Abstract
Chinese water chestnuts (CWCs) have high nutritional value and are important in the field of food and medicine. However, there may be significant differences between CWCs from different regions. This study focused on the differences in nutritional quality and functional active substance content of CWCs from nine different origins in China and established an evaluation method to assess these differences. It was found that the total phenolic and total flavonoid contents of CWCs peel were much higher than those of pulp, with the peel of CWCs from origin II having the highest total phenolic content and a high DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical scavenging rate. The starch content of the pulp of CWCs from origin V was significantly higher than that in the other origins, and the peel from origin IX and the pulp from origin IV had the highest soluble sugar levels. Principal component analysis (PCA) revealed that the peel quality was primarily affected by the browning degree, soluble solids, and antioxidant capacity, while the pulp exhibited a stronger correlation with total flavonoids and protein. The comprehensive evaluation demonstrated that origin I, origin VII, and origin IX were relatively high-quality peel resources, while origin II, origin V, and origin VIII had better pulp quality than the other groups. This study aimed to elucidate the differences between various production areas of CWCs and provide a reference for the deep processing of CWCs.
1. Introduction
Chinese water chestnuts (CWCs, Eleochairis toberosa) are perennial aquatic herbaceous plants of the genus CWCs in the Salix family. They are a type of aquatic vegetable with high nutritional value, containing many functionally active components, such as proteins, fats, crude fibers, flavonoids, and sterols, among others, and they are cultivated all over the world [1]. CWCs are mainly grown in southern Chinese regions such as Guangxi, Hubei, and Guangdong provinces [2]. They are popular among Chinese people due to their sweet taste, crunchy texture, and high nutritional value [3]. In traditional Chinese medicine, CWCs were considered a good food for treating coughs, pharyngitis, and high blood pressure [4]. Additionally, the starch in CWCs has been extracted for use as a natural thickener or stabilizer in the food industry [5].
The peel of CWCs is rich in phenols, flavonoids, polysaccharides, phenolic glycosides, and other active ingredients, with antibacterial, antioxidant, antitumor, immunomodulation, nitrite scavenging, and acrylamide formation inhibition, among other active functions [6,7,8,9,10]. It can be used to extract a variety of active substances, including brown pigment and polysaccharides. It is clinically used for phlegm-heat cough, sore throat, and dysuria, among other conditions [11]. The processing industry of CWCs has undergone significant development in recent years, and a variety of deep-processed products, including canned CWCs, have been emerging. Fresh-cut CWCs are popular because of their distinctive flavor, and canned CWCs are sold all over the world [12]. CWCs peel, a by-product of CWCs processing, is rich in flavonoids and polyphenols and exhibits potent antioxidant and acrylamide-forming properties [13]. Most CWCs peels are typically discarded after CWC production, with only a small portion being used as livestock feed, rendering them a waste product that is difficult to recycle in the environment. Consequently, strengthening the development and utilization of CWCs peel resources can effectively enhance the economic benefits of the CWCs industry.
CWCs germplasm resources from different regions of China are being collected, identified, and classified to construct a database of genetic diversity of CWCs and analyze it based on molecular marker technology. This helps provide a scientific basis for the conservation breeding and genetic improvement of CWCs, in addition to having practical significance for the protection and utilization of agricultural genetic resources in China [14]. Wu Peng et al. [15] compared the sensory scores and primary nutrients of different varieties of CWCs, and found that Guilin CWCs and Xiaogan CWCs varieties differed in terms of moisture, starch, total soluble sugar, protein, carbohydrate, and calories. They concluded that Guangdong CWCs had the best overall quality, as they were sweet and juicy in the mouth, had high contents of moisture and total sugar, and simultaneously had relatively high levels of starch, protein, and carbohydrates, which imparted high food value. Wang et al. [16] investigated the effects of variety, transplanting density, and potash on the yield and soluble solids content of CWCs and revealed that the variety of CWCs had the most significant effect on these parameters.
In recent years, a number of studies have focused on differences in the same crop in different varieties or under different growing conditions. Curi et al. [17] showed that different varieties of figs differed in antioxidant activity and content of bioactive compounds and significantly affected the color of fig jelly, which in turn had an impact on the consumer acceptance of the final product. Tian et al. [18] showed that different varieties of prunes significantly affected the processing characteristics (e.g., juice yield, sugar-acid ratio), phenolic content, and sensory quality of prune juice and clearly screened Wandao and Biqi as the optimal processing varieties. Müller et al. [19] systematically evaluated the differences in nutrient composition of 15 different hazelnut varieties grown in Germany, demonstrated that variety is a key determinant of hazelnut nutritional quality, and screened five potential varieties for subsequent long-term studies.
Previously, there were also studies on the differences between CWCs from different origins or different varieties, but the studies were only limited to the differences in the nutrient composition of CWCs and did not pay much attention to the functional active substance composition and did not screen out CWCs that were suitable for processing or with high functional active compositions. Therefore, a comprehensive analysis of the functional activities and nutrients of CWCs from different origins or varieties is necessary.
This study utilized CWCs grown in Wuhu, Anhui Province; Hefei, Anhui Province; Yichang, Hubei Province; Enshi, Hubei Province; Ganzhou, Jiangxi Province; Shaoguan, Guangdong Province; Nanning, Guangxi Province; Hezhou, Guangxi Province; and Guilin, Guangxi Province as the subjects. This study aimed to evaluate the differences in appearance (color, degree of browning, and soluble quinone content), texture, nutrients (total soluble solids, moisture content, soluble protein, soluble sugar, starch, and vitamin C (VC), functionally active constituents (total phenolics and total flavonoids), and antioxidant capacity (DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical scavenging rate) among the different places of origin and to establish a scientifically effective evaluation method. This assessment method will help to fully understand the quality of CWCs from a scientific point of view and provide methods to provide strategies for the further processing of CWCs.
2. Materials and Methods
2.1. Plant Material and Reagents
Materials: CWCs are planted around July each year, the CWCs bulb begins to expand around October, and harvesting begins around November, and the harvesting period can last until April of the following year. The raw materials used in this experiment were collected from local farmers in each production area to ensure the accuracy of the sample source. CWCs grown in Wuhu, Anhui Province, Hefei, Anhui Province, Yichang, Hubei Province, Enshi, Hubei Province, Ganzhou, Jiangxi Province, Shaoguan, Guangdong Province, Nanning, Guangxi Province, Hezhou, Guangxi Province, and Guilin, Guangxi Province were procured for this study. CWCs from various locations were transported to the laboratory via room temperature for 1–2 days. The acquired CWCs were obtained after removing any individuals with mechanical damage, deterioration, or severe decay. Samples of relatively uniform size were selected and stored at 4 °C for 24 h for subsequent index determination. The selected CWCs were washed, surface sediment was removed, and the 1–2 mm thick surface of the CWCs was peeled off as the peel (the outer reddish-brown portion of the CWCs), while the remaining portion (the inner rice-white portion) was tested as the pulp.
Table 1 displays the CWCs for each origin, along with the corresponding numbers in the images used in this study.

Table 1.
The origin of CWCs (Chinese water chestnuts) and their corresponding number.
Experimental reagents: analytical pure: anhydrous ethanol, foraminol, gallic acid, rutin, anhydrous sodium dihydrogen phosphate, anhydrous disodium hydrogen phosphate, anhydrous sodium carbonate, glacial acetic acid, sodium hydroxide, sodium nitrite, aluminum nitrate, DPPH standard, phenol, concentrated sulfuric acid; Chromatographic purity: methanol; Detection kits: Vitamin C (VC)/Ascorbic Acid Determination Kit (NanJing JianCheng Bioengineering Institute, Nanjing, China), Protein Quantification (TP) determination kit (NanJing JianCheng Bioengineering Institute, Nanjing, China), and starch content detection kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China).
2.2. Methods
2.2.1. Appearance Quality of CWCs
Three CWCs were randomly selected and photographed in a small studio (80 × 80 × 80 cm) using a Canon camera (Canon EOS 550D) [20].
2.2.2. Color Measurement
A colorimeter was used to determine the surface color difference values (L*, a*, and b*) of the CWCs’ peel and pulp, respectively, referring to the method described by Li et al. [11]. The color measurement was determined immediately after the end of 24 h of storage at 4 °C.
2.2.3. Measurement of Moisture Content
The moisture content was measured using the direct drying method as specified in GB 5009.3-2016, “Determination of Moisture in Foods, National Standard for Food Safety.” 3 g of chopped CWCs tissue was placed in a drying dish and dried at 105 °C until constant weight (the difference in mass between the two times before and after was not more than 2 mg), and it was calculated according to the calculation method in GB 5009.3-2016, “Determination of Moisture in Foods, National Standard for Food Safety.”, and the moisture content was expressed as %.
