Effects of Different Storage Conditions on Physiological, Biochemical, and Microbial Community Traits of Michelia macclurei Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Determination of Seed Water Content Rate, Viability, and Germination Rate
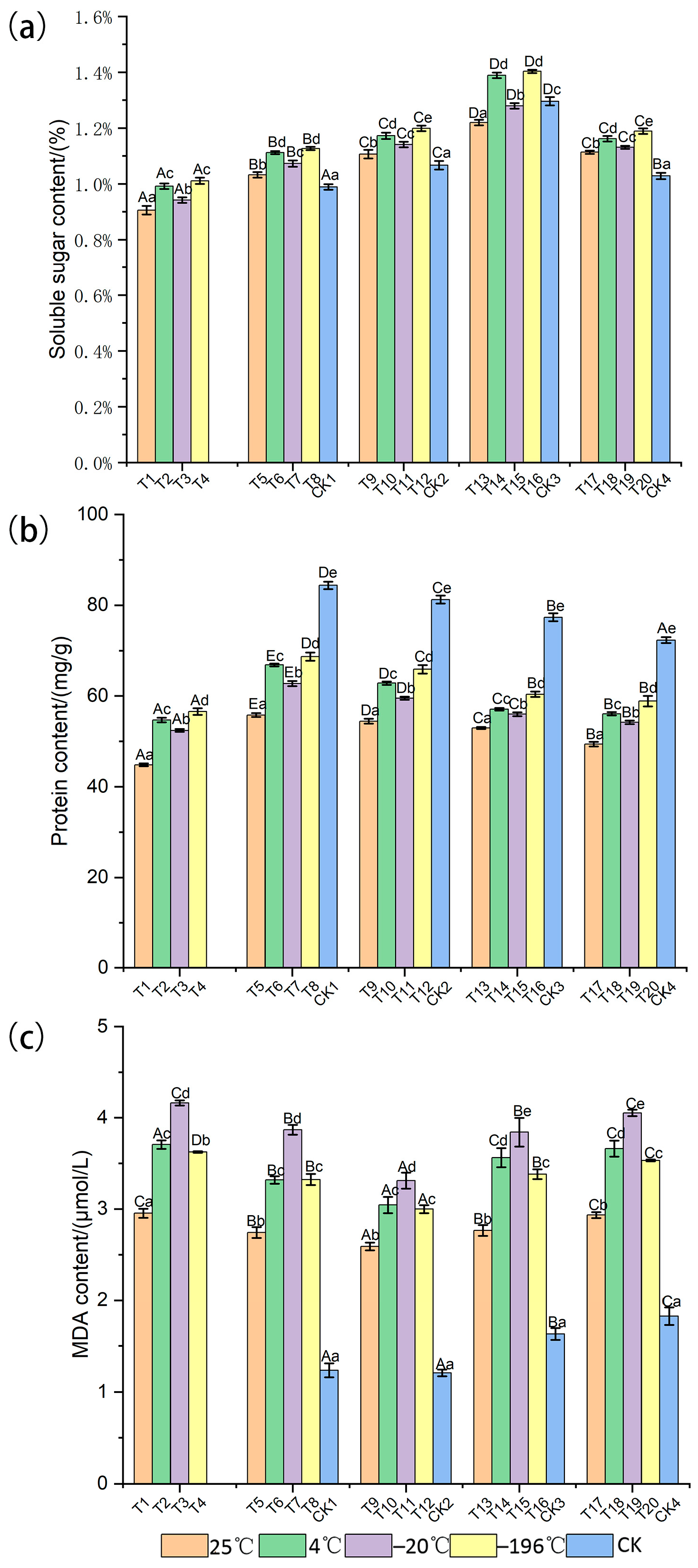
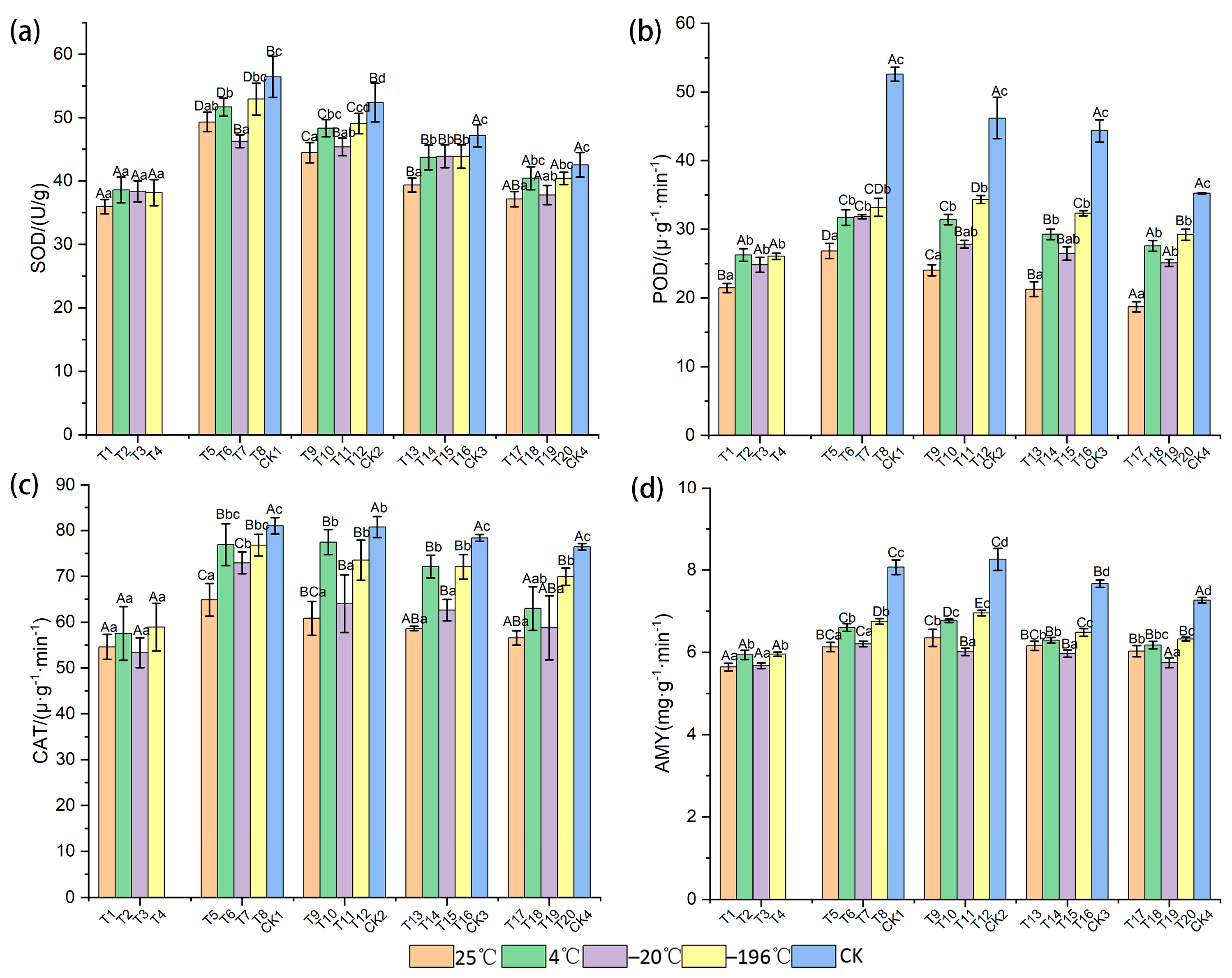
2.4. Determination of Soluble Sugar, Protein, MDA, and Enzyme Activity in Seeds
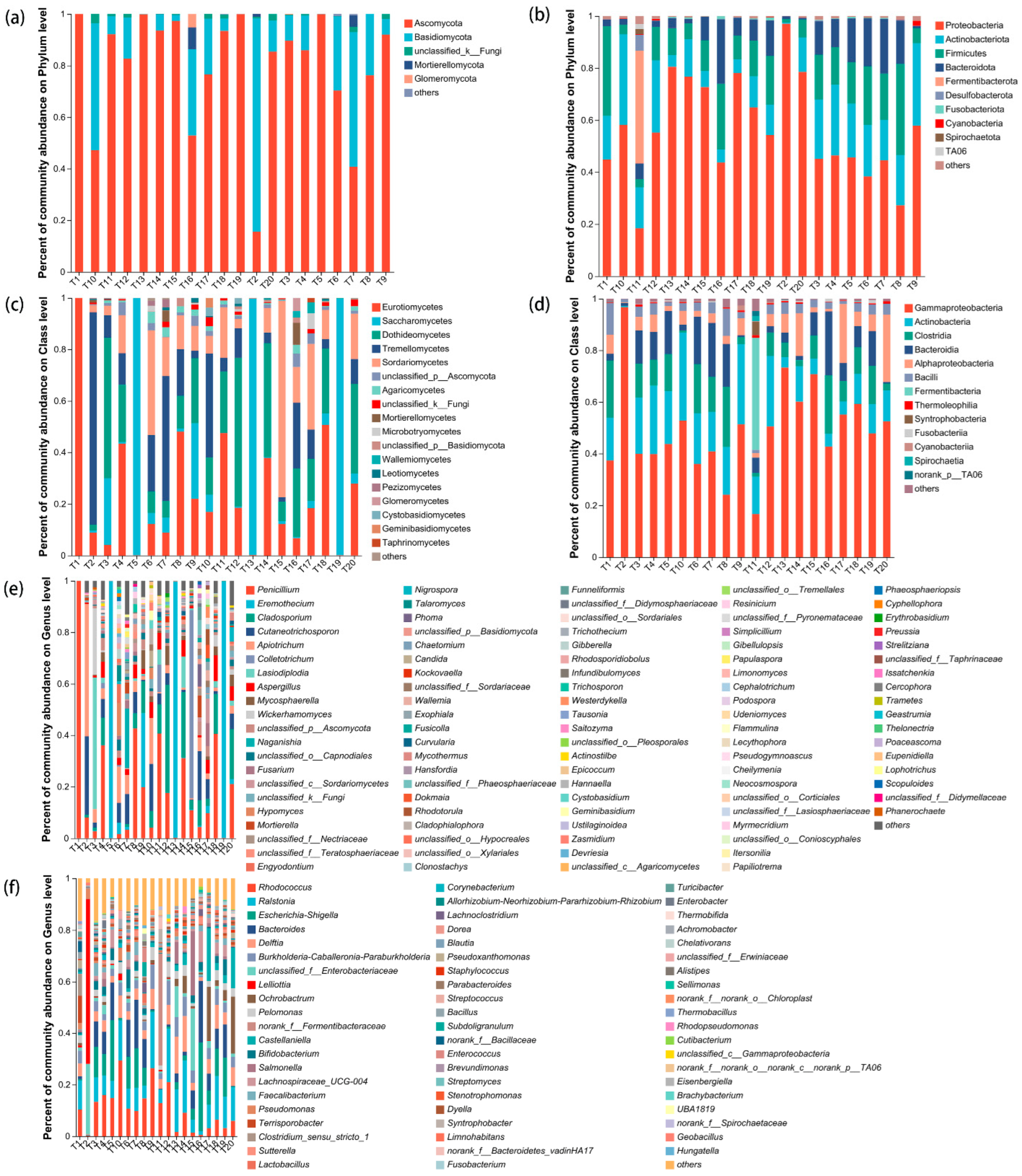
2.5. Analysis of Seed Microbial Community
2.6. Data Analysis
3. Results
3.1. Response of Physiological and Biochemical Indexes to Storage Conditions
3.2. Principal Component Analysis on Different Treated Samples
3.3. Response of Seed Microbial Community to Storage Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pradhan, B.K.; Badola, H.K. Seed germination response of populations of Swertia chirayita [(Roxb. ex Fleming) H. Karst] following periodical storage. Seed Technol. 2008, 30, 63–69. [Google Scholar]
- Pradhan, B.K.; Badola, H.K. Effect of storage conditions and storage periods on seed germination in eleven populations of Swertia chirayita: A critically endangered medicinal herb in Himalaya. Sci. World J. 2012, 2012, 128105. [Google Scholar] [CrossRef]
- Gianella, M.; Balestrazzi, A.; Ravasio, A.; Mondoni, A.; Börner, A.; Guzzon, F. Comparative seed longevity under genebank storage and artificial ageing: A case study in heteromorphic wheat wild relatives. Plant Biol. 2022, 24, 836–845. [Google Scholar] [CrossRef]
- Trusiak, M.; Plitta-Michalak, B.P.; Michalak, M. Choosing the right path for the successful storage of seeds. Plants 2022, 12, 72. [Google Scholar] [CrossRef]
- Mamedi, A.; Sharifzadeh, F.; Maali-Amiri, R.; Divargar, F. Physiological and biological responses of Ca2+-primed Quinoa seed longevity stored at different hermetic storage conditions. J. Plant Growth Regul. 2024, 43, 1967–1984. [Google Scholar] [CrossRef]
- Nazreen, S.; Khan, B.R.; Mohmand, A.S. The effect of storage temperature, storage period and seed moisture content on seed viability of soybean. Pak. J. Biol. Sci. 2000, 3, 2003–2004. [Google Scholar] [CrossRef]
