Abstract
The systemic delivery of oxytetracycline (OTC) by trunk injection has emerged as a viable strategy to manage huanglongbing (HLB, also known as citrus greening), a bacterial disease devastating citrus production around the world. This study examines the efficacy of delivering OTC systemically into the trunk of young, HLB-affected citrus trees using a drill-based or a drill-free system to improve tree health and productivity. Two field trials were conducted in two commercial production sites in Florida. Trees were four years old at the start of the study and composed of ‘Valencia’ or ‘OLL-8’ sweet orange (Citrus sinensis) scion grafted on X-639 (C. reticulata × Poncirus trifoliata) rootstock. Injections were performed in spring or late summer/early fall in 2022 and 2023. Using the drill-based system, 0.79 g of OTC was administered into each tree, whereas 0.15 g or 0.3 g was administered using the drill-free system. Delivering a higher dose of OTC by drill-based injection increased fruit yield and improved juice quality more than delivering lower doses by drill-free injection, though responses varied between cultivars. Injections in late summer/early fall increased the juice total soluble solids content considerably more than injections in spring. However, fall injections resulted in OTC fruit residues exceeding the maximum allowed level. Trunk injury was more extensive when OTC was applied with the drill-free system than when it was applied with the drill-based system.
1. Introduction
Citrus production around the world is affected by numerous pests and diseases, among which huanglongbing (HLB), also known as citrus greening, is arguably the most devastating. The disease is associated with difficult-to-culture bacteria in the genus Candidatus Liberibacter that are insect-vectored and reside in the phloem of affected trees [1,2]. The most widespread and destructive species is Candidatus Liberibacter asiaticus (CLas), vectored by the Asian citrus psyllid, Diaphorina citri [3]. Infection results in disturbances of plant carbohydrate metabolisms and other metabolic pathways [4,5,6], resulting in phloem collapse and starch accumulation [7]. A recent study suggested that HLB is a pathogen-triggered immune disease resulting in the production of reactive oxygen species and cell death, in turn producing other aberrations and ultimately resulting in the characteristic symptoms associated with the disease [8]. These symptoms include yellowing and/or asymmetric blotchy mottling of the leaves, canopy die-back, and production of small, misshapen fruits that do not color properly and drop prematurely [9]. A multi-pronged approach, including rigorous vector control and the removal of infected trees, was implemented in Brazil, where the disease was discovered in 2004, resulting in little loss of production [10]. However, HLB incidence has now begun to reach critical levels [11]. In Florida, the removal of infected trees was not given serious consideration, mainly because of the canker eradication program that destroyed millions of trees in the years preceding 2005, when HLB was discovered [12]. Consequently, HLB spread quickly and was considered endemic in 2013 [13]; it is the main reason for the drastic decline in citrus production in Florida from more than 7 million metric tons in 2004–2005 to 905,000 metric tons in 2023–2024 [14].
In the absence of genetic resistance among commercially relevant citrus varieties, various mitigation strategies have been tried in Florida, ranging from enhanced nutrition to thermotherapy [15]. None of these, however, has proven effective. Eliminating the disease vector remains one of the most important aspects of a successful HLB management program but has been hampered in Florida by the large abundance of the psyllids, costs, and increasing resistance to the available insecticides [16]. Psyllid exclusion by growing citrus trees under protective screens (CUPS) has been successfully implemented for fresh varieties, i.e., grapefruits and some mandarins [17], but is not applicable to the large-scale production of processing oranges predominant in Florida. Young trees may be protected with individual protective covers (IPCs) for up to three or four years after planting [18] but become infected within several months after removal [19].
The most effective curative measure to treat bacterial diseases is using antibiotics. Only three antibiotics, streptomycin, oxytetracycline, and kasugamycin, are currently registered for use in plant agriculture [20,21]. In 2016, oxytetracycline hydrochloride and streptomycin sulfate were granted an emergency exemption (https://www.federalregister.gov/documents/2016/12/15/2016-30175/pesticide-emergency-exemptions-agency-decisions-and-state-and-federal-agency-crisis-declarations) (accessed on 8 July 2025) to be used for HLB management by foliar spray application. Yet, because of the vascular location of the disease-associated bacteria and the difficulty of penetrating the leaf cuticle [22], these applications were not effective. One way to systemically deliver pesticides is by trunk injection, also referred to as ‘endotherapy’ [23,24,25]. Trunk injection of antibiotics to manage HLB was already explored decades ago in South Africa and other countries where the disease was prevalent [26,27,28,29]. Although antibiotic injections resulted in the remediation of disease symptoms, they were not pursued as a large-scale management strategy.
Interest in antibiotic injections re-emerged with the increasing prevalence of HLB in Florida. Zhang et al. [30,31] screened various antibiotics for their efficacy to suppress CLas in plant-based assays and identified the combination of penicillin and streptomycin as most effective. Hu and Wang [32] demonstrated that trunk injection of oxytetracycline can effectively suppress CLas populations in leaves and roots of HLB-affected trees under field conditions but used relatively high concentrations of OTC. More recently, Archer et al. [33,34] conducted a series of field studies confirming the efficacy of vascular-delivered oxytetracycline (OTC) in improving tree health, productivity, and fruit quality of HLB-affected citrus trees. In response to the severity of the HLB crisis, a 24 (c) special local need label was issued in October 2022 (https://www.tjbiotechllc.com/_files/ugd/9835f7_109e90aa3aea49e29e2f71d935ebba6e.pdf) (accessed on 8 July 2025), permitting the systemic delivery of OTC by trunk injection in commercial citrus in Florida and suggesting a drill-based system as the recommended application method. In contrast to foliar sprays, the targeted delivery of pesticides into the trunk of a tree by injection reduces any potential negative impacts on the environment and the risk to human health [35].