2.2.4. Measurement of Soluble Solids Content
The soluble solids content was determined according to the method described by Xu et al. [21]. 10 g of CWCs tissue was ground in a mortar and pestle, the ground sample was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken for determination using a digital saccharimeter (Abbey refractometer), and the soluble solids content was expressed as %.
2.2.5. Measurement of Total Flavonoid Content
The total flavonoid content was determined using the method described by Chen et al. [22]. The CWCs tissue was mixed with 60% ethanol at a ratio of 1:10 (w/v), homogenized for 2 min at 10,000 r/min under the condition of an ice bath, and then centrifuged at 10,000 r/min for 10 min at low temperature, and the supernatant was collected and set aside. To 2 mL of supernatant, 0.15 mL of 5% NaNO2 was added, mixed, and left to stand for 6 min, then 0.15 mL of 10% AlNO3 was added, mixed, and left to stand for 6 min. Finally, 2 mL of 1 mmol/L NaOH was added to the reaction system, and the solution was fixed to 5 mL with 80% ethanol, mixed, and left to stand for 15 min. The absorbance was measured at 510 nm and zeroed with 80% ethanol. The total flavonoid content was calibrated with rutin, and the total phenol content was expressed as mg/100 g.
2.2.6. Measurement of Total Phenol Content
The total phenol content of CWCs was determined using the method described by Min et al. [23]. The CWCs tissue was mixed with 60% ethanol solution in the ratio of 1:10 (w/v), homogenized at 10,000 r/min for 3 min under ice bath conditions, followed by low-temperature centrifugation at 10,000 r/min for 10 min, and the supernatant was collected and set aside. The supernatant was collected and set aside. 0.25 mL of supernatant and 1 mL of distilled water (1.25 mL of distilled water for control) were pipetted into a 10 mL centrifuge tube, and 0.25 mL of forchlorfenuron was added, mixed well, and reacted at room temperature for 6 min. 2.5 mL of 7% Na2CO3 and 2 mL of distilled water were added to the tube, and the reaction was carried out at 25 °C and protected from light for 90 min. The absorbance of the reaction system was measured at 760 nm. Gallic acid was used to calibrate the total phenol content, and the total phenol content was expressed as mg/100 g.
2.2.7. Measurement of Soluble Quinone Content
The soluble quinone content was determined according to the method of Wang et al. [24]. The CWCs tissue was homogenized in an ice bath, and the supernatant was collected after centrifugation. The absorbance was measured at 437 nm, and the soluble quinone content was expressed as A437nm/g.
2.2.8. Measurement of Browning
The browning degree of CWCs was determined using the method described by Min et al. [23]. The CWC tissues were homogenized in an ice bath, and the supernatant was collected after centrifugation. The supernatant was placed in a water bath at 25 °C for 5 min. The absorbance was measured at 410 nm, and the browning degree was expressed as A410nm × 10.
2.2.9. Measurement of DPPH Free Radical Scavenging Rate
The DPPH radical scavenging rate was determined using the method described by Yi et al. [25] with slight modifications. CWCs tissue was mixed with anhydrous ethanol in the ratio of 2:25 (w/v), homogenized for 1 min at 10,000 r/min in an ice bath, and then sonicated for 30 min at 50 °C, followed by centrifugation at a low temperature of 10,000 r/min for 10 min. The absorbance of the samples was measured at 517 nm, and the samples were adjusted to zero using anhydrous ethanol. The results are expressed as %.
2.2.10. Measurement of VC Content
VC content was determined using the VC/ascorbic acid assay kit (NanJing JianCheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions. Samples were homogenized with phosphate buffer (0.1 mol/L, pH = 7.0) at a ratio of 1:9 under ice bath conditions. After centrifugation at 4 °C for 10 min at 10,000 r/min, the supernatant was saved for later use. The absorbance of each sample was measured at 536 nm and the results were expressed as µg/g.
2.2.11. Measurement of Starch Content
The starch content was determined using a starch content assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The results are expressed as mg/g.
2.2.12. Measurement of Protein Content
Protein content was determined using the quantitative protein assay kit (NanJing JianCheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s instructions. Samples were homogenized with phosphate buffer (0.1 mol/L, pH = 7.0) at a ratio of 1:9 under ice bath conditions. After centrifugation at 4 °C for 10 min at 10,000 r/min, the supernatant was saved for later use. The absorbance of each sample was measured at 595 nm, and the results were expressed as mg/g.
2.2.13. Measurement of Soluble Sugar Content
The soluble sugar content was determined by using the method described by Yang et al. [26], which was slightly modified by calibrating the soluble sugar content with anhydrous glucose. Add 3 g of CWCs tissue to 30 mL of distilled water, homogenize at 10,000 r/min for 3 min under the condition of an ice bath, and then put into a boiling water bath to extract for 30 min, followed by centrifugation at 10,000 r/min for 5 min at low temperature, and take the supernatant for spare. To the residue, 10 mL of distilled water was added and extracted in a boiling water bath for 10 min, followed by centrifugation at 10,000 r/min for 5 min at low temperature, and the supernatant was taken. Combine the two supernatants and transfer to a volumetric flask to 50 mL. 0.05 mL of the supernatant, add 1.95 mL of distilled water (2 mL of distilled water for blank) and 1 mL of 9% phenol, add 5 mL of concentrated sulfuric acid within 5–20 s and mix, let stand for 30 min, and then measure the absorbance at 485 nm. The results are expressed as ‰.
2.2.14. Measurement of Hardness and Brittleness
A previously reported method was used to determine the hardness and brittleness [27]. The pulp of CWC was cut into cubes (approximately 1 × 1 × 1 cm). The texture analyzer was set to TPA mode, and the P/45 probe was used; the trigger force was set at 100 gf, and the velocities were as follows: initial velocity of 10.0 mm/s, compression velocity of 0.5 mm/s, and end rise velocity of 10.0 mm/s. A dwell time of 5 s was set between two compressions, and the maximum deformation was set to 35%. The results are expressed as gf.
2.2.15. Statistical Analyses
Three biological replicates were tested for each indicator, and each biological replicate contained at least three CWCs and data are expressed as mean ± standard error. The data were analyzed using Microsoft Excel 2016 (Microsoft Inc., Redmond, WA, USA) for basic data analysis. Origin 32-bit software (2024) was used for data visualization. Principal component analysis and one-way analysis of variance (ANOVA) using IBM SPSS Statistics (27.0), combined with Duncan’s multiple comparisons test to assess the differences between the groups, with a significance level of p < 0.05.
3. Results
3.1. Appearance of CWCs
The peel of CWCs was purplish-black, the pulp was white [15], and the pulp had a fine and uniform texture, while the peel had a more uneven color. The surface of the CWCs had some links, characterized by a short, blunt bud tip at the top and a shallower ring mark surrounding the bud tip. Supplementary Figure S1 shows images of the appearance of CWCs from nine different origins.
The L* value in the color difference measurement mainly reflects the degree of brightness and darkness of the color, the a* value indicates the degree of offset of the color in the red-green direction, and the b* value represents the degree of offset of the color in the yellow-blue direction. The color difference between the peel and pulp of CWCs is displayed in Table 2 and Table 3, respectively. Since the peel of CWCs was darker in color, the L* values were smaller and ranged from 23.33 to 27.41. Moreover, due to the inconsistency in the color of CWCs peel of various origins, the a* and b* values also varied, with the a* values roughly distributed between 8.17 and 18.08, and the b* values roughly distributed between 1.36 and 3.60. The L* value of CWC’s pulp was larger (80.82–85.41), the a* value was roughly distributed between 2.46 and 3.42, and the b* value was roughly distributed between 10.02 and 14.13. Genetic and environmental variables may play a role in the color variances between the peel and pulp of CWCs.

Table 2.
Color difference values of CWCs peel.

Table 3.
Color difference values of CWCs pulp.