- Hezewijk, M.V.; Beem, A.V.; Verkleij, J.A.C.; Pieterse, A.H. Germination of Orobanche crenata seeds, as influenced by conditioning temperature and period. Can. J. Bot. 1993, 71, 786–792. [Google Scholar] [CrossRef]
- Müller, E.; Cooper, E.J.; Alsos, I.G. Germinability of arctic plants is high in perceived optimal conditions but low in the field. Botany 2011, 89, 337–348. [Google Scholar] [CrossRef]
- Butola, J.S.; Badola, H.K. Seed germination improvement using chemicals in Heracleum candicans Wall, a threatened medicinal herb of Himalaya. Indian For. 2004, 130, 565–572. [Google Scholar]
- Chen, S.Y.; Kuo, S.R.; Chien, C.T. Storage behaviour of seeds of Cinnamomum osmophloeum and Neolitsea aciculata var. variabillima (Lauraceae). Seed Sci. Technol. 2007, 35, 237–243. [Google Scholar] [CrossRef]
- Ferguson, J.M. AOSA perspective of seed vigor testing. J. Seed Technol. 1993, 17, 101–104. [Google Scholar]
- Anguelova-merhar, V.S.; Calistru, C.; Berjak, P. A study of some biochemical and histopathological responses of wet-stored recalcitrant seeds of Avicennia marina infected by Fusarium moniliforme. Ann. Bot. 2003, 92, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Q.; Chen, Y.; Zhong, C.; Zhang, Y.; Chen, Z.; Pinyopusarerk, K.; Bush, D. Arbuscular mycorrhizal fungi enhanced growth of Magnolia macclurei (Dandy) Figlar seedlings grown under glasshouse conditions. For. Sci. 2017, 63, 441–448. [Google Scholar] [CrossRef]
- Li, X.; Liao, L.N.; Yang, H.P.; Huang, Y.Z.; He, A.J.; Ye, S.M. Spatial pattern of artificial mixed forest of Cunninghamia lanceolata and Michelia macclurei. J. Southwest. For. Univ. 2020, 40, 1–9. [Google Scholar]
- Zhao, J.; Chen, H.; Li, G.; Jumaturti, M.A.; Yao, X.; Hu, Y. Phylogenetics study to compare chloroplast genomes in four Magnoliaceae species. Curr. Issues Mol. Biol. 2023, 45, 9234–9251. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, B.; Fu, Y. A study of the chemical composition and biological activity of Michelia macclurei Dandy heartwood: New sources of natural antioxidants, enzyme inhibitors and bacterial inhibitors. Int. J. Mol. Sci. 2023, 24, 7972. [Google Scholar] [CrossRef]
- Lan, X.; Liang, Y.; Wei, Z.; Yu, G. Biological characteristics of Michelia macclurei Dandy and its utilization prospects. Guangxi Agric. Sci. 2010, 41, 253–255. [Google Scholar]