Drilling holes into the trunk of a tree can cause considerable injury and result in the invasion of pathogens followed by decay. Trees can compartmentalize or ‘seal off’ injuries through a process referred to as CODIT (compartmentalization of decay/damage in trees), established by Shigo and Marx [36] and refined by Morris et al. [37,38]. This process, however, may be hindered by the application of xenobiotics (chemicals that are not naturally found within an organism) such as OTC. Early injection studies to mitigate ‘citrus greening’ in South Africa noted large areas of discoloration in the wood after tetracycline injection [27], which likely contributed to the lack of adoption of this management strategy [29]. More recently, Archer and Albrecht [39,40] confirmed this observation and documented the relatively larger damage OTC causes at the injection site compared to water. Drill-free systems to deliver pesticides have been designed as an alternative to drill-based systems to reduce trunk injury and deliver therapies more precisely (nearer to the active sapwood), therefore requiring lower doses of the active ingredient. One such system is described in Girelli et al. [41] and Grandi et al. [42] and has been used in olive trees to deliver therapies targeting the xylem-limited bacterial pathogen associated with Olive Quick Decline Syndrome. A similar system was recently registered for management of HLB in commercial citrus (https://invaiocitrus.com/news/invaio-achieves-first-registration-for-citrus-greening-solution-featuring-trecise-technology-invaio-sciences/) (accessed on 8 July 2025).
This study was designed to compare the drill-free delivery system now registered for use in Florida with a drill-based injection system previously used in our program [33,34] regarding their efficacy to mitigate HLB in young sweet orange trees under commercial citrus production conditions, and to assess their advantages and disadvantages.
2. Materials and Methods
2.1. Experimental Area and Design
Two field trials were conducted from May 2022 to May 2024. Trial 1 was in a commercial citrus grove in St. Lucie County, Florida, USA (27°28′32.3″ N 80°35′01.3″ W). The average air temperature during the study period near the trial location was 23.5 °C with an average minimum and maximum of 11.6 °C and 35.2 °C, respectively. The average humidity was 82.8%, and the total rainfall was 241 cm. Meteorological data were retrieved from the Florida Automated Weather Network (https://fawn.ifas.ufl.edu/) (accessed on 8 July 2025) from the nearest FAWN station. The trees were ‘Valencia’ sweet orange (Citrus sinensis) grafted on X-639 rootstock (C. reticulata × Poncirus trifoliata). They were planted in November 2018 in double rows on raised beds at a spacing of 8 ft (2.4 m) within and 24 ft (7.3 m) between rows, for a density of 227 trees per acre (561 trees per hectare). The soil at the location was a poorly drained Spodosol [43].
Trial 2 was in a commercial citrus grove in Highlands County, Florida, USA (27°07′13.8″ N 81°18′05.2″ W). The average air temperature during the study period near the trial location was 23.5 °C with an average minimum and maximum of 11.5 °C and 34.5 °C, respectively. The average humidity was 82.8%, and the total rainfall was 291 cm. Meteorological data were retrieved from the nearest FAWN station. The trees were ‘OLL-8’ sweet orange (C. sinensis) grafted on X-639 rootstock. They were planted in May 2018 in single rows at the same spacing as trial 1. The soil at the location was a sandy, well-drained Entisol [43].
Tree care was according to the grower collaborators’ standards and included pest and weed management, regular irrigation, and fertilization. Each trial was set up in a randomized complete block design (RCBD) with six replications and each replicate consisting of two contiguous trees. HLB has been endemic in Florida since 2013 [13], and all trees displayed moderate symptoms typical of HLB [9].
2.2. Treatments
There were seven treatments (T0-T6), which are summarized in Table 1. Trees in T0 remained non-injected throughout the study to serve as the control. Trees in T1 received oxytetracycline (OTC) by foliar spray. The formulation used was FirelineTM (FireLine 17 WP (AgroSource, Tequesta, FL, USA; 18.3% oxytetracycline hydrochloride), applied at the recommended rate of 24 oz. per acre (1.75 L/ha) or 4.4 oz. active ingredient (a.i.) per acre (0.322 L/ha) in combination with an adjuvant (Methylated Seed Oil, Southern Ag, Palmetto, FL, USA) at 0.1% (v/v). Spraying was conducted with a battery-powered backpack sprayer set to 18 psi. Trees were sprayed until run-off. Buffer trees were left on either side of the sprayed trees to prevent carry-over into neighboring treatments.

Table 1.
Summary of injection treatments.
Trees in T2 and T3 were injected using a drill-free microinjection system (TreciseTM, Invaio Sciences Inc., Cambridge, MA, USA) (Figure 1A). This system consists of an arrowhead-shaped (9 mm wide, 8.5 mm long, and 1.5 mm thick) microinjection tip connected to a pressurized canister containing the treatment formulation (https://invaiocitrus.com/solutions/) (accessed on 8 July 2025). The system was inserted into the tree using an impact hammer so that the wide side of the tip was perpendicular to the length of the trunk. Once connected to the canister and activated, the liquid inside the canister is released at low pressure through two small slits in the microinjection tip, which typically takes several hours. The OTC formulation exclusively licensed for use with the TreciseTM system and used here was ArborBioticTM (MGF Scientific, Inc., West Palm Beach, FL, USA; 39.6% oxytetracycline hydrochloride). Two injection devices, one on each opposite side of the trunk, were used per tree per application. In T2, each device contained 37.5 mg of OTC-HCL in 60 mL of distilled water (final concentration: 0.625 mg/mL). Two applications were made each year, and the total amount of OTC-HCl delivered to each tree per year was 150 mg, as recommended according to the Trecise label (https://citrusrdf.org/wp-content/uploads/2024/10/SLN-Label_Revised_SLNFL230002.approval.pdf) (accessed on 8 July 2025). In T3, each device contained 75.1 mg of OTC-HCL in 60 mL of distilled water (final concentration: 1.25 mg/mL). Two applications were made each year, and the total amount of OTC-HCl delivered to each tree per year was 300 mg. In May 2022, only 60 mL was used mistakenly, reducing the total amount of OTC-HCL applied to each tree in that year to 112.5 mg instead of 150 mg for T2 and 225 mg instead of 300 mg for T3. In 2022, the first application was made in May and the second one in August. In 2023, the first application was made in May and the second one in September. All applications were made into the rootstock trunk. After the first application, devices were offset by 90° from the previous application sites.