3.2. Moisture Content
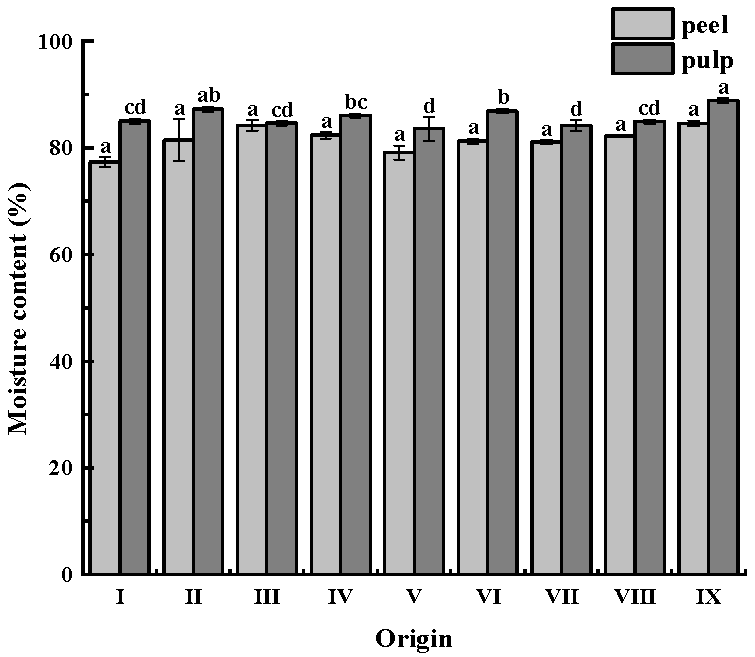
The moisture content of CWCs is usually high, accounting for about 80–90% of their total weight. The moisture content of CWCs is displayed in Figure 1. The moisture content of CWCs peel (77.36–84.22%) was lower than that of CWCs pulp (83.60–88.88%), and there was no significant difference between the moisture content of CWCs peel (p > 0.05). The lower water content of the CWCs peel was related to its physiological function of directly contacting the outside world and assuming protection of internal tissues. In contrast, CWCs pulp mainly stored nutrients and water, and its water content was higher.
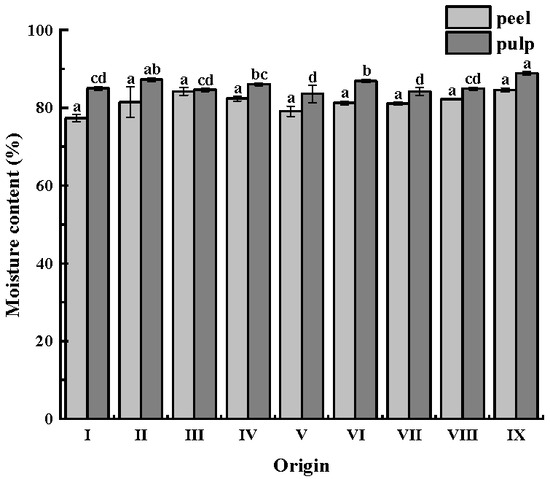
Figure 1.
Moisture content of CWCs. Different letters such as a, b, c, etc. indicate significant differences between different origins of the same part (p < 0.05).
3.3. Soluble Solids Content
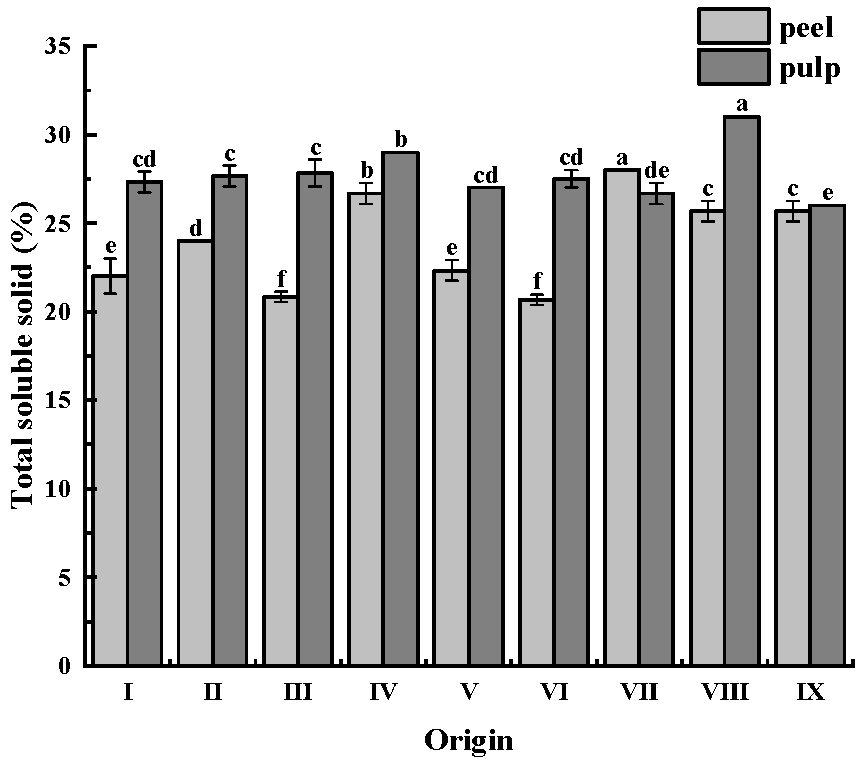
The soluble solids in CWCs mainly include sugars (such as sucrose, glucose, and fructose, among others), starch, organic acids (including citric acid and malic acid, among others), minerals (such as ionic forms of potassium, sodium, calcium, magnesium, and others), vitamins (including VC and vitamin B, among others), amino acids, and a few other water-soluble small molecules. These soluble solids have a significant effect on the taste, flavor, and nutritional value of CWCs. Soluble solids content is an important indicator for evaluating the freshness and ripeness of fruits and vegetables [28].
The soluble solids content of CWCs is displayed in Figure 2. The soluble solids content of the CWCs peel ranged from 20.67% to 26.67%, while that of the CWCs pulp was higher than that of CWCs peel, ranging from 26.00% to 31.00%. The highest soluble solids content was found in CWCs peel from origin VII (28.00%) and in CWCs pulp from origin VIII (31.00%).
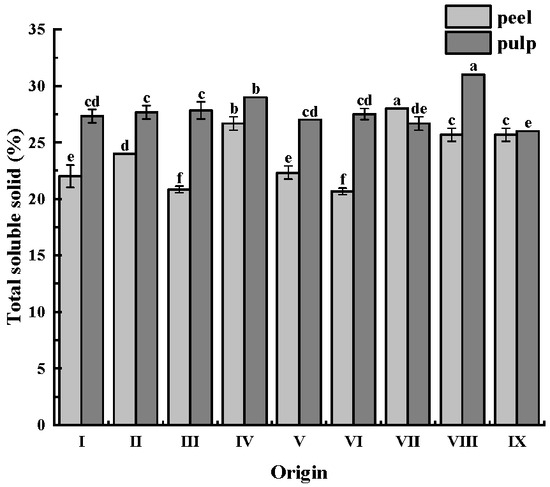
Figure 2.
Soluble solids content of CWCs. Different letters such as a, b, c, etc. indicate significant differences between different origins of the same part (p < 0.05).
3.4. Hardness and Brittleness
According to previous studies, the textural characteristics of food products have a significant impact on consumer choice [29]. Table 4 indicates that the hardness of CWCs was slightly greater than their crispness, except for CWCs from origin I, whose hardness was consistent with their crispness.

Table 4.
Measurement of the hardness and brittleness of CWCs pulp.
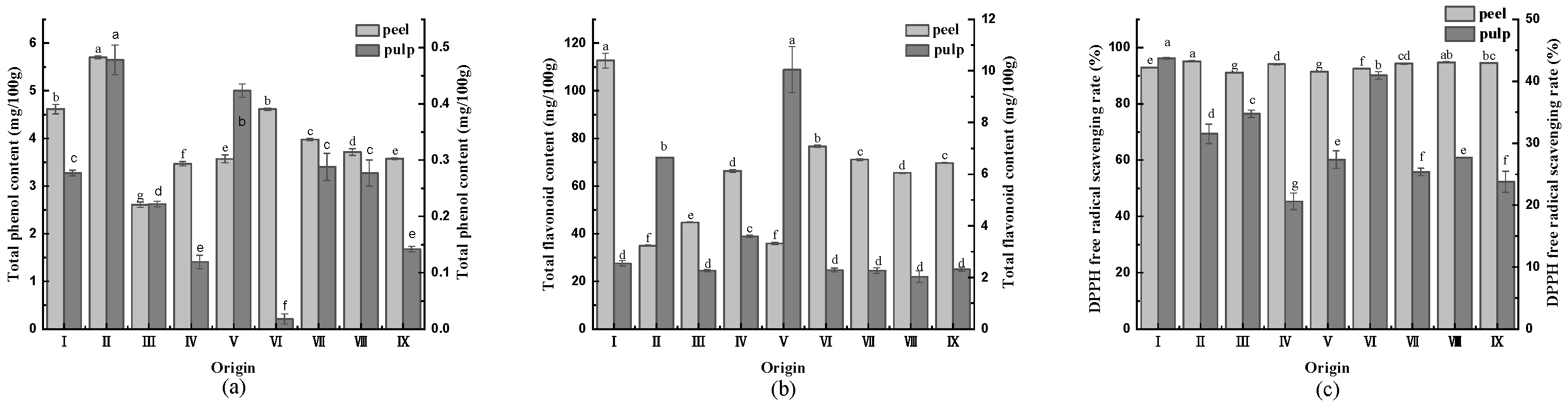
3.5. Total Phenolic and Flavonoid Contents, and DPPH Radical Scavenging Rate
Phenols are essential substrates for the enzymatic browning of vegetables and fruits and also have antioxidant properties [30]. The total phenolic content of CWCs is illustrated in Figure 3a. The total phenolic content of the CWCs peel was approximately much higher than that of the pulp. The peel of CWCs in origin II (5.701 mg/100 g) had the highest total phenolic content, and that in origin III (2.608 mg/100 g) was the lowest, with a difference of 2.18 times. Additionally, the pulp of CWCs in origin II (0.478 mg/100 g) had the highest total phenolic content, and the pulp in origin VI (0.018 mg/100 g) had the lowest, with a difference of 26.56 times.