- Fang, X.Y.; Shen, W.H. Effect of natural dehydration of Michelia macclurei seeds on germination rate. Contemp. Hortic. 2021, 44, 14–15. (In Chinese) [Google Scholar]
- Han, Y.; Gao, H.; Wang, Y.; Zhang, L.; Jia, J.; Ma, H. Storage time affects the viability, longevity, and germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability 2023, 15, 8576. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Y.; Shi, F.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of Ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Ren, M.; Tan, B.; Xu, J.; Yang, Z.; Zheng, H.; Tang, Q.; Zhang, X.; Wang, W. Priming methods affected deterioration speed of primed rice seeds by regulating reactive oxygen species accumulation, seed respiration and starch degradation. Front. Plant Sci. 2023, 14, 1267103. [Google Scholar] [CrossRef]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. MiSeq: A Next Generation Sequencing Platform for Genomic Analysis. In Disease Gene Identification: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 223–232. [Google Scholar]
- Yan, H.F.; Xia, F.S.; Mao, P. Research progress of seed aging and vigor repair. Chin. Agric. Sci. Bull 2014, 30, 20–26. (In Chinese) [Google Scholar]
- Mira, S.; Estrelles, E.; González-Benito, M.E. Effect of water content rate and temperature on seed longevity of seven Brassicaceae species after 5 years of storage. Plant Biol. 2015, 17, 153–162. [Google Scholar] [CrossRef]
- Liava, V.; Ntatsi, G.; Karkanis, A. Seed germination of three milk thistle (Silybum marianum (L.) Gaertn.) populations of Greek origin: Temperature, duration, and storage conditions effects. Plants 2023, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H. Vitality of tomato seeds during storage. Beijing Agric. Sci. 1999, 17, 24–26. (In Chinese) [Google Scholar]
- Ma, X.; He, C.; Luo, F.; Xu, W.; Duan, X. Effects of hydro-priming on the vigor of Setaria sphacelate cv. Narok’s seeds in different storage period. Chin. J. Grassl. 2017, 39, 16–23. [Google Scholar]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, V. Impact of seed color and storage time on the radish seed germination and sprout growth in plasma agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, X.C. Study on storage and germination characteristics of Impatiens seeds. China Seed Ind. 2007, 8, 47–49. (In Chinese) [Google Scholar]