Figure 1.
Drill-free (Trecise) injection system (A) and drill-based (Chemjet) injection system (B). The inset in A shows the arrowhead-shaped injection tip of the Trecise system.
Trees in T4, T5, and T6 were injected following the method used in previous studies [33,34] using Chemjet Tree Injectors (Logical Result LLC, Interlochen, MI, USA) (Figure 1B). Chemjets are spring-loaded syringes that release liquid at low pressure (approximately 20 psi) after activation. Each Chemjet can hold a maximum volume of 20 mL. The oxytetracycline (OTC) formulation used was Arbor-OTC (Arborjet, Inc., Woburn, MA, USA; 39.6% oxytetracycline hydrochloride). Two injectors were used per tree per application. In all treatments, each injector contained 396 mg of OTC-HCL (1 g of formulation) in 20 mL of distilled water (final OTC concentration: 19.8 mg/mL). One application was made each year, and the total amount of OTC-HCl delivered to each tree per year was 792 mg. In 2022, applications were made in May (T4 and T5) or in August (T6). In 2023, applications were made in May (T4 and T5) or in September (T6). Applications were either made into the scion trunk (T4) or the rootstock trunk (T5 and T6). At each application, devices were placed on opposite sides of the trunk. Year-2 applications were offset by 90° and 3–5 cm higher than year-1 applications. Holes were drilled to a depth of 15 mm with an 11/64 in (4.3 mm) Brad point drill bit and a 20 V battery-powered drill (Dewalt, Baltimore, MD, USA). The injectors were inserted into the drilled hole at an angle of 20–30° and were removed once the full amount of liquid was taken up by the tree, which usually took 20–60 min. Treatments were applied on the same day and at the same time as T1–T3.
The pH of both injected OTC formulations was 1.8–2.0 after dissolving the OTC in distilled water. Although both products are marketed under different names, the products are essentially the same (based on personal communication with an industry representative).
2.3. Candidatus Liberibacter Asiaticus Detection (CLas)
CLas titers were determined in May 2023 and 2024, one year after each injection, to assess the long-term efficacy of injected OTC. Six mature, fully expanded leaves from the most recent flush were collected from each of the two trees in each plot and pooled for analysis. Midveins and petioles were excised from the leaf blades and pulverized under liquid nitrogen using a mortar and pestle. DNA extractions were performed using 100 mg of pulverized tissue and using the Plant DNeasy Mini Kit (Quiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) TaqMan assays were carried out using primers HLBas and HLBr and probe HLBp [44]. The latter was labeled with the reporter dye 6-carboxy-fluorescein (FAM) at the 5′-terminal nucleotide and with Black Hole Quencher (BHQ)-1 at the 3′-terminal nucleotide. Assays were conducted with the BioRad CFX96 Real-Time PCR systems (Bio-Rad, Hercules, CA, USA) and the iTaq Universal Probes Supermix (Bio-Rad) following the manufacturer’s instructions. Amplifications were performed for 15 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s in a reaction volume of 20 µL and using 2 µL (20–30 ng) of extracted DNA.
2.4. Tree Size
Tree height, canopy width, and scion and rootstock trunk diameters were measured in May/June 2024. Trunk circumferences were measured 5 cm above (scion) and 5 cm below (rootstock) the graft union and converted to diameters by dividing the circumference by π. Canopy width was measured in two perpendicular directions. The canopy volume was determined with the formula (width2 × height)/4 [45].
2.5. Pre-Harvest Fruit Drop and Yield
Pre-harvest fruit drop was assessed in the week before harvest by counting the number of fruits underneath each tree. The number of fruits remaining on each tree was estimated by dividing the yield by the average fruit weight. The percentage of fruit drop was calculated as the percentage of dropped fruit relative to the sum of dropped fruits and fruits on the tree.
At the end of September 2022, Hurricane Ian swept across Florida. Although neither trial site was exposed to the hurricane’s maximum force winds, higher-than-usual winds with a maximum speed greater than 25 m/s were measured for several hours near each trial site (https://fawn.ifas.ufl.edu/) (accessed on 8 July 2025). The resulting fruit drop was estimated at the end of October 2022 by counting the number of dropped fruits and calculating the percentage relative to the sum of dropped fruits and fruits on the tree at harvest.
In trial 1, fruits were harvested on 20 March 2023 and on 2 April 2024. In trial 2, fruits were harvested on 13 March 2023 and on 14 March 2024. Fruits were weighed with a 52 × 40 cm portable digital floor scale (CWP-300, CAS Corporation USA, East Rutherford, NJ, USA), and the yield was expressed as kg of fruits per tree.
2.6. Fruit and Juice Quality
In 2023, 24–32 randomly collected fruits from each replicate were collected. Due to the lower yield in 2023, analyses were conducted at the SWFREC laboratory using standard procedures [46]. The average fruit weight was determined using a digital scale. Juice total soluble solids (TSS) were determined by measuring degrees Brix (°Bx) with a digital refractometer (Hanna Instruments, Smithfield, RI, USA). Juice titratable acidity (TA) was measured by titrating sodium hydroxide to a phenolphthalein endpoint, and the TSS/TA ratio was determined.
In 2024, 40–50 randomly collected fruits from each replicate were collected. Fruit weight, percent juice, TSS, and TA were determined at the Juice Processing Pilot Plant, Citrus Research and Education Center, University of Florida, Institute of Food and Agricultural Sciences, Lake Alfred, FL, USA. The average fruit weight was determined using a digital scale. Juice was extracted using a pinpoint extractor (JBT, Chalfont, PA, USA), and the fruit juice percentage was calculated. Total soluble solids and TA were determined using standard commercial procedures [46], and the TSS/TA was determined.