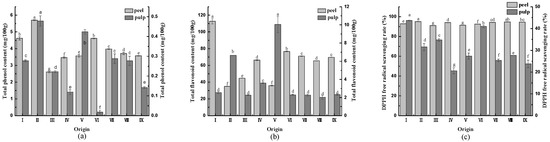
Figure 3.
(a) Total phenol content of CWCs; (b) total flavonoid content of CWCs; (c) DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical scavenging rate of CWCs. Different letters such as a, b, c, etc. indicate significant differences between different origins of the same part (p < 0.05).
The total flavonoid content of CWCs is displayed in Figure 3b. The total flavonoid content of the CWC peel was higher than that of the fruit pulp. The highest total flavonoid content was found in the peel of origin I (112.714 mg/100 g), which was 3.22-fold higher than the lowest. Moreover, the highest total flavonoid content in fruit pulp was in the origin V (10.048 mg/100 g), which was 4.96-fold higher than the lowest.
The DPPH radical scavenging rate is an indicator of the antioxidant capacity of fruits and vegetables [22]. As illustrated in Figure 3c, the DPPH radical scavenging rates of CWC peel were all higher than 90%. Origin II had the highest DPPH radical scavenging rate (95.01%). It ranged from 20% to 45% in the pulp of CWCs. The origin with the highest DPPH radical scavenging rate was origin I (43.72%).
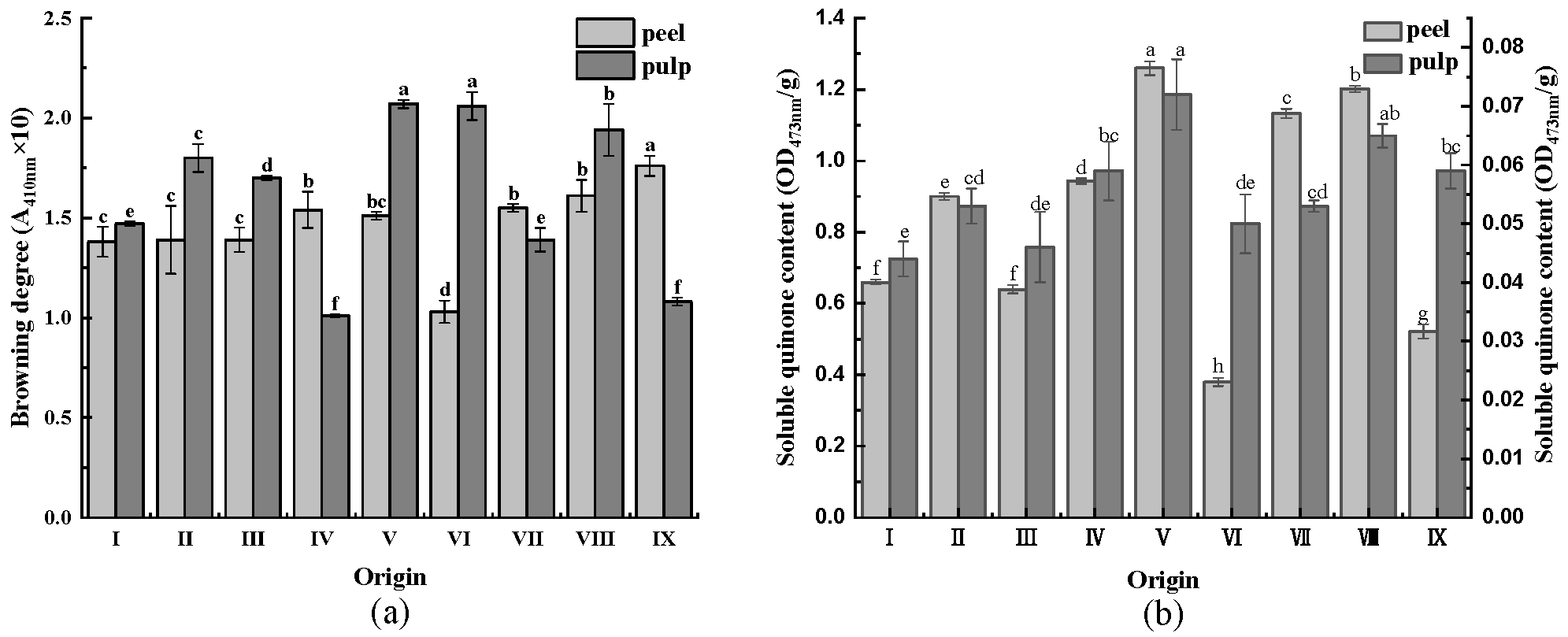
3.6. Browning Degree and Soluble Quinone Content
The degree of browning is one of the most important indicators for evaluating the quality of fruits and vegetables [31]. As displayed in Figure 4a, the browning degree of most CWC pulp was higher than that of the peel, which can be attributed to the high total phenolic and flavonoid contents of CWC peel, which exerted antioxidant effects and reduced the browning degree of the CWC peel.
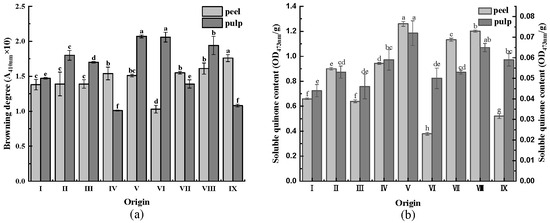
Figure 4.
(a) Browning degree of CWCs; (b) soluble quinone content of CWCs. Different letters such as a, b, c, etc. indicate significant differences between different origins of the same part (p < 0.05).
The primary reason for the formation of tanning pigments on the surface of fruits and vegetables is the oxidation of phenolics to quinones, which are the products of phenolic oxidation in the enzymatic browning reaction [32]. The content of soluble quinone gradually increases during the storage period of fruits and vegetables. Figure 4b illustrates that the soluble quinone content of CWCs peel was higher than that of CWCs pulp. Additionally, the origin V had the highest soluble quinone content in CWCs peel and pulp, consisting of 1.26 OD473nm/g in the peel and 0.072 OD473nm/g in the pulp.