- Cai, Q.; Hu, Y.; Zhang, J.; Xie, H. Preliminary study on physiological characteristics for rice seed after aging. Fujian J. Agric. Sci. 2012, 27, 1061–1066. (In Chinese) [Google Scholar]
- Long, J.; Zheng, Q.; Yang, Z.; Hao, L.; Zhang, F.; Chang, R.; Zhang, H.; Wang, Y. Effect of seed longevity and storage material in Pugionium Gaertn at Different storage years. Seed 2017, 36, 15–20. [Google Scholar]
- Zhang, J.; Chen, S.; Rui, H. Study on the relationship between malondialdehyde content and stress resistance in different alfalfa varieties. Heilongjiang Anim. Sci. Vet. Med. 2008, 8, 53–54. (In Chinese) [Google Scholar]
- Lie, G.W.; Ye, L.H.; Xue, L. Effects of ozone stress on major plant physiological functions. Acta Ecol. Sin. 2014, 34, 294–306. [Google Scholar] [CrossRef]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J. Physiological and biochemical parameters of lupin seed subjected to 29 years of storage. Crop Sci. 2017, 57, 2149–2159. [Google Scholar] [CrossRef]
- Zheng, G.H. A brief discussion on the key issues of seed storage. Seed 1984, 4, 46–48. (In Chinese) [Google Scholar]
- Chang, H.; Zhang, F.; Yang, Z.; Kong, D.; Zheng, Q.; Hao, L. Physiological and biochemical responses of Allium mongolicum seeds to storage aging. Plant Physiol. J. 2015, 51, 1075–1081. [Google Scholar]
- Hao, Y.C. The effect of storage time on characteristics of Pinus tabulaeformis seed germination and physiological changes. Prot. For. Sci. Technol. 2016, 3, 34–35. (In Chinese) [Google Scholar]
- Dickie, J.B.; Ellis, R.H.; Kraak, H.L.; Ryder, K.; Tompsett, P.B. Temperature and seed storage longevity. Ann. Bot. 1990, 65, 197–204. [Google Scholar] [CrossRef]
- Han, C.; Long, C. Seed dormancy, germination and storage behavior of Magnolia wilsonii (Magnoliaceae), an endangered plant in China. Acta Bot. Yunnanica 2010, 32, 47–52. [Google Scholar] [CrossRef]
- Han, C.Y.; Sun, W.B. Seed storage behaviour of Magnolia odoratissima. Seed Sci. Technol. 2013, 41, 143–147. [Google Scholar] [CrossRef]