Fruit (peel) color was determined in both years using a CR-400 chroma meter (Konica Minolta, Ramsey, NJ, USA). Peel color is presented as the a*/b* color ratio based on the CIE L*a*b* color system. More negative a*/b* ratio values indicate a deeper green color, and more positive values indicate a deeper red (orange) color.
2.7. Fruit OTC Residue Analysis
Ten randomly collected fruits from each replicate were collected at each harvest. Fruits from two replicates were combined for a total of three replicates per treatment. Fruits were quartered, and one quarter of each fruit from each replicate was combined and homogenized in a 1400-Watt power blender (Ninja Professional Plus Kitchen System, SharkNinja, Needham, MA, USA). A 5 mL sample of each homogenized sample was sent to the USDA AMS National Sciences Laboratory in Gastonia, NC, USA, an ISO/IEC accredited laboratory (https://www.ams.usda.gov/services/lab-testing/nsl) (accessed on 8 July 2025), for OTC residue analysis using LC-MS/MS.
2.8. Trunk Injury
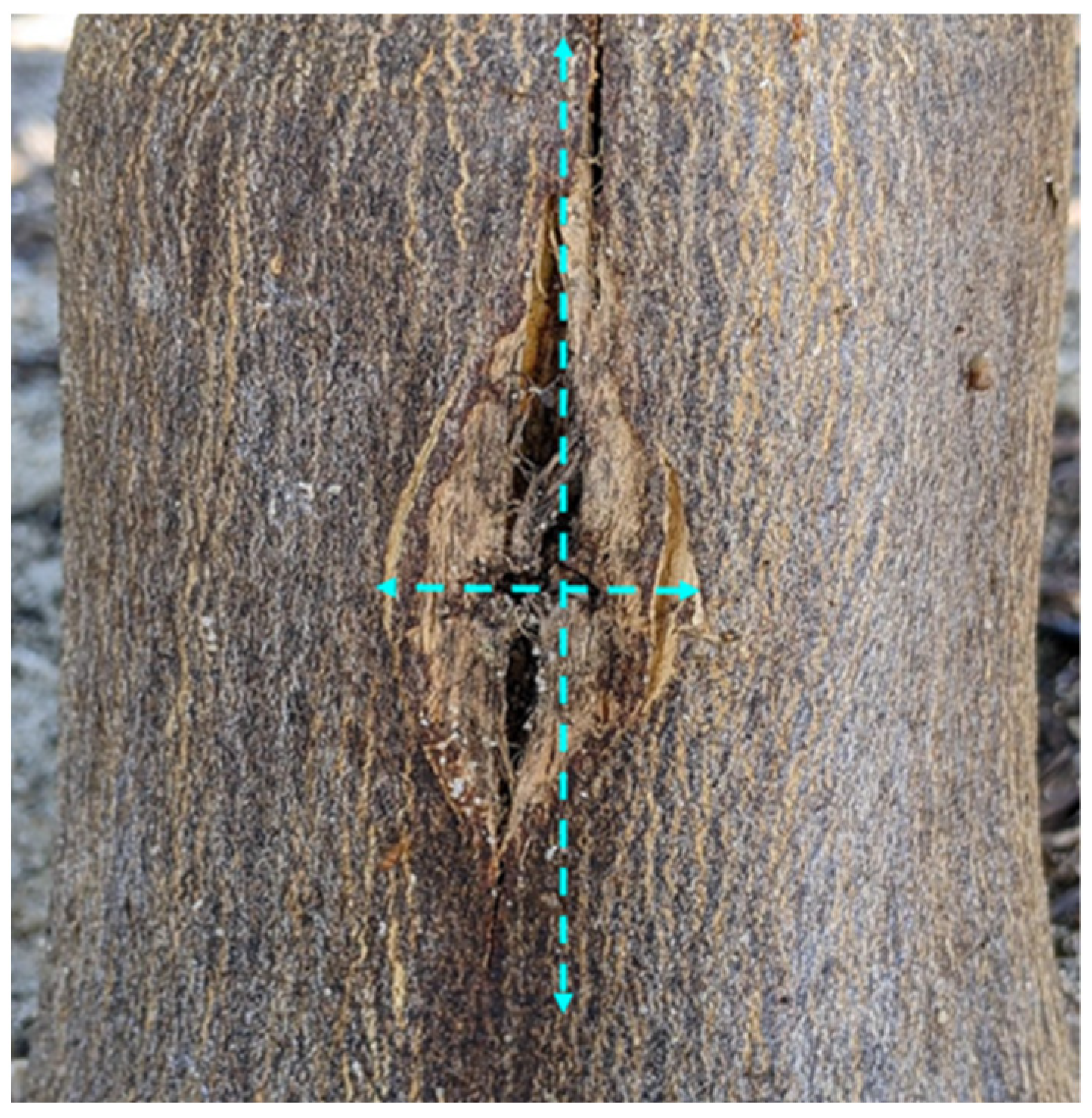
One year after each injection event, trunk injury was assessed by determining wound closure and wound size. Wound size was assessed by measuring the wound/bark crack length and width at its widest point (Figure 2) and expressed in cm. Drill hole closure was visually rated on a scale of 1 to 3, with 1 = hole fully closed, 2 = hole partially closed, and 3 = hole fully open. The values of all wounds caused in a year were averaged for statistical analysis.
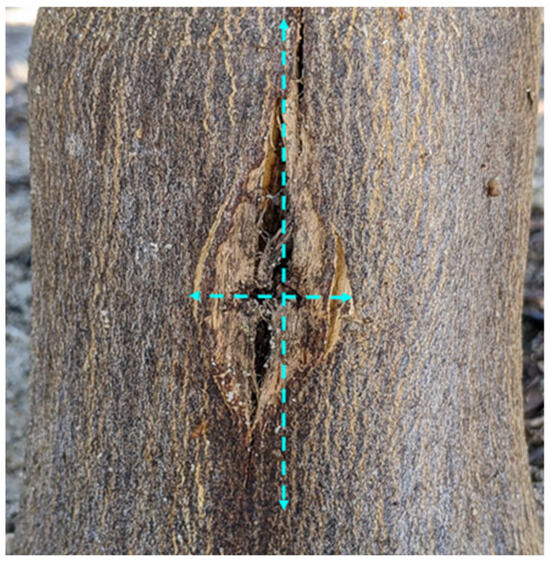
Figure 2.