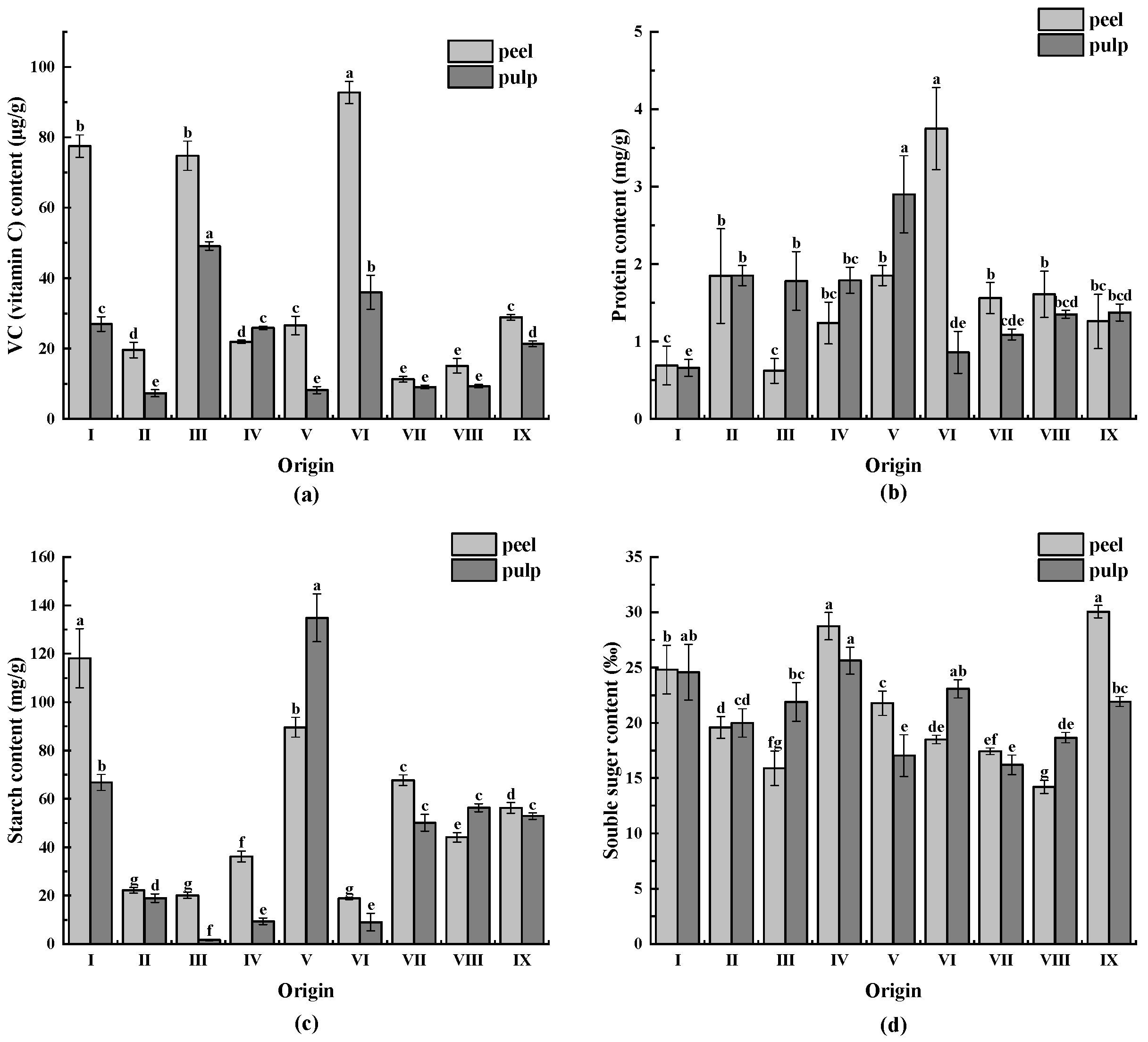
3.7. VC, Protein, Starch, and Soluble Sugar Contents
As illustrated in Figure 5a, the VC content of CWCs varied significantly between different origins. The VC content of CWCs peel ranged from 11.25 to 92.77 µg/g, with origin VI having the highest VC content (92.77 µg/g) and origin VII having the lowest VC content (11.25 µg/g), representing an 8.25-fold difference. The highest VC content in CWCs pulp ranged from 7.29 to 49.15 µg/g in origin III (49.15 µg/g), and the lowest VC content was in origin II (7.29 µg/g), resulting in a 6.74-fold difference between the two. The VC content of CWCs peel and pulp differed significantly, with the peel having a higher VC content.
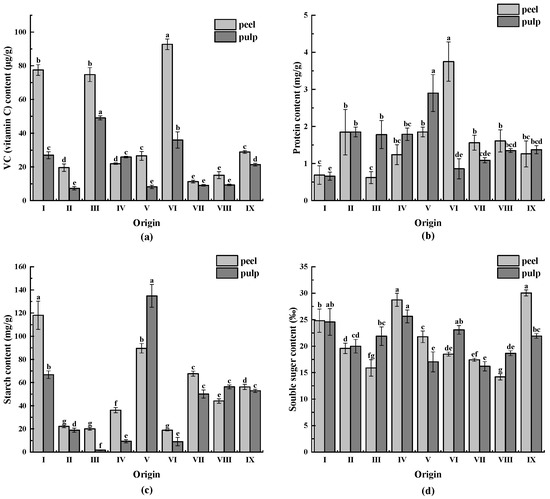
Figure 5.
(a) VC (vitamin C) content of CWCs; (b) protein content of CWCs; (c) starch content of CWCs; (d) soluble sugar content of CWCs. Different letters such as a, b, c, etc. indicate significant differences between different origins of the same part (p < 0.05).
As demonstrated in Figure 5b, the highest protein content in CWCs peel was found in origin VI (3.75 mg/g), and the origin with the lowest protein content was origin III (0.62 mg/g), with a difference of 6.05 times. Moreover, the origin with the highest protein content in CWC pulp was V (2.90 mg/g), and the origin with the lowest protein content was origin I (0.66 mg/g), with a difference of 4.39 times. There was no major difference between the protein content of CWCs peel and pulp from different origins, except for origin VI.
The starch content of CWCs varied significantly among different origins, as illustrated in Figure 5c. The starch content of CWCs peel ranged from 18.86 to 118.83 mg/g, with the origin with the highest starch content being origin I (118.83 mg/g), which was 6.3-fold higher than the lowest. The starch content of CWCs pulp ranged from 1.65 to 134.84 mg/g, with the origin V having the highest starch content (134.84 mg/g), which was 81.72-fold higher than the lowest. Starch is the main carbohydrate in sugarcane bulbs.
The soluble sugar content of CWCs peels ranged from 14.21 ‰ to 30.04 ‰, with the peel from origin IX having the highest and origin VIII having the lowest soluble sugar content, resulting in a 2.12-fold difference. Similarly, the soluble sugar content of CWCs pulp ranged from 16.20 ‰ to 25.63 ‰, with the pulp from origin IV having the highest content and pulp from origin VII having the lowest, resulting in a difference of 1.58-fold. This is illustrated in Figure 5d.
3.8. PCA (Principal Component Analysis)
PCA can simplify multiple related variables into a few comprehensive indicators [33], which can reflect the essential components and representational characteristics of the samples [34]. Its advantage is that it can explain most of the information with less data, thus downscaling a large amount of data and significantly reducing its complexity. PCA has been widely used in the food industry. Yue et al. [35] used PCA to evaluate the quality of turnip fleshy roots. Wen et al. [36] used PCA to analyze the comprehensive traits of 25 varieties of chili peppers and found six advantageous varieties. Suo et al. [37] explored the differences in flavor substances during the alcoholic fermentation of “Ziqiu” wine using PCA. Consequently, PCA can be used to effectively analyze the strengths and weaknesses of CWCs from various origins and identify high-quality CWCs resources.
PCA was used to analyze the 13 indicators of CWCs peel and pulp from nine origins, and each one produced six principal components. The eigenvalues, contribution rates, and cumulative contribution rates of each principal component of CWCs peel and pulp are displayed in Table 5 and Table 6, respectively.

Table 5.
Variance eigenvalues, contribution rate, and loading matrix values of principal components of CWCs peel from different origins.

Table 6.
Variance eigenvalues, contribution rate, and loading matrix values of principal components of CWCs pulp from different origins.
According to Table 5, the first principal component was primarily associated with the browning degree, soluble solids content, and DPPH radical scavenging rate. The second principal component was mainly related to total phenol and protein contents, while the third principal component was primarily related to total flavonoid content. Data processing of the principal component factor scores using factor composite scores. According to Table 7, yielded the following formula:
where F1, F2, F3, and F are the first, second, third, and composite principal components, respectively, and X1–X6 represent browning degree, soluble solids content, DPPH radical scavenging rate, total phenolic content, protein content, and total flavonoid content, respectively.
F1 = 0.580X1 + 0.575X2 + 0.449X3 − 0.146X4 − 0.332X5 + 0.018X6
F2 = −0.182X1 + 0.196X2 + 0.517X3 + 0.663X4 + 0.439X5 + 0.173X6
F3 = −0.114X1 − 0.077X2 − 0.047X3 + 0.023X4 − 0.357X5 + 0.923X6
F = 0.457F1 + 0.338F2 + 0.205F3
F = 0.181X1 + 0.313X2 + 0.370X3 + 0.162X4 − 0.077X5 + 0.255X6

Table 7.
Score coefficients of PCA (Principal component analysis) of nutrients in the CWCs peel of different origins.
Table 6 demonstrates that the first principal component was primarily related to total flavonoid, protein, and total phenol contents. The second principal component was primarily related to the DPPH radical scavenging rate and browning degree, while the third principal component was mainly related to the soluble solids content. Data processing of the principal component factor scores using factor composite scores. According to Table 8, yielded the following formula:
where F1, F2, F3, and F are the first, second, third, and combined principal components, respectively, and X1–X6 represent total flavonoid, protein, total phenol contents, DPPH radical scavenging, browning degree, and soluble solids content, respectively.
F1 = 0.587X1 + 0.561X2 + 0.482X3 − 0.152X4 + 0.291X5 − 0.030X6
F2 = −0.006X1 − 0.100X2 − 0.050X3 + 0.709X4 + 0.674X5 + 0.176X6
F3 = −0.151X1 + 0.031X2 + 0.032X3 − 0.332X4 + 0.114X5 + 0.923X6
F = 0.496F1 + 0.289F2 + 0.215F3
F = 0.257X1 + 0.256X2 + 0.231X3 + 0.058X4 + 0.364X5 + 0.234X6

Table 8.
Score coefficients of PCA of nutrients in the CWCs pulp of different origins.