- José, A.C.; Da Silva, E.A.A.; Davide, A.C.; Melo, A.J.S.; Toorop, P.E. Effects of drying rate and storage time on Magnolia ovata Spreng. seed viability. Seed Sci. Technol. 2011, 39, 425–434. [Google Scholar] [CrossRef]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Walters, C.; Wesley-Smith, J.; Crane, J.; Hill, L.M.; Chmielarz, P.; Pammenter, N.W.; Berjak, P. Cryopreservation of recalcitrant (i.e., Desiccation-Sensitive) Seeds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Norman, M.N.; Chin, H.F.; Hor, Y.L. Desiccation and cryopreservation of embryonic axes of Hevea brasiliensis. Pertanike 1986, 9, 299–303. [Google Scholar]
- Zhao, W. Study on Components and Function of the Aril and Sarcotesta in Seven Plants. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Kim, S.I.; Roh, J.Y.; Kim, D.H.; Lee, H.S.; Ahn, Y.J. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 2003, 39, 293–303. [Google Scholar] [CrossRef]
- Sun, X.; Mantri, N.; Ge, J.; Du, Y.; Wang, G.; Lu, J.; Jiang, W.; Lu, H. Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Torreya grandis Merrilli aril. Plant Omics 2014, 7, 337–344. [Google Scholar]
- Berg, G.; Raaijmakers, J.M. Saving seed microbiomes. ISME J. 2018, 12, 1167–1170. [Google Scholar] [CrossRef]
- Torres-Cortés, G.; Bonneau, S.; Bouchez, O.; Genthon, C.; Briand, M.; Jacques, M.A.; Barret, M. Functional microbial features driving community assembly during seed germination and emergence. Front. Plant Sci. 2018, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, R.; Wu, X.; Xu, T.; Ahmad, S.; Zhang, X.; Zhao, J.; Liu, Y. An endophytic strain of the genus Bacillus isolated from the seeds of maize (Zea mays L.) has antagonistic activity against maize pathogenic strains. Microb. Pathog. 2020, 142, 104074. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, A.J.; Zong, Q.; Du, Z.; Guo, Q.; Liu, T.; Chen, W.; Gao, L. Microbiome signature of endophytes in wheat seed response to wheat dwarf bunt caused by Tilletia controversa Kühn. Microbiol. Spectr. 2023, 11, e00390-22. [Google Scholar] [CrossRef]
- Zhang, J.B. The Study of Bacterial Abundance and Community Structure in Rice Seeds and Their Influencing Factors. Master’s Thesis, Hebei University, Baoding, China, 2023. (In Chinese). [Google Scholar]