Trunk injury assessments. Bark crack length and width were measured at the widest point (dashed lines).
2.9. Data Analysis
All statistical analyses were performed using RStudio Version 4.2.2 [47]. Prior to analysis, data were inspected for normality and homogeneity of variance. Data were log-transformed if assumptions were not met, but averages were presented in the non-transformed state. Treatment effects were analyzed using analysis of variance (ANOVA), with treatments as fixed factors and blocks as random factors. Differences were considered significant when the p value was smaller than 0.05. A post hoc comparison of means was conducted using Tukey’s honestly significant difference (HSD) test, and significant (p < 0.05) differences among means were indicated by different letters. Trunk injury data were analyzed using the Aligned Rank Transform (ART) procedure for non-parametric data [48], followed by post hoc pairwise comparison using Tukey’s honestly significant difference (HSD) test, with significant differences (p < 0.05) among means indicated by different letters.
3. Results
3.1. Candidatus Liberibacter Asiaticus (CLas) Detection
CLas titers are expressed as cycle threshold (Ct) values, with low Ct-values indicating high bacterial titers and high Ct-values indicating low titers. In May 2023, one year after the first injection, Ct-values ranged from 22.8 to 24.4 in trial 1 and from 26.5 to 28.5 in trial 2, and there was no significant difference among treatments (Table 2). In May 2024, one year after the second injection, Ct-values in trial 1 ranged from 24.4 to 25.5, and there were also no significant differences among treatments. In trial 2, there was a significant treatment effect. The highest Ct-value (34.9) and therefore lowest CLas titer was measured for trees injected with 792 mg in August (T6), while the lowest Ct-value (27.7) and therefore highest CLas titer was measured for trees sprayed with OTC (T1), followed by T0 (29.3).

Table 2.
Candidatus Liberibacter asiaticus detection in ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments.
3.2. Tree Size
The canopy volume of trees measured in June 2024 ranged from 2.2 m3 to 2.8 m3 in trial 1 and from 2.5 m3 to 2.9 m3 in trial 2, but there were no significant differences among treatments (Table 3). Neither were there any significant differences among treatments for the scion and rootstock trunk diameters, which were 7.9–8.5 cm (trial 1) and 7.4–7.6 cm (trial 2) and 10.5–11.8 cm (trial 1) and 9.7–10.4 cm (trial 2), respectively (Table 3).

Table 3.
Size of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments two years after start of treatments.
3.3. Fruit Drop
The percentage of fruit that dropped following Hurricane Ian in 2022 was 11.6–29.5% in trial 1 and 51.7–74.3% in trial 2 (Table 4). There were significant differences among treatments. In trial 1, the most hurricane-associated fruit drop (31.8% and 29.5%, respectively) was measured in trees that had been sprayed with OTC (T1) or not received any OTC (T0), and the lowest (11.6%) was in trees that had been injected with 792 mg of OTC in August (T6). In trial 2, the most hurricane-associated fruit drop (74.3%) was measured in T0, and the lowest (51.7%) was in trees that had been injected with 792 mg OTC in May in the scion (T4).

Table 4.
Percent fruit drop of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments after Hurricane Ian in 2022.