The score for each origin can be calculated using the formulae above. As displayed in Table 9, the three origins with higher scores were origin I, origin VII, and origin IX according to the evaluation method of CWCs peel. Similarly, origin II, origin V, and origin VIII were the three origins with higher scores according to the evaluation method of CWC pulp.

Table 9.
Scores of CWCs from different origins.
4. Discussion
This study identified that the peel and pulp of CWCs from nine origins differed in physicochemical indexes, functional active substance content, and nutrient content.
According to the dictionary of traditional Chinese medicine, the area between the peel and the pulp of CWCs is rich in CWCs puchiin [38], a class of complex mixtures of heat-resistant and antimicrobial activity. It contains phenolics, alkaloids, organic acids, saponins, and free flavonoids that have antimicrobial and anti-inflammatory effects [39]. In addition, CWCs contain a large number of phenolics and flavonoids, which have a wide range of development prospects in the fields of food production, nutraceutical processing, and biotechnology research and development. This study demonstrated that the content of functionally active substances in the peel of CWCs was much higher than that in the pulp. Total phenol and flavonoid contents of the peel ranged from 2.608 mg/100 g to 5.701 mg/100 g and 35.00 mg/100 g to 112.714 mg/100 g, respectively. In contrast, the pulp contained these contents at levels ranging from 0.018 mg/100 g to 0.478 mg/100 g and 2.024 mg/100 g to 10.048 mg/100 g, respectively. The total phenol content varied across different parts of the fruit. The study by Horas et al. [40] demonstrated that the phenolic content of bitter melon pulp was significantly higher than that of the internal tissues and seeds, with the seeds having the lowest phenolic content. The total phenolic content within the same origin depended on several factors, including environmental conditions, maturity, location, and soil conditions. It has been confirmed that the total flavonoid content of different parts of pomegranate varies at different stages of growth and development, and the highest total flavonoid content was found in its placenta part [41]. The CWCs of origin I and origin II had a relatively high content of total phenols and total flavonoids in the peel and the pulp, and the DPPH free radical scavenging rate of CWCs from these two origins was also relatively high, indicating that they contained a high content of functional active substances, strong antioxidant capacity, and a greater potential for the development of functional foods. Phenolic compounds possess a unique antioxidant capacity, enabling plants to adapt to adversity [3]. Meanwhile, previous studies confirmed that flavonoids and their extracts in CWC peels are excellent natural antioxidants [2]. The experiments on the antioxidant activity of flavonoids in CWCs peels revealed that flavonoids with 3′,4′-dihydroxy B-rings have potent antioxidant activity. The high DPPH radical scavenging rate of CWCs peel was attributed to its high total phenolic and flavonoid contents. This result is also in agreement with that of another study [41], which demonstrated that the antioxidant activity of all parts of pomegranate was positively correlated with the total phenolic, ankyrin, and flavonoid contents. The antioxidant activity of all parts of the pomegranate was significantly and positively correlated with total flavonoids, except for the peel. However, there are differences in secondary metabolites between different varieties of CWCs. Li et al. [42] compared the metabolic profiles of CWCs pericarp collected in different years and identified a total of 321 metabolites. Among them, 87 flavonoids, 25 phenylpropanoids, and 33 organic acids and their derivatives were identified, and there was a significant difference in the content of these metabolites between “Big CWCs” (a fresh variety) and “Little CWCs” (a starch variety) [43]. Consequently, the detection and analysis of functional active substances in CWCs from different origins and varieties are conducive to the systematic planning of CWCs resource utilization pathways, providing a reference for the development and utilization of CWCs resources.
The moisture content of CWC’s pulp was higher than that of the peel in all nine origins. The pulp is the main edible part and contains sufficient juice; therefore, its moisture content is higher. There was no significant difference in the moisture content of the peel. The moisture content of CWCs was higher in origin IX, which has sufficient water and more precipitation, both of which are favorable for the growth of CWCs. In addition, irrigation practices can affect the water content of CWCs. The water content of CWCs varies depending on the irrigation methods used when CWCs are planted. Zhou [44] demonstrated that CWCs with intermittent irrigation had a lower water content, which decreased by 1.43% compared to conventional irrigation, which might be the reason for the differences in water content of CWCs from different origins.
There were significant differences between the soluble solids content, hardness, and crispness of CWCs from different origins. The differences in soluble solids content may be caused by differences in origin and slight differences in cultivation methods in different regions. Previous studies have confirmed that different weather conditions cause variations in the soluble solids content during plant growth [45,46,47]. Moderate precipitation and sufficient light promote the accumulation of photosynthetic products and adequate synthesis of starch and sugar, resulting in high soluble solids content of CWCs, but strict water control is needed during the expansion of CWCs bulbs to avoid extremely high water content of the bulbs and soluble solids Insufficient accumulation. The reason for the superior soluble solids content of both the peel and the pulp of the fruit at origin IV may be related to the local climate of Enshi, Hubei. Enshi, Hubei, has abundant precipitation and sufficient light in the early stage of CWCs growth, providing better environmental conditions for the growth of CWCs. Meanwhile, local precipitation decreases in the late stage of CWCs growth and during the harvest period, which is favorable for the accumulation of soluble solids. Additionally, the differences in crop location and load lead to increasing differences in the soluble solids content during fruit development [48,49], and this may be the reason for the differences in the content of soluble solids between CWCs from different origins. The brittleness of CWCs is generally less than or equal to their hardness. This finding is consistent with the results of previous studies, where Lu et al. [50] revealed that the hardness of CWCs was slightly greater than their crispness, and both demonstrated a decreasing trend with increasing cooking time.
The VC data revealed that the CWC peel had a higher VC content than the fruit pulp. Some studies revealed that CWCs have a high VC content, and the pulp of CWCs contains six vitamins, with VC having the greatest concentration at 70.0 mg/kg. This is higher than its content in certain fruits, such as pears (50.0 mg/kg), apples (30.0 mg/kg), and grapes (40.0 mg/kg).
Regarding the protein content, the peel of origin VI contained 3.75 mg/g of protein, while the pulp contained 0.86 mg/g. Among all varieties, the difference in protein content between the peel and the pulp was the most significant in this place of origin, and the difference in protein content between the peel of this origin and the other varieties was also significant (p < 0.05). The results of past studies have shown that aspartic acid is the most abundant of the 11 amino acids found in CWC peels, and four of them are necessary for adults [51].
CWCs are rich in starch, protein, fat, crude fiber, carotene, and vitamins, as well as calcium, iron, phosphorus, and other nutrients [52]. Among these, CWCs are rich in starch, making them suitable as a raw material for starch extraction. In this study, it was found that the starch content of CWCs from different origins varied greatly, which may be due to the variations in planting irrigation methods [44] and variety selection. The starch content of CWCs peel ranges from 18.86 to 118.83 mg/g, and that of CWCs pulp ranges from 1.65 to 134.84 mg/g. The pulp of origin V had the highest mean value of starch content (134.84 mg/g), which was significantly different from that of the pulp of CWCs from other origins. Previous studies have also shown differences in the starch content of CWCs from different origins. Zhang et al. [51] demonstrated that the average starch content in CWCs was 65.7 g/kg, but there was a significant difference in starch content across origins (p < 0.05). Chen et al. [53], while investigating the choice of a new variety of starchy-type CWCs, identified that the starch content of CWCs from Panyu water hooves in Guangzhou was about 12% higher than that of CWCs from Panyu, Guangzhou. Currently, CWCs’ starch is primarily used in the production of horseshoe cake; however, some scholars have recently attempted to develop other applications of CWCs’ starch. Deng et al. [54] modified the CWCs starch, which has good emulsifying properties, and the emollient lotion prepared after addition has a fine texture and a good moisturizing effect. Wu et al. [55] added CWCs starch to the surimi products of silver carp, which significantly improved the gelatinization characteristics of silver carp surimi and obtained good gelatinous products. CWC’s starch exhibits excellent gel properties and can be used in food processing and other fields as a substitute for other expensive food gels or mixed with other food gels to enhance the colloidal properties of the products. In recent years, new varieties of starch-based CWCs have been cultivated to meet market demand. Consequently, finding high-quality CWC starch resources and having a comprehensive understanding of the starch content of CWCs at each place of origin are crucial for the deep processing of CWCs.