- Chen, Y.; Lu, J.X.; Fan, H.D.; Li, C.H. Separation, purification and initiative identification of mildew bacteria from mildew dry jujube. J. Northwest Univ. Natl. (Nat. Sci.) 2012, 6, 75–80. (In Chinese) [Google Scholar]
- Yang, S.X.; Liu, J.X.; Jiang, Y. Preliminary study on mildew mechanism of peanut seeds. Hubei Agric. Sci. 2020, 59, 72. (In Chinese) [Google Scholar]


| Treatment Code | Temperature (°C) | Dry (Day) | Arils Removed | Storage (Month) |
|---|---|---|---|---|
| T1 | 25 | 0 | yes | 1 |
| T2 | 4 | 0 | yes | 1 |
| T3 | −20 | 0 | yes | 1 |
| T4 | −196 | 0 | yes | 1 |
| T5 | 25 | 0 | no | 1 |
| T6 | 4 | 0 | no | 1 |
| T7 | −20 | 0 | no | 1 |
| T8 | −196 | 0 | no | 1 |
| CK1 | 25 | 0 | no | 0 |
| T9 | 25 | 1 | no | 1 |
| T10 | 4 | 1 | no | 1 |
| T11 | −20 | 1 | no | 1 |
| T12 | −196 | 1 | no | 1 |
| CK2 | 25 | 1 | no | 0 |
| T13 | 25 | 3 | no | 1 |
| T14 | 4 | 3 | no | 1 |
| T15 | −20 | 3 | no | 1 |
| T16 | −196 | 3 | no | 1 |
| CK3 | 25 | 3 | no | 0 |
| T17 | 25 | 5 | no | 1 |
| T18 | 4 | 5 | no | 1 |
| T19 | −20 | 5 | no | 1 |
| T20 | −196 | 5 | no | 1 |
| CK4 | 25 | 5 | no | 0 |
| Indicator | Prin.1 | Prin.2 | Prin.3 |
|---|---|---|---|
| Viability | 0.423 | 0.812 | −0.102 |
| Germination | 0.456 | 0.792 | 0.056 |
| Water content rate | 0.312 | 0.756 | 0.412 |
| Soluble sugar | 0.765 | −0.312 | 0.412 |
| Protein | 0.892 | 0.102 | −0.212 |
| MDA | −0.782 | 0.412 | 0.102 |
| SOD | 0.843 | 0.325 | −0.102 |
| POD | 0.912 | −0.123 | 0.056 |
| CAT | 0.876 | 0.156 | −0.312 |
| AMY | 0.801 | −0.212 | 0.301 |
| Eigenvalues | 4.82 | 2.15 | 1.23 |
| Variance contribution rate (%) | 48.20 | 21.50 | 12.3 |
| Cumulative contribution rate (%) | 48.20 | 69.70 | 82.00 |
| Treatment | PC1 Score | PC2 Score | PC3 Score | Composite Score (F) | Rank |
|---|---|---|---|---|---|
| T6 | 2.45 | 1.78 | 0.56 | 2.12 | 1 |
| T10 | 2.12 | 2.01 | 0.45 | 1.98 | 2 |
| T8 | 2.23 | 1.45 | 0.67 | 1.89 | 3 |
| T12 | 1.98 | 1.67 | 0.52 | 1.76 | 4 |
| T7 | 1.67 | 1.23 | 0.45 | 1.45 | 5 |
| Microbe | Samples | Sequence Number | Index of Diversity | Coverage | |||
|---|---|---|---|---|---|---|---|
| Shannon Index | Simpson Index | ACE Index | Chao Index | ||||
| Fungi | T1 | 67123 | 0.340 | 0.817 | 31.4 | 14.0 | 0.99994 |
| T2 | 73150 | 1.800 | 0.312 | 57.2 | 56.5 | 0.99997 | |
