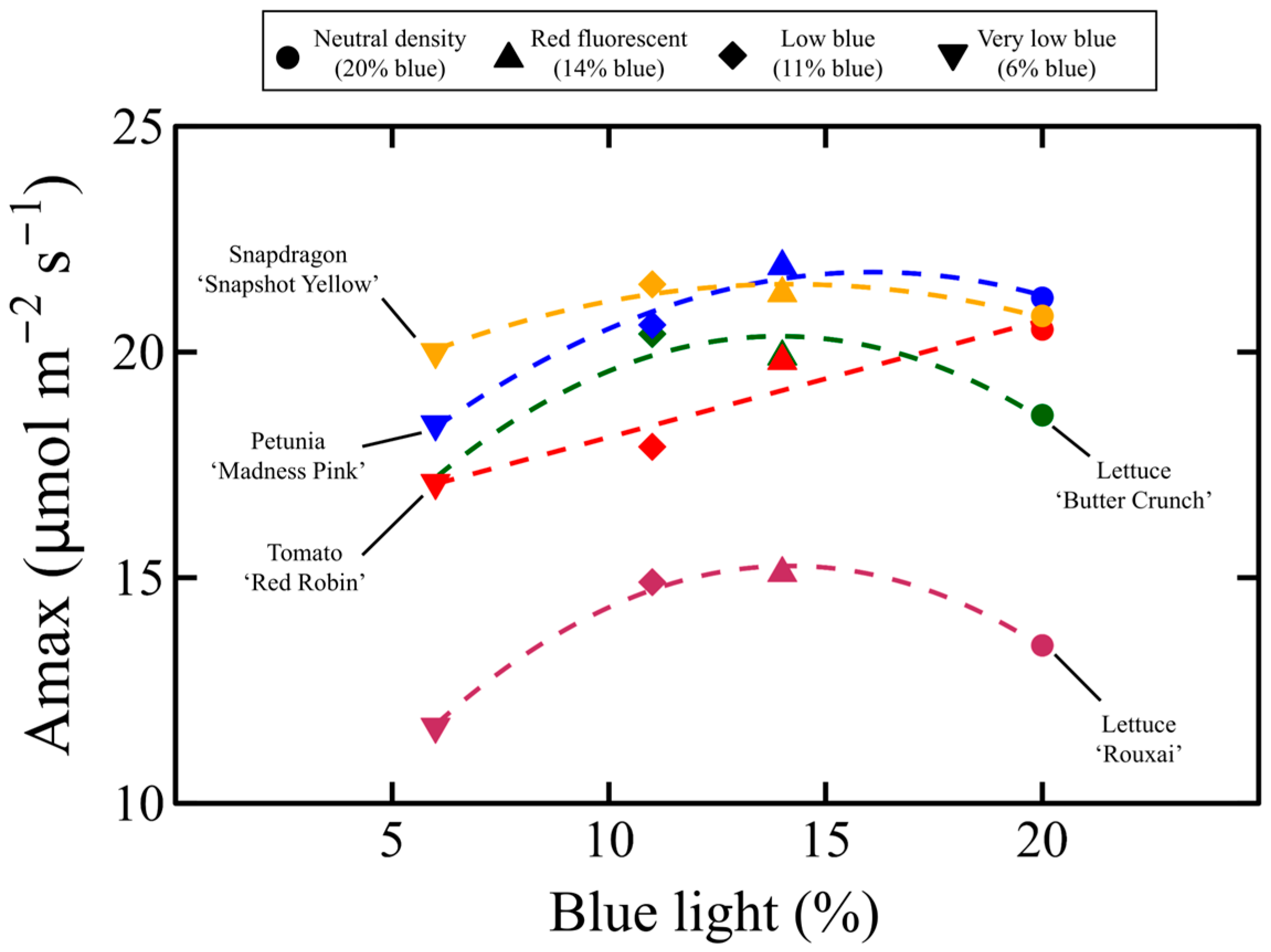
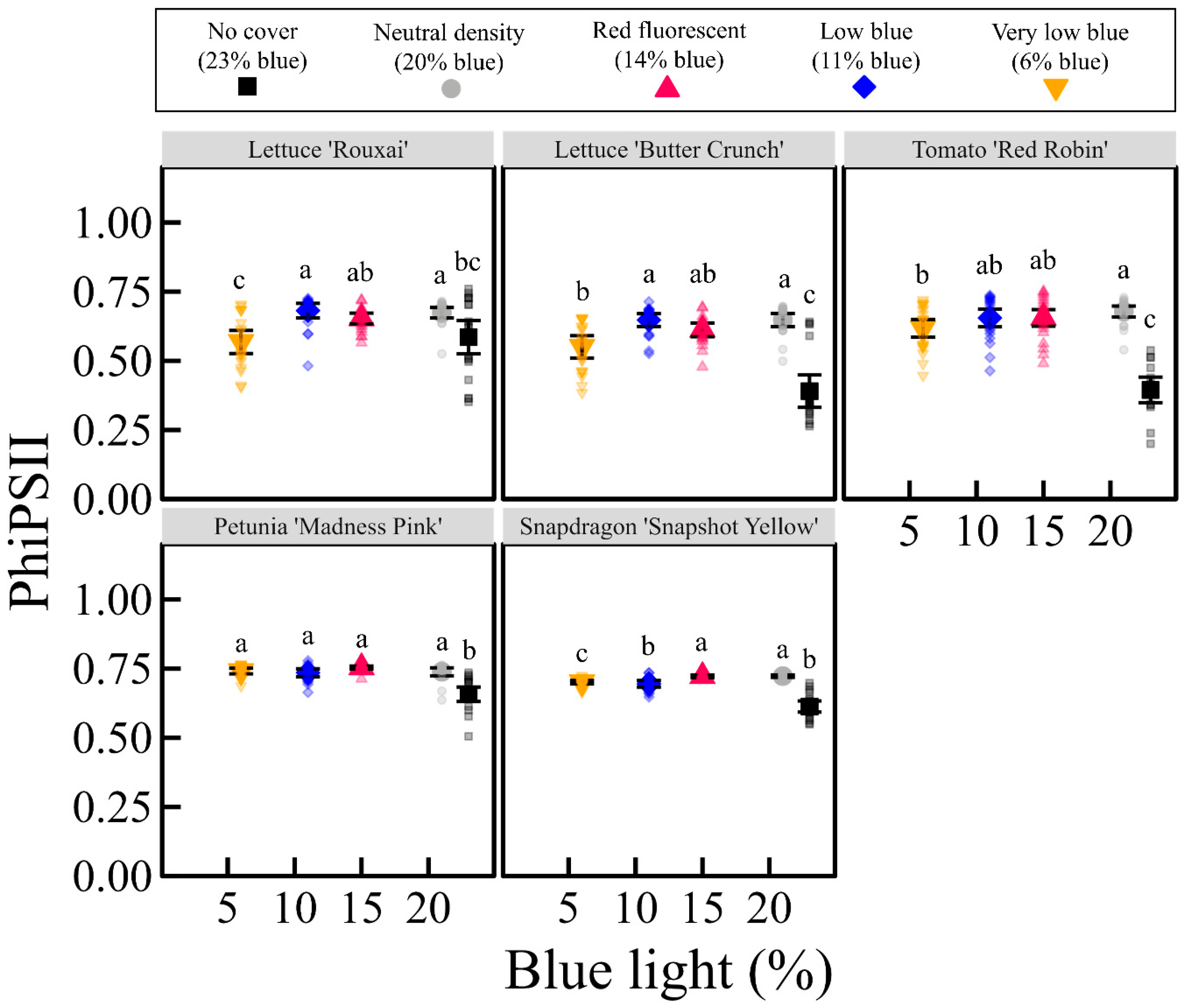
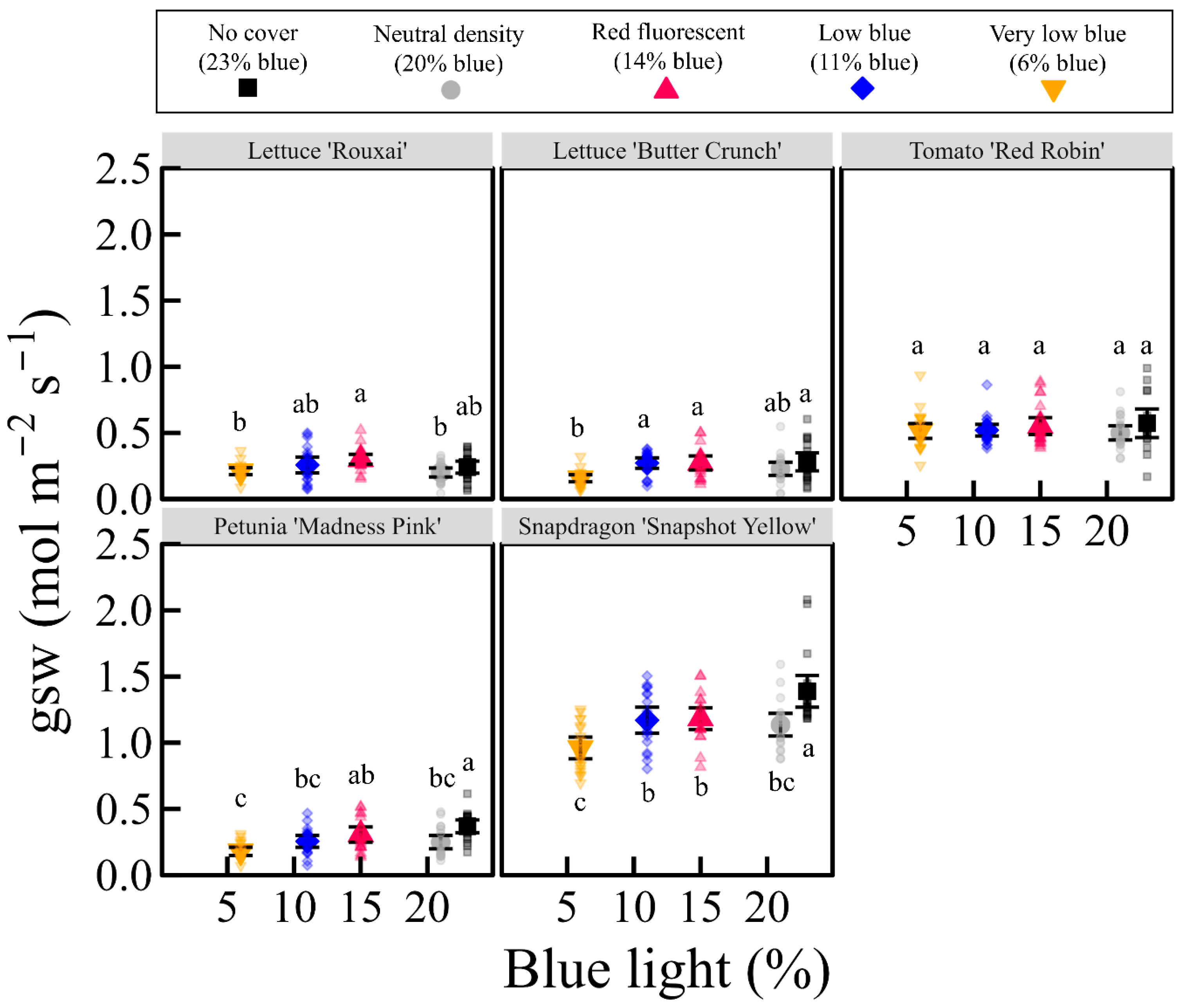
3.3. Light Response Curves, Quantum Yield, and Stomatal Conductance
4. Discussion
4.1. Transmission PPFD Influenced Crop Morphology
4.2. Decreasing the Percentage of B Light Slightly Increased Extension Growth in a Species-Specific Manner
4.3. Effects of DLI on Crop Yield
4.4. Effects of Photon Spectrum on Biomass Accumulation
4.5. Maximum Rate of Photosynthesis Is Affectred by DLI in a Species-Specifc Manner
4.6. Maximux Rate of Photosynthesis Is Affected by the Transmission of B Light
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV | Ultraviolet; 280–399 nm |
| B | Blue; 400–499 nm |
| G | Green; 500–599 nm |
| R | Red; 600–699 nm |
| FR | FR; 700–750 nm |
| PPFD | Photosynthetic photon flux density; 400–700 nm |
| Chl | Chlorophyll |
| PPE | Phytochrome photoequilibrium |
| SFM | Shoot fresh mass |
| SDM | Shoot dry mass |
| FFM | Fruit fresh mass |
| PCA | Projected canopy area |
| EC | Electrical conductivity |
| DLI | Daily light integral |
| PAR | Photosynthetically active radiation |
| LED | Light-emitting diode |
| SLA | Specific leaf area |
| RUE | Radiation use efficiency |
| PhiPSII | Quantum yield of photosystem II |
| Amax | Maximum rate of photosynthesis |
| gsw | Stomatal conductance |
References
- Wittwer, S.H.; Castilla, N. Protected Cultivation of Horticultural Crops Worldwide. HortTechnology 1995, 5, 6–23. [Google Scholar] [CrossRef]