The pre-harvest fruit drop was significantly affected by the treatments in both years (Table 5). In trial 1, in 2023, significantly more fruit drop (70.7% and 75.0%, respectively) was observed for trees from T0 and T1 than for trees that had been injected with 792 mg of OTC (T4-T6; 43.1–51.8%). In 2024, significantly more fruit drop was measured for trees from T1 (55.0%) than for trees from T5 and T6 (38.4% and 35.3%, respectively). In trial 2, in both years, more fruit drop was measured for non-injected and OTC-sprayed trees compared to the other treatments, but differences among treatments were not statistically significant.

Table 5.
Pre-harvest fruit drop, yield, and fruit quality of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments.
3.4. Fruit Yield and Fruit Quality
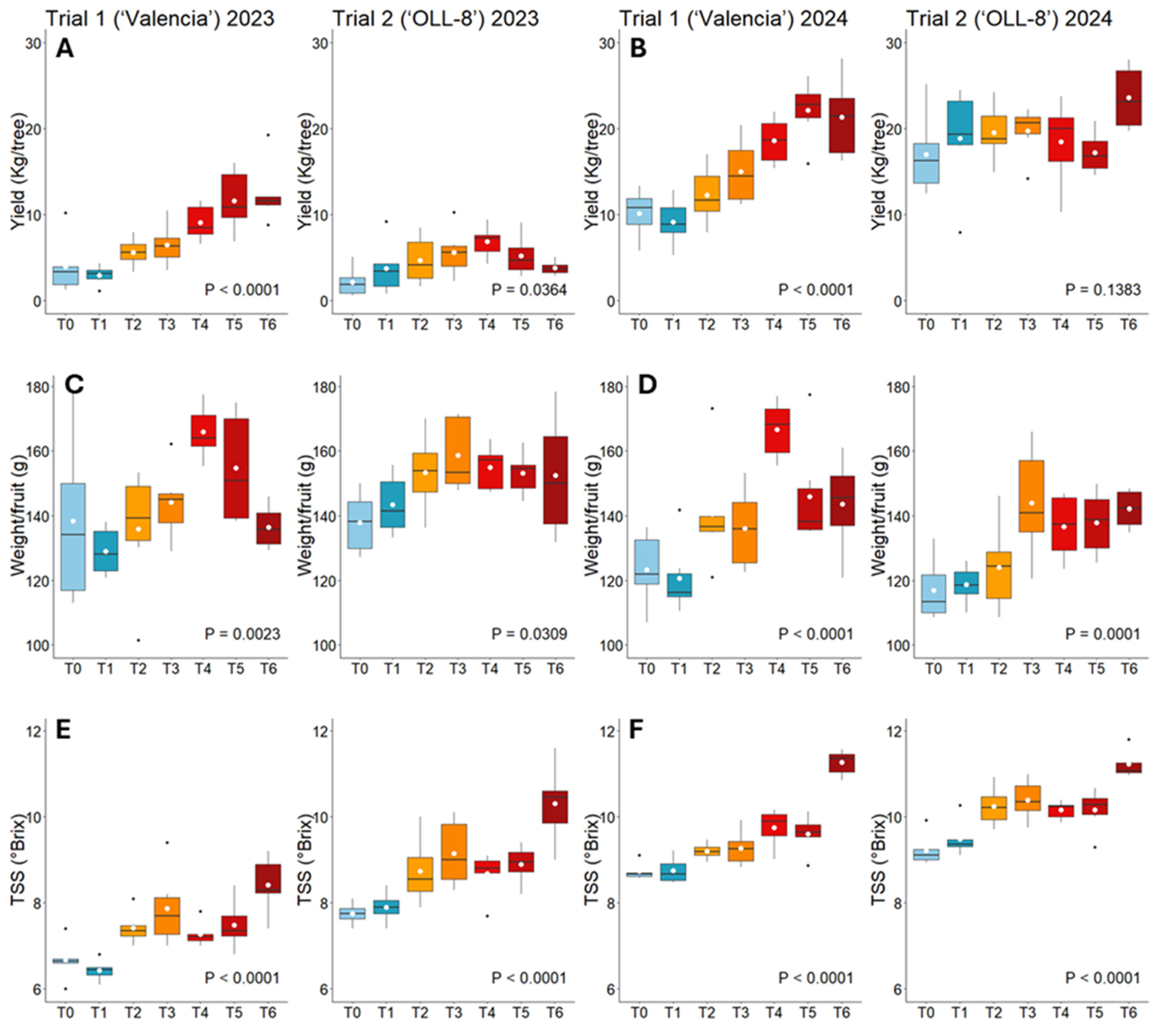
The fruit yield was significantly affected by the treatments in both years (Table 5, Figure 3A,B). In trial 1, in 2023, trees injected with 792 mg of OTC (T4–T6) produced significantly more fruit (9.1–12.4 kg/tree) compared to non-injected (T0) and sprayed trees (T1), which produced 4.0 kg/tree and 3.0 kg/tree, respectively. Trees injected with 150 mg and 225 mg of OTC using the drill-free system (T2 and T3) produced significantly less fruit (5.6 kg/tree and 6.5 kg/tree, respectively) than trees from T5 and T6 injected with 792 mg of OTC using the drill-based system (11.6 kg/tree and 12.4 kg/tree, respectively). The same effect was observed in 2024; the highest yields were measured for T5 and T6 (22.1 kg/tree and 23.6 kg/tree), followed by T4 (18.6 kg/tree), and the lowest by T0 and T1 (9.g kg/tree and 9.2 kg/tree, respectively). In trial 2, in 2023, the highest yield (6.9 kg/tree) was measured when trees received 792 mg of OTC administered into the scion in May (T4), which was significantly more compared to non-injected trees (T0; 2.2 kg/tree). In 2024, the average yield was 17.0–23.6 kg/tree, but there were no significant differences among treatments.
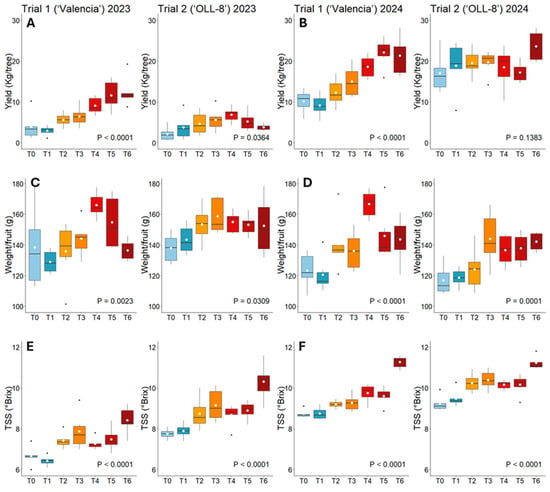
Figure 3.
Graphical depiction of selected variables. Yield (A,B), fruit weight (C,D), and TSS (E,F) of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments measured in 2023 and 2024. For statistical details, refer to Table 5 and Table 6. Data are presented as boxplots with median, interquartile range, and outliers.
The weight per fruit was significantly affected by the treatments (Table 5, Figure 3C,D). In trial 1, in 2023, the highest weight was measured for fruit from T4 (170 g), followed by T5 (160 g), and the lowest was measured for fruit from T1 (117 g) and T0 (124 g). The same effect was measured in 2024 with fruit from T4 and T5 weighing 167 g and 146 g, respectively, compared to 123 g and 121 g for fruit from T0 and T1, respectively. In trial 2, in 2023, fruit from T3 weighed significantly more than fruit from T0. In 2024, fruit from T3 weighed the most (144 g), followed by fruits from T5 and T6 (138 g and 142 g, respectively), while fruit from non-injected and OTC-sprayed trees weighed the least (117 g and 119 g, respectively).
The peel color was very green overall in 2023 and not significantly affected by the treatments in either trial (Table 5), although there was a trend for fruit from T6 being less green (−0.8) than fruit from the other treatments (−3.3 to −4.9) in trial 2. In 2024, in trial 1, the best (most orange) peel color was measured for fruit from trees injected in August with 792 mg of OTC (T6; 0.4). The worst (greenest) color was measured for T0 (−4.6) and T1 (−4.4). In trial 2, fruits were greener overall than in trial 1, but like in that trial, the best peel color was measured with T6 (−3.3) and the worst with T0 and T1 (−10.5 and −10.9, respectively).