| T3 | 58681 | 1.871 | 0.319 | 75.0 | 75.0 | 1.00000 | |
| T4 | 67135 | 3.565 | 0.051 | 105.4 | 105.0 | 0.99999 | |
| T5 | 57888 | 0.016 | 0.997 | 20.3 | 20.0 | 0.99998 | |
| T6 | 62504 | 3.315 | 0.057 | 51.4 | 50.0 | 0.99998 | |
| T7 | 63386 | 3.547 | 0.073 | 96.1 | 93.5 | 0.99997 | |
| T8 | 57818 | 2.891 | 0.112 | 39.8 | 39.0 | 0.99998 | |
| T9 | 57549 | 3.088 | 0.115 | 100.4 | 100.0 | 0.99998 | |
| T10 | 51234 | 3.029 | 0.070 | 35.0 | 35.0 | 1.00000 | |
| T11 | 67780 | 3.284 | 0.064 | 0.0 | 76.0 | 0.99994 | |
| T12 | 59372 | 2.826 | 0.145 | 68.9 | 69.0 | 0.99997 | |
| T13 | 72869 | 0.052 | 0.989 | 68.1 | 66.5 | 0.99986 | |
| T14 | 62555 | 3.259 | 0.094 | 113.2 | 113.0 | 0.99998 | |
| T15 | 70114 | 1.555 | 0.540 | 123.4 | 123.5 | 0.99996 | |
| T16 | 57491 | 3.130 | 0.064 | 41.0 | 40.0 | 0.99998 | |
| T17 | 50627 | 3.454 | 0.037 | 50.8 | 48.0 | 0.99994 | |
| T18 | 65171 | 3.368 | 0.073 | 151.3 | 151.0 | 0.99999 | |
| T19 | 67347 | 0.027 | 0.994 | 78.8 | 60.1 | 0.99969 | |
| T20 | 68124 | 3.598 | 0.055 | 88.4 | 88.0 | 0.99999 | |
| Bacteria | T1 | 67079 | 3.973 | 0.047 | 528.9 | 521.2 | 0.99869 |
| T2 | 66037 | 1.109 | 0.479 | 252.3 | 211.9 | 0.99920 | |
| T3 | 66684 | 4.083 | 0.047 | 370.8 | 347.3 | 0.99966 | |
| T4 | 64410 | 4.007 | 0.047 | 295.5 | 287.3 | 0.99958 | |
| T5 | 69109 | 3.334 | 0.088 | 311.1 | 242.0 | 0.99961 | |
| T6 | 70348 | 4.069 | 0.040 | 301.5 | 311.5 | 0.99973 | |
| T7 | 71063 | 3.810 | 0.054 | 324.0 | 329.1 | 0.99972 | |
| T8 | 48888 | 4.201 | 0.040 | 392.0 | 407.6 | 0.99928 | |
| T9 | 54786 | 3.122 | 0.130 | 201.4 | 204.5 | 0.99987 | |
| T10 | 61412 | 3.086 | 0.125 | 192.0 | 175.8 | 0.99977 | |
| T11 | 71424 | 2.757 | 0.213 | 220.3 | 222.0 | 0.99989 | |
| T12 | 61991 | 3.428 | 0.100 | 284.0 | 267.2 | 0.99963 | |
| T13 | 49768 | 3.511 | 0.073 | 354.2 | 365.2 | 0.99888 | |
| T14 | 61543 | 3.449 | 0.080 | 346.4 | 334.1 | 0.99899 | |
| T15 | 52688 | 3.240 | 0.122 | 343.6 | 352.0 | 0.99922 | |
| T16 | 54109 | 2.980 | 0.130 | 235.6 | 237.4 | 0.99946 | |
| T17 | 55083 | 3.169 | 0.101 | 277.3 | 285.9 | 0.99942 | |
| T18 | 67386 | 3.747 | 0.059 | 306.4 | 288.3 | 0.99963 | |
| T19 | 57395 | 3.833 | 0.049 | 264.3 | 269.0 | 0.99974 | |