- Cuesta Roble Releases 2019 Global Greenhouse Statistics. Produce Grower, 10 January 2020. Available online: https://www.producegrower.com/news/cuesta-roble-2019-global-greenhouse-statistics/ (accessed on 1 May 2025).
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue Radiation Interacts with Green Radiation to Influence Growth and Predominantly Controls Quality Attributes of Lettuce. J. Amer. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Stallknecht, E.J.; Herrera, C.K.; Yang, C.; King, I.; Sharkey, T.D.; Lunt, R.R.; Runkle, E.S. Designing Plant–Transparent Agrivoltaics. Sci. Rep. 2023, 13, 1903. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-Red Radiation Promotes Growth of Seedlings by Increasing Leaf Expansion and Whole-Plant Net Assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Kelly, N.; Runkle, E.S. Ultraviolet A and Blue Light Transiently Regulate Total Phenolic and Anthocyanin Concentrations in Indoor-Grown Red-Leaf Lettuce. HortScience 2023, 58, 1595–1602. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of Green Light to Plant Growth and Development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological Responses of Cucumber Seedlings under Different Blue and Red Photon Flux Ratios Using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Kushunina, M.A.; Zhigalova, T.V. Effects of Red and Blue LED Light on the Growth and Photosynthesis of Barley (Hordeum Vulgare L.) Seedlings. J. Plant Growth Regul. 2023, 42, 1804–1820. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red Photons Have Equivalent Efficiency to Traditional Photosynthetic Photons: Implications for Redefining Photosynthetically Active Radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, D.; Yoon, H.I.; Son, J.E. Growth, Morphology, and Photosynthetic Activity of Chinese Cabbage and Lettuce Grown under Polyethylene and Spectrum Conversion Films. Hortic. Environ. Biotechnol. 2023, 64, 593–603. [Google Scholar] [CrossRef]
- Parrish, C.H.; Hebert, D.; Jackson, A.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Optimizing Spectral Quality with Quantum Dots to Enhance Crop Yield in Controlled Environments. Commun. Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lou, R.; Park, Y.; Guo, Y.; Stallknecht, E.J.; Xiao, Y.; Rieder, D.; Yang, R.; Runkle, E.S.; Yin, X. Increasing Greenhouse Production by Spectral-Shifting and Unidirectional Light-Extracting Photonics. Nat. Food 2021, 2, 434–441. [Google Scholar] [CrossRef]
- Shoji, S.; Saito, H.; Jitsuyama, Y.; Tomita, K.; Haoyang, Q.; Sakurai, Y.; Okazaki, Y.; Aikawa, K.; Konishi, Y.; Sasaki, K.; et al. Plant Growth Acceleration Using a Transparent Eu3+-Painted UV-to-Red Conversion Film. Sci. Rep. 2022, 12, 17155. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing Plant Productivity by Changing the Solar Spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Stallknecht, E.J.; Runkle, E.S. An Experimental Red Fluorescent Film Has Cultivar-Specific Effects on Lettuce Yield and Morphology. HortScience 2025, 60, 1132–1141. [Google Scholar] [CrossRef]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Ag. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Inada, K. Action Spectra for Photosynthesis in Higher Plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R. Plant Canopy Architecture. In Physiology of Crop Production; CRC Press: Boca Raton, FL, USA, 2006; pp. 11–32. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Minich, A.; Minich, I.; Shaitarova, O.; Permyakova, N.; Zelenchukova, N.; Ivanitskiy, A.; Filatov, D.; Ivlev, G. Vital Activity of Lactuca sativa and Soil Microorganisms under Fluorescent Films. Tomsk State Pedagog. Univ. Bull. 2011, 8, 74–84. [Google Scholar]
- Nishimura, Y.; Wada, E.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. The effect of spectrum conversion covering film on cucumber in soilless culture. Acta Hortic. 2012, 956, 481–487. [Google Scholar] [CrossRef]
- González, A.; Rodríguez, R.; Bañón, S.; Franco, J.A.; Fernández, J.A.; Salmerón, A.; Espí, E. Strawberry and cucumber cultivation under fluorescent photoselective plastic films cover. Acta Hortic. 2003, 614, 407–413. [Google Scholar] [CrossRef]
- Hemming, S.; Os, E.A.V.; Hemming, J.; Dieleman, J.A. The Effect of New Developed Fluorescent Greenhouse Films on the Growth of Fragaria × Ananassa “Elsanta”. Acta Hortic. 2006, 71, 145–154. [Google Scholar] [CrossRef]