3.5. Juice Quality
The TSS was significantly affected by the treatments in both years (Table 6, Figure 3E,F). In trial 1, in 2023, the highest TSS (8.4) was measured when trees were injected with 792 mg of OTC in August (T6). The lowest TSS (6.7 and 6.4, respectively) was measured for non-injected trees and OTC-sprayed trees (T0 and T1). In 2024, TSS was considerably higher overall than in 2023. Like in 2023, the highest TSS was measured for T6 (11.3), followed by T4 (9.8) and T5 (9.6). The lowest TSS (8.7) was measured for T0 and T1. In trial 2, in 2023, T6 also induced the highest TSS (10.3), followed by T3 (9.2). The lowest TSS (7.7 and 7.9, respectively) was measured for T0 and T1. In 2024, T6 induced a significantly higher TSS (11.5) compared to all other treatments, and T0 and T1 produced a significantly lower TSS (9.2 and 9.5, respectively) than T2–T5 (10.2–10.4).

Table 6.
Juice quality of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments.
The TA was significantly affected by treatments only in trial 1 in 2023, when it was significantly lower in T4 (0.79%) compared to all other treatments (0.94–1.1%) except T3.
In trial 1, in 2023, the TSS/TA ratio was highest for T4 (9.3), followed by T3 and T6 (8.6), and lowest for T0 (7.0) and T1 (6.8). In 2024, ratios were higher overall. The highest ratio (13.9) was measured for T6, followed by T4 (11.6). The lowest ratio (9.7) was measured for T1. In trial 2, there was no significant difference among treatments in 2023, although in general, ratios increased with increasing doses of OTC. In 2024, like in trial 1, the highest TSS/TA ratio (13.9) was measured for T6, and the lowest (10.0) was measured for T0. The ratio for the other treatments ranged from 10.6 (T1) to 12.4 (T3–T4) and 12.6 (T5).
The juice percentage and juice color were only measured in 2024 (Table 6). There was no significant difference among treatments for the juice percentage in either trial. In trial 1, the highest juice color index was measured for fruits from T4 and T6 (37.6–37.7), followed by T5 (37.5), and the lowest was measured for T1. In trial 2, the highest juice color index was measured for fruits from T6 (38.4) compared to most other treatments (37.7–37.9).
3.6. Fruit OTC Residue Analysis
Fruit OTC residues were significantly influenced by the treatment (Table 7). In trial 1, in 2023, fruit residues were highest (10.8 ppb) when trees had been injected with 792 mg in August (T6) and lowest (0.0 ppb) when they were sprayed with OTC. In 2024, residues in fruits from T6 were 13.7 ppb, which was significantly higher compared to the other treatments (0.0–3.3 ppb).

Table 7.
Parts per billion of OTC detected at harvest in whole fruits of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments.
In trial 2, in 2023, the highest OTC residues (8.0 ppm) were detected in fruits from T6, followed by T3 (5.7 ppb). No residues were detected for T1. Fruit residues from treatments in which trees received 792 mg of OTC in May were 3.0 ppb (T4) and 1.7 ppb (T5). In 2024, the highest OTC residues (30.3 ppb and 26.0 ppb) were detected when trees were injected with 300 mg, delivered in two injections (May and September, T3), and with 792 mg in September (T6). Significantly lower residues (0.0–3.0 ppb) were found in fruits from the other treatments, except T2 (14.0 ppb).
3.7. Trunk Injury
In both trials, by May 2023, most of the drill holes created in the previous year were completely closed, and there was no significant difference among treatments (Table 8). In 2024, there was a significant treatment effect for hole closure in both trials, but differences among means were not significant.

Table 8.
Wound closure and bark crack size of ‘Valencia’ and ‘OLL-8’ trees subjected to different injection treatments.
There was a significant treatment effect for bark cracking in both trials and both years (Table 8). In trial 1, in 2023 bark cracks were significantly longer (5.1 cm and 6.8 cm, respectively) and wider (3.1 cm and 2.6 cm) when OTC was delivered using the drill-free system (T2 and T3) than when it was delivered with the drill-based system (T4–T6), which resulted in cracks with a length of 1.3–1.9 cm and a width of 1.1–1.3 cm. In 2024, the longest cracks were measured in response to T3 (6.5 cm), followed by T2 (4.5 cm), while the shortest cracks (2.0 cm) were measured when the highest dose of OTC was injected into the scion using the drill-based system (T4). Bark cracks were significantly wider (1.8–2.1 cm) in response to T2, T3, and T6 than to T4 and T5 (1.1 cm and 1.2 cm, respectively). In trial 2, in 2023, the longest bark cracks (8.5 cm) were measured in response to T3, followed by T2 (5.6 cm), while the shortest were measured in response to T4 (1.9 cm). Cracks were wider (2.3 cm and 2.4 cm, respectively) when the drill-free system (T2 and T3) was used than when the drill-based system was used (1.3–1.7 cm). Like in 2023, in 2024, T3 followed by T2 produced the longest bark cracks (8.1 cm and 5.1 cm, respectively), but the shortest cracks were measured for T5 (2.1 cm). Bark crack width was 1.0–1.4 cm and not significantly affected by the treatments in that year. Typical wound phenotypes are shown in Figure 4.

Figure 4.
Typical wound phenotypes after drill-free (A–C) and drill-based injection (D,E). (A–E), rootstock injection; (F) = scion injection. Note moderate to no bark cracking in (A,D–F) and more extensive bark cracking in (B,C).
Aside from the bark cracking, no deleterious effects were observed. This includes yellowing or bronzing of the leaves and other phytotoxic effects often observed after OTC injections [49].
4. Discussion
Antibiotics are important tools for the management of bacterial diseases of tree crops and have been used in plant agriculture since the 1950s [50]. Of the three antibiotics currently allowed in plant agriculture, streptomycin (registered since 1958) has been the most used, primarily to manage fire blight, a bacterial disease caused by Erwinia amylovora [51]. Streptomycin and oxytetracycline (OTC) were approved for HLB management in Florida in 2016, but they were found to be of limited use when applied by spray. The systemic delivery of OTC by trunk injection was approved in Florida in 2022 in a desperate attempt to manage this destructive disease.