| T20 | 42490 | 3.236 | 0.100 | 213.2 | 208.8 | 0.99958 | |
| Microbe | Group | Index of Diversity | |||
|---|---|---|---|---|---|
| Shannon Index | Simpson Index | ACE Index | Chao Index | ||
| Fungi | Group 1 | 1.519 | 0.499 | 57.9 | 54.1 |
| Group 2 | 3.174 | 0.086 | 64.5 | 63.5 | |
| Group 3 | 2.195 | 0.367 | 74.7 | 89.6 | |
| Group 4 | 2.715 | 0.245 | 82.1 | 77.4 | |
| Group A | 2.517 | 0.250 | 31.0 | 45.0 | |
| Group B | 2.907 | 0.142 | 68.2 | 66.8 | |
| Group C | 2.045 | 0.374 | 83.6 | 82.9 | |
| Group D | 2.485 | 0.314 | 99.4 | 94.5 | |
| Group E | 2.050 | 0.416 | 66.8 | 66.6 | |
| Bacteria | Group 1 | 3.301 | 0.142 | 351.7 | 321.9 |
| Group 2 | 3.658 | 0.078 | 282.2 | 285.7 | |
| Group 3 | 3.277 | 0.118 | 309.7 | 308.1 | |
| Group 4 | 3.393 | 0.088 | 259.4 | 257.9 | |
| Group A | 3.445 | 0.107 | 321.6 | 323.0 | |
| Group B | 2.879 | 0.184 | 284.4 | 273.5 | |
| Group C | 3.885 | 0.055 | 355.8 | 352.1 | |
| Group D | 3.603 | 0.077 | 276.9 | 273.7 | |
| Group E | 3.224 | 0.109 | 265.2 | 244.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Chen, Z.; Li, B.; Xue, H.; Zhang, S.; Chen, H.; Qu, C.; Jiang, Q. Effects of Different Storage Conditions on Physiological, Biochemical, and Microbial Community Traits of Michelia macclurei Seeds. Horticulturae 2025, 11, 975. https://doi.org/10.3390/horticulturae11080975
Tian S, Chen Z, Li B, Xue H, Zhang S, Chen H, Qu C, Jiang Q. Effects of Different Storage Conditions on Physiological, Biochemical, and Microbial Community Traits of Michelia macclurei Seeds. Horticulturae. 2025; 11(8):975. https://doi.org/10.3390/horticulturae11080975
Chicago/Turabian StyleTian, Shenghui, Zhaoli Chen, Baojun Li, Haoyue Xue, Shida Zhang, Haijun Chen, Chao Qu, and Qingbin Jiang. 2025. "Effects of Different Storage Conditions on Physiological, Biochemical, and Microbial Community Traits of Michelia macclurei Seeds" Horticulturae 11, no. 8: 975. https://doi.org/10.3390/horticulturae11080975
APA StyleTian, S., Chen, Z., Li, B., Xue, H., Zhang, S., Chen, H., Qu, C., & Jiang, Q. (2025). Effects of Different Storage Conditions on Physiological, Biochemical, and Microbial Community Traits of Michelia macclurei Seeds. Horticulturae, 11(8), 975. https://doi.org/10.3390/horticulturae11080975