Carbohydrates found in fruits mainly include soluble and insoluble sugars; soluble sugar can determine the sweetness of fruits, while insoluble sugar is mainly starch that can be converted into soluble sugar during the ripening process of fruits [56]. Accordingly, soluble sugar content affects the taste of CWCs. This study found that the soluble sugar content in the peel of most varieties of CWCs was higher than that in the pulp. In addition, the peel from origin IX and the pulp from origin IV were remarkable in terms of soluble sugar content, and thus, they had superior sweetness and were more preferred by consumers. Meanwhile, soluble sugar is the main component of soluble solids. and its mechanism of influence by climate is similar to that of soluble solids, requiring abundant precipitation and sufficient sunlight in the early stage and strict water control in the late stage of growth. The climatic conditions in origin IV are sufficiently favorable to meet these conditions. The peel and pulp of origin IV had a high content of soluble sugar and also exhibited a more prominent soluble solids content, resulting in a sweet flavor and a high-quality option for freshly consumed CWCs. Wu Peng et al. [15] compared the nutrient composition of different varieties of CWCs and found that light-rich climatic conditions may be favorable for increasing the soluble sugar content of CWCs. Differences in the growth environment and light conditions of CWCs of various origins may contribute to the variations in their soluble sugar content. Meanwhile, previous studies have shown that there are seasonal changes in the carbohydrate composition of plant tissues, and the starch in CWCs is gradually converted to soluble sugar as maturity increases, and CWCs has the highest starch content in autumn and the lowest in winter [57]. Differences in CWCs harvesting time may also contribute to differences in soluble sugar content between CWC production areas. Meanwhile, irrigation methods may also affect the soluble sugar content of CWCs [44]. Clarifying the differences in soluble sugar content of CWCs from different origins will help accurately screen high-sugar-type fresh CWCs varieties, classify, and use them with high-amylose-type processed CWCs, thereby promoting the specialized and refined development of CWCs resources and enhancing their industrial value.
5. Conclusions
This study investigated the quality characteristics of CWCs from nine different regions in China, including basic indicators such as appearance, moisture content, soluble solids, and textural properties, as well as nutritional quality and functional bioactive compound content, such as total phenols, total flavonoids, DPPH radical scavenging activity, browning degree, soluble quinones, VC, protein, starch, and soluble sugars. The results indicated regional differences in the nutritional quality and functional bioactive compound content of CWCs.
Additionally, the total phenol and total flavonoid contents of CWCs peel were significantly higher than those of the pulp. The highest total phenol content in CWCs peel was from origin II, and the highest total phenol content in CWCs pulp was from origin II, with a difference of 11.93 times. The highest total flavonoid content in CWCs peel was from origin I, and the highest total flavonoid content in CWCs pulp was from origin V, with a difference of 11.22 times. Meanwhile, the fruit peel also exhibited high antioxidant capacity, particularly the peel of the origin II and the pulp of the origin I, which were exceptional in terms of antioxidant capacity. The starch content of origin V was 2.019–81.72 times higher than that of other origins. The soluble sugar content was highest in IX peel and IV pulp, which were 1.89 and 1.58 times higher than the lowest content, respectively. PCA revealed that the nutritional components of CWCs peel and pulp were mainly concentrated in different principal components. Comprehensive score assessment identified that the highest scores for peel were from origin I, origin VII, and origin IX, while the highest scores for pulp were from origin II, origin V, and origin VIII. This study provides a scientific basis for the selection and deep processing of CWCs’ origins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11080979/s1, Figure S1. Appearance pictures of CWCs from nine origins.
Author Contributions
Conceptualization, Y.W.; methodology Y.W. and T.M.; software, Y.Y.; validation, Y.W. and H.L.; formal analysis, Y.W. and Y.A.; investigation, Y.Y. and H.L.; resources, L.W. and T.M.; data curation, H.W. and T.M.; writing—original draft preparation, Y.W.; writing—review and editing, T.M., Y.Y. and H.W.; visualization, Y.A. and H.W.; supervision, L.W. and Y.Y.; project administration, Y.W.; funding acquisition, T.M., H.W. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Department of Hubei Province [2024BBB027].
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript. The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| CWCs | Chinese Water Chestnuts |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| PCA | Principal component analysis |
References
- Nie, H.; Chen, H.; Li, G.; Su, K.; Song, M.; Duan, Z.; Li, X.; Cao, X.; Huang, J.; Huang, S.; et al. Comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut processed with different methods. Food Chem. 2021, 335, 127662. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, X.; He, J.; Su, J.; Peng, L.; Wu, X.; Du, R.; Zhao, Q. Isolation, characterisation, and antioxidant activities of flavonoids from chufa (Eleocharis tuberosa) peels. Food Chem. 2014, 164, 30–35. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, W.; Murtaza, A.; Iqbal, A.; Li, J.; Zhang, J.; Li, J.; Kong, X.; Pan, S. Eugenol treatment delays the flesh browning of fresh-cut water chestnut (Eleocharis tuberosa) through regulating the metabolisms of phenolics and reactive oxygen species. Food Chem. X 2022, 14, 100307. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.-C.; Zhou, A.-M. Preliminary Study on Functional Component and Functional Activities of Waste Slurry Derived in Processing Water Chestnut Starch. Food Sci. 2006, 27, 251–256. [Google Scholar]
- Xiao, Y.; Mu, L.; Deng, Y.; Liu, Y.; Zhang, A.; Lao, Y.; Liu, W.; Wang, S.; Xia, X.; Li, Y. Study on the inhibition of intermittent warm water treatments on the browning of fresh-cut water chestnuts. J. Hubei Norm. Univ. (Nat. Sci.) 2023, 43, 18–25. [Google Scholar]
- You, Y.; Duan, X.; Wei, X.; Su, X.; Zhao, M.; Sun, J.; Ruenroengklin, N.; Jiang, Y. Identification of Major Phenolic Compounds of Chinese Water Chestnut and their Antioxidant Activity. Molecules 2007, 12, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Pan, L.; Tu, K.; Jiao, S. Antitumor, Antioxidant, and Nitrite Scavenging Effects of Chinese Water Chestnut (Eleocharis dulcis) Peel Flavonoids. J. Food Sci. 2016, 81, H2578–H2586. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Pan, L.; Mao, S.; Zhang, W.; Wei, Y.; Tu, K. Study on antibacterial properties and major bioactive constituents of Chinese water chestnut (Eleocharis dulcis) peels extracts/fractions. Eur. Food Res. Technol. 2014, 238, 789–796. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Nie, H.; Huang, S.; Li, X.; Gong, J.; Wu, F.; Yin, J.; Liao, Y.; Wu, S.; Luo, Y. Identification of compounds from chufa (Eleocharis dulcis) peels with inhibitory acrylamide formation activity. Rev. Bras. Farmacogn. 2019, 29, 483–487. [Google Scholar] [CrossRef]
- Li, F.; Hu, Y.; Shan, Y.; Liu, J.; Ding, X.; Duan, X.; Zeng, J.; Jiang, Y. Hydrogen-rich water maintains the color quality of fresh-cut Chinese water chestnut. Postharvest Biol. Technol. 2022, 183, 111743. [Google Scholar] [CrossRef]
- Song, M.; Wu, S.; Shuai, L.; Duan, Z.; Chen, Z.; Shang, F.; Fang, F. Effects of exogenous ascorbic acid and ferulic acid on the yellowing of fresh-cut Chinese water chestnut. Postharvest Biol. Technol. 2019, 148, 15–21. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; He, J.; Peng, L.; Wu, X.; Du, R.; Zhao, Q. Phenolic constituents and antioxidant activity of Eleocharis tuberosa peels. Nat. Prod. Res. 2013, 25, 1615–1620. [Google Scholar]
- Liu, X.; Meng, X. Progress Research on Resource Development and Utilization of Water Chestnut. Food Res. Dev. 2021, 42, 212–217. [Google Scholar] [CrossRef]
- Wu, P.; Chen, C.; Gao, Z.; Yang, T.; Cong, Y. Comparison of Nutritional Components of Different Varieties of Water Chestnuts. China Food Saf. Mag. 2017, 36, 123. [Google Scholar] [CrossRef]