- Hidaka, K.; Yoshida, K.; Shimasaki, K.; Murakami, K.; Yasutake, D.; Kitano, M. Spectrum Conversion Film for Regulation of Plant Growth. J. Fac. Agric. Kyushu Univ. 2008, 53, 549–552. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade Avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, D.; Bachy, C.; Sudek, S.; Wong, C.-H.; Jiménez, V.; Rockwell, N.C.; Martin, S.S.; Ngan, C.Y.; Reistetter, E.N.; Van Baren, M.J.; et al. Marine Algae and Land Plants Share Conserved Phytochrome Signaling Systems. Proc. Natl. Acad. Sci. USA 2014, 111, 15827–15832. [Google Scholar] [CrossRef]
- Lin, C.; Shalitin, D. Cryptochrome Structure and Signal Transduction. Annu. Rev. Plant Biol. 2003, 54, 469–496. [Google Scholar] [CrossRef]
- Loik, M.E.; Carter, S.A.; Alers, G.; Wade, C.E.; Shugar, D.; Corrado, C.; Jokerst, D.; Kitayama, C. Wavelength-Selective Solar Photovoltaic Systems: Powering Greenhouses for Plant Growth at the Food-Energy-Water Nexus. Earth’s Future 2017, 5, 1044–1053. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kang, J.H.; Kang, W.H.; Son, J.E. Subtle Changes in Solar Radiation under a Green-to-Red Conversion Film Affect the Photosynthetic Performance and Chlorophyll Fluorescence of Sweet Pepper. Photosynthetica 2020, 58, 1107–1115. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Skolik, P.; Morais, C.L.M.; Martin, F.L.; McAinsh, M.R. Determination of Developmental and Ripening Stages of Whole Tomato Fruit Using Portable Infrared Spectroscopy and Chemometrics. BMC Plant Biol. 2019, 19, 236. [Google Scholar] [CrossRef]
- R: A Language for Statistical Computing. 2025. Available online: https://cran.r-project.org/bin/windows/base/old/4.4.3/ (accessed on 1 July 2025).
- Marshall, B.; Biscoe, P.V. A Model for C3 Leaves Describing the Dependence of Net Photosynthesis on Irradiance. J. Exp. Bot. 1980, 31, 29–39. [Google Scholar] [CrossRef]
- Casal, J.J. Shade Avoidance. Arab. Book 2012, 10, e0157. [Google Scholar] [CrossRef]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Muszyńska, E.; Gajewski, Z.; Mazur, S.; Kunicki, E.; Jeremiasz, O.; Sobik, P.; Nowak, P.; et al. Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study. Cells 2023, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Runkle, E.S. Blue Radiation Attenuates the Effects of the Red to Far-Red Ratio on Extension Growth but Not on Flowering. Environ. Exp. Bot. 2019, 168, 103871. [Google Scholar] [CrossRef]
- Craver, J.K.; Nemali, K.S.; Lopez, R.G. Acclimation of Growth and Photosynthesis in Petunia Seedlings Exposed to High-Intensity Blue Radiation. J. Am. Soc. Hortic. Sci. 2020, 145, 152–161. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-Day Plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, E.M.F.M.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–104. [Google Scholar] [CrossRef]
- Dorais, M. The Use of Supplemental Lighting for Vegetable Crop Production: Light Intensity, Crop Response, Nutrition, Crop Management, Cultural Practices. In Proceedings of the Canadian Greenhouse Conference, Niagara Falls, ON, Canada, 9 October 2003; Volume 9, pp. 115–133. [Google Scholar]
- Faust, J.E.; Logan, J. Daily Light Integral: A Research Review and High-Resolution Maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Albright, L.D.; Both, A.J.; Chiu, A.J. Controlling greenhouse light to a consistent daily integral. Trans. ASAE 2000, 43, 421–431. [Google Scholar] [CrossRef]
- Masabni, J.; Sun, Y.; Niu, G.; Del Valle, P. Shade Effect on Growth and Productivity of Tomato and Chili Pepper. HortTechnology 2016, 26, 344–350. [Google Scholar] [CrossRef]
- Gould, K.S. Nature′s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. BioMed Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Klouvatou, A.; Vekkos, K.-A.; Manetas, Y.; Karabourniotis, G. The Photoprotective Role of Epidermal Anthocyanins and Surface Pubescence in Young Leaves of Grapevine (Vitis Vinifera). Ann. Bot. 2006, 98, 257–265. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Battey, N.H.; Hadley, P. The Influence of Ultraviolet Radiation on Growth, Photosynthesis and Phenolic Levels of Green and Red Lettuce: Potential for Exploiting Effects of Ultraviolet Radiation in a Production System. Ann. App. Biol. 2010, 156, 357–366. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E.; Torre, S. A High Proportion of Blue Light Increases the Photosynthesis Capacity and Leaf Formation Rate of Rosa × Hybrida but Does Not Affect Time to Flower Opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of Blue and Red Light in Stomatal Dynamic Behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/Blue Light Ratios Induce Morphology and Physiology Alterations Differently in Cucumber and Tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.B.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How Plant Architecture Affects Light Absorption and Photosynthesis in Tomato: Towards an Ideotype for Plant Architecture Using a Functional–Structural Plant Model. Ann. Bot. 2011, 108, 1065–1073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stallknecht, E.J.; Runkle, E.S. Towards Understanding the Promotion of Plant Growth Under an Experimental Red-Fluorescent Plastic Film. Horticulturae 2025, 11, 980. https://doi.org/10.3390/horticulturae11080980
Stallknecht EJ, Runkle ES. Towards Understanding the Promotion of Plant Growth Under an Experimental Red-Fluorescent Plastic Film. Horticulturae. 2025; 11(8):980. https://doi.org/10.3390/horticulturae11080980
Chicago/Turabian StyleStallknecht, Eric J., and Erik S. Runkle. 2025. "Towards Understanding the Promotion of Plant Growth Under an Experimental Red-Fluorescent Plastic Film" Horticulturae 11, no. 8: 980. https://doi.org/10.3390/horticulturae11080980
APA StyleStallknecht, E. J., & Runkle, E. S. (2025). Towards Understanding the Promotion of Plant Growth Under an Experimental Red-Fluorescent Plastic Film. Horticulturae, 11(8), 980. https://doi.org/10.3390/horticulturae11080980