- Wang, B.; Lai, X.; Chen, W.; Pan, X.; Huang, S.; Wang, J.; Kang, X. Effects of Variety, Transplanting Density and Potassium Fertilizeron Yield and Soluble Solid Content of Chufa (Eleocharis dulcis). Acta Agric. Jiangxi 2017, 29, 20–24. [Google Scholar] [CrossRef]
- Curi, P.N.; Albergaria, F.C.; Pio, R.; Schiassi, M.C.E.V.; Tavares, B.d.S.; Souza, V.R.d. Characterisation and jelly processing potential of different fig cultivars. Br. Food J. 2019, 121, 1686–1699. [Google Scholar] [CrossRef]
- Tian, J.; Cao, Y.; Chen, S.; Fang, Z.; Chen, J.; Liu, D.; Ye, X. Juices processing characteristics of Chinese bayberry from different cultivars. Food Sci. Nutr. 2019, 7, 404–411. [Google Scholar] [CrossRef]
- Müller, A.; Helms, U.; Rohrer, C.; Möhler, M.; Hellwig, F.; Glei, M.; Schwerdtle, T.; Lorkowski, S.; Dawczynski, C. Nutrient Composition of Different Hazelnut Cultivars Grown in Germany. Foods 2020, 9, 1596. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, J.; Chen, J.; Gong, J.; Peng, L.; Yi, Y.; Ai, Y.; Hou, W.; Wang, H.; Min, T. Melatonin maintains the storage quality of fresh-cut Chinese water chestnuts by regulating phenolic and reactive oxygen species metabolism. Food Qual. Saf. 2022, 6, fyac002. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, Y.; Chen, J.; Yi, Y.; Ai, Y.; Hou, W.; Wang, L.; Wang, H.; Min, T. Mechanisms of ethanol treatment on controlling browning in fresh-cut lotus roots. Sci. Hortic. 2023, 310, 111708. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Lu, Y.; Miao, Z.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Effect and mechanism of eugenol on storage quality of fresh-peeled Chinese water chestnuts. Front. Plant Sci. 2022, 13, 965723. [Google Scholar] [CrossRef]
- Min, T.; Xie, J.; Zheng, M.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Wang, H. The effect of different temperatures on browning incidence and phenol compound metabolism in fresh-cut lotus (Nelumbo nucifera G.) root. Postharvest Biol. Technol. 2017, 123, 69–76. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef]
- Yi, Y.; Han, M.M.; Huang, F.; Wang, L.M.; Min, T.; Wang, H.X. Effects of a Lysine-Involved Maillard Reaction on the Structure and In Vitro Activities of Polysaccharides from Longan Pulp. Molecules 2019, 24, 972. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, R.; Han, Y.; Wu, W.; Fang, X.; Mu, H.; Gao, H.; Chen, H. Critical taste substances and regulatory pathways of fresh lotus seed pulps at different ripeness stages. Postharvest Biol. Technol. 2023, 205, 112522. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Regulation and mechanism of ethylene treatment on storage quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices. Sci. Hortic. 2023, 313, 111900. [Google Scholar] [CrossRef]
- Li, J.L.; Sun, D.W.; Cheng, J.H. Recent Advances in Nondestructive Analytical Techniques for Determining the Total Soluble Solids in Fruits: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.; Guerrero, L.; Gratacós-Cubarsí, M.; Claret, A.; Argyris, J.; Garcia-Mas, J.; Hortós, M. Textural properties of different melon (Cucumis melo L.) fruit types: Sensory and physical-chemical evaluation. Sci. Hortic. 2016, 201, 46–56. [Google Scholar] [CrossRef]
- Teng, Y.; Murtaza, A.; Iqbal, A.; Fu, J.; Ali, S.W.; Iqbal, M.A.; Xu, X.; Pan, S.; Hu, W. Eugenol emulsions affect the browning processes, and microbial and chemical qualities of fresh-cut Chinese water chestnut. Food Biosci. 2020, 38, 100716. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, S.; Kang, S.; Qi, Y.; Zhu, Z.; Yie, L.; Chen, B.; Tong, J. Comprehensive evaluation of straw forage quality of different soybeanvarieties based on principal component analysis and membership function analysis. Feed. Res. 2025, 48, 143–148. [Google Scholar] [CrossRef]
- Wang, J. Microanalysis of the Application of Stoichiometry in Food Analysis. Mod. Food. 2020, 21, 184–186+190. [Google Scholar] [CrossRef]
- Yue, L.; Zhuang, H.; Maimait, Z.; Wang, J.; Mao, H.; Zhang, Y.; Yadikar, N.; Yu, M. Comprehensive evaluation of the texture quality of turnip succulent root based on principal component analysis and cluster analysis. Acta Agric. Zhejiangensis 2025, 37, 1057–1071. [Google Scholar]
- Wen, Y.; Xie, S.; Guo, Z.; Wei, Y.; Cao, L.; Chen, L.; Liu, K.; Wang, P.; Wang, J. Growth Adaptation of Pepper Varieties Based on Principal ComponentAnalysis and Cluster Analysis. China Fruit. Veg. 2025, 45, 41–47. [Google Scholar] [CrossRef]
- Suo, H.; Wang, J.; Bai, W. Analysis of Volatile Components Changes of ‘Ziqiu’ Grape WineDuring Alcoholic Fermentation. China Fruit. Veg. 2025, 45, 13–22. [Google Scholar]
- Zhang, Y.; Hu, Y.; Li, X. Study on nutritional ingredients and health care value of Chinese water chestnut. Technol. Pioneers 2013, 6, 198. [Google Scholar]
- Li, Z.; Shao, J. Research Progress on Bioactive Substances in Chinese Water Chestnut Peel. Food Nutr. China 2009, 6, 60–62. [Google Scholar]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total Phenolic Contents and Phenolic Acid Constituents in 4 Varieties of Bitter Melons (Momordica charantia) and Antioxidant Activities of their Extracts. J. Food Sci. 2006, 70, C275–C280. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Yu, G.; Pu, J.; Tian, K.; Tang, X.; Du, Y.; Wu, H.; Hu, J.; Luo, X.; et al. Comparative analysis of the phenolic contents and antioxidant activities of different parts of two pomegranate (Punica granatum L.) Cultivars: ‘Tunisia’ and ‘Qingpi’. Front. Plant Sci. 2023, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, S.; Li, X.; Luo, Y.; Nie, H. Identification of compounds from chufa (Eleocharis dulcis) peels by widely targeted metabolomics. Food Sci. Nutr. 2023, 11, 545–554. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Li, J.; Wang, A.; Le, Y. Water Vegetables Answer Farmers’ Questions (62-1): What are the Utilization Values of Chinese Water Chestnuts? J. Change Veg. 2023, 11, 46–49. [Google Scholar]
- Zhou, G. Effect of different condition of water on quality of Eleocharis tuberosa. Genom. Appl. Biol. 2000, 2, 94–96. [Google Scholar]
- Sugiura, T.; Ogawa, H.; Fukuda, N.; Moriguchi, T. Changes in the taste and textural attributes of apples in response to climate change. Sci. Rep. 2013, 3, 2418. [Google Scholar] [CrossRef]
- Warmund, M.; Starbuck, C.; Al-Khatib, K. Changes in fruit quality parameters of ‘Jonathan Rasa’ and ‘Delicious Flanagan’ apples in response to elevated temperatures. Trans. Kans. Acad. Sci. 2007, 110, 259–267. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Lannard, B. Dry matter content association with time of on-tree maturation, quality at harvest, and changes in quality after controlled atmosphere storage for ‘Royal Gala’ apples. Can. J. Plant Sci. 2021, 101, 98–106. [Google Scholar] [CrossRef]
- Meland, M. Effects of different crop loads and thinning times on yield, fruit quality, and return bloom in Malus × domestica Borkh. ‘Elstar’. J. Hortic. Sci. Biotechnol. 2009, 84, 117–121. [Google Scholar] [CrossRef]
- Anthony, B.; Serra, S.; Musacchi, S. Optimizing Crop Load for New Apple Cultivar: “WA38”. Agronomy 2019, 9, 107. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, S.; Jia, C.; Xu, Y.; Zhang, B.; Niu, M. Textural Properties of Chinese Water Chestnut (Eleocharis dulcis) during Steam Heating Treatment. Foods 2022, 11, 1175. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Hu, Z.; Yang, G.; Yu, X.; Chen, Q.; Zheng, L.; Yan, Z. Eleocharis dulcis corm: Phytochemicals, health benefits, processing and food products. J. Sci. Food Agric. 2022, 102, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, C.; Wang, Q.; Jiang, Z.; Jiang, K.; Luo, X.; Cui, H. The nutritional value of Chinese water chestnuts and the processing technology for canned fresh Chinese water chestnuts. Light. Ind. Sci. Technol. 2025, 41, 48–50+72. [Google Scholar]
- Chen, L.; Wen, J.; Cai, B.; Meng, P.; Bi, Z.; Zhai, P.; Gui, J.; Su, B.; Su, G.; Zhang, L.; et al. A Starch-based Water Chestnut Variety —‘Guifenti No 1′. China Veg. 2011, 2, 107–109. [Google Scholar] [CrossRef]
- Deng, C.; Chen, Z.; Su, H.; Wei, Y.; Duan, Z. Application study of Chinese water chestnut OSA modified starch in emollientotion. J. Hezhou Univ. 2019, 35, 145–149. [Google Scholar]
- Wu, P.; Gan, Q.; Chen, H.; He, R. The effect of radon starch on the quality of white herring products. J. Huanggang Norm. Univ. 2023, 43, 27–32. [Google Scholar]
- Tian, X.; Zhu, L.; Zou, H.; Li, B.; Ma, F.; Li, M. Research Progress on Accumulation Pattern and Regulation of SolubleSugar in Fruit. Acta Hortic. Sin. 2023, 50, 885–895. [Google Scholar] [CrossRef]
- Ashworth, E.N.; Stirm, V.E.; Volenec, J.J. Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L. Tree Physiol. 1993, 13, 379–388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).