The results from this study confirmed the results from previous studies documenting the efficacy of trunk-injected OTC on yield as well as fruit and juice quality in HLB-affected citrus trees [33,34,52]. It also showed that higher doses of OTC are needed to maximize the beneficial effects of the antibiotic. In the ‘Valencia’ trial, in both production seasons, the least pre-harvest fruit drop and the highest yield were measured when trees received the highest dose of OTC (792 mg) with the drill-based system. Delivering moderate doses of OTC (300 mg) with the drill-free system also produced beneficial effects, but at a lesser magnitude. The least benefits were derived from the 150 mg dose, which is the maximum dose recommended for use with the drill-free system currently. A recent study on 10-year-old ‘Valencia’ trees also showed that a higher dose of OTC produces higher yields and better-quality fruits and considerably larger differential benefits in terms of dollars per acre compared to a lower dose [52]. However, the effects of OTC injections in the ‘OLL-8’ trial were less evident. The hurricane in 2022 caused considerable fruit drop at this location, likely because of its more exposed location on Florida’s Central Ridge, an ancient sand dune system with a higher elevation than the rest of the Florida peninsula. Despite this, an increase in yield was observed when trees received the highest dose of OTC in May using the drill-based system. No such effect was observed in 2024. One possible explanation for the lack of effect in that production season was the higher productivity of the ‘OLL-8’ trees—nearly double compared to ‘Valencia’—which may have limited additional improvement. OLL-8 trees also had lower leaf CLas titer levels in both years compared to the ‘Valencia’ trees. This suggests a higher tolerance of ‘OLL-8’ compared to ‘Valencia’, although orchard management and other local conditions may have contributed to the lower CLas titers and higher productivity.
Despite the increased yield in response to the injections in the ‘Valencia’ trial, considerable fruit drop was noted shortly before the harvest. This suggests that the effect of the antibiotic did not last long enough to completely prevent HLB-induced pre-harvest fruit drop, one of the most serious consequences of the disease in Florida. Harvesting the fruits earlier may have prevented fruit loss, though at the expense of juice quality (TSS). As Florida citrus growers are paid based on the soluble solids content of their fruits, harvesting must be timed to achieve the highest possible TSS while safeguarding yield. Combining OTC injections with practices to promote fruit retention, such as the application of plant growth regulators [53], might enhance the benefits derived from the antibiotic. The absence of a long-lasting effect of OTC injections was also reflected in the lack of notable differences in leaf CLas titers measured 12 months after injection, except in the ‘OLL-8’ trial in 2024, when titers were lower in response to the highest dose of OTC, particularly when administered in September. The lack of a long-term effect of one single annual OTC injection on CLas titers was also observed in a recent study conducted on mature ‘Valencia’ trees in a commercial citrus production environment [52]. Like in that study, no growth differences were measured after two years of treatment in this study, suggesting that more years of injection may be needed for growth effects to manifest under well-managed commercial growing conditions.
Previous field studies documented that OTC effects on CLas titers are most detectable during the early months after injection [32,33]. It may therefore be assumed that the positive effects of the injected OTC were the result of a temporary suppression of the bacteria during the early months after injection. Greater and longer-lasting effects can be achieved by multiple injections and/or higher OTC doses within the same production season [32,33,54]. However, the maximum annual amount of OTC must not exceed the dose specified on the label. Ultimately, bacterial resistance may occur, although Glusberger et al. [55] suggested that resistance may develop slowly, if at all, because of the restrictive habitat of the vector and pathogen and because they found a low frequency of spontaneous mutations in a culturable relative of CLas, Liberibacter crescens, exposed to the antibiotic. In any case, additional therapies, whether they be antimicrobial peptides, flavonoids, immune inducers, or other molecules [56,57], are needed to reduce the likelihood of resistance development.
One of the most serious consequences of HLB is the reduction in fruit and juice quality. Fruits from affected trees are smaller, do not color properly, and contain fewer soluble solids compared to fruits from healthy trees [58,59]. This has major economic consequences as producers of processing oranges are paid by the amount of TSS [14]. OTC injections improved most fruit and juice quality variables in this study, with the most prominent effect observed for the fruit weight and TSS. TSS was higher overall in ‘OLL-8’ fruits compared to ‘Valencia’ fruits, suggesting this variety as a promising alternative for juice processing in Florida. However, different soil properties, orchard management, and other local conditions could also have contributed to differences between trials. While all injection treatments increased the fruit weight (and concomitantly, fruit size), August and September injections resulted in the highest TSS.
Fruit development in citrus can be divided into three stages or phases [60]: phase I, from anthesis to the end of physiological fruitlet abscission (June drop), during which cell division predominates and growth is moderate; phase II, from the end of June drop to shortly before fruit color change (in Florida, around September), during which cells enlarge rapidly and fruits reach their maximum size; and phase III, during which growth is halted and fruits begin a non-climacteric ripening process. Our results suggest that injections occurring during the cell division phase (e.g., May) promote cell division and cell elongation, while injections occurring during or near the end of phase II (e.g., August or September) have less effect on growth and promote the accumulation of sugars [61]. Another reason for the growth effect on fruits may be a consequence of the OTC to increase the abundance of certain plant growth-promoting microbes, as recently demonstrated in a field study in Florida [62]. That study also discovered that changes in the plant microbiome are most evident during the early days or weeks after injection and diminish thereafter. Other studies are in progress to determine the longer-term effects of OTC trunk injections on the whole tree microbiome.
In addition to fruit weight and TSS, peel color, juice color, and the TSS/TA ratio were improved in both trials, with larger OTC doses generally having larger effects and late injections producing a better peel color. The larger effects of higher doses of OTC on fruit and juice quality were also observed in a recent field study [52]. Early investigations in South Africa also found considerably fewer symptomatic fruits following trunk injection of antibiotics [26,27].
Injection of antibiotics into fruit trees may raise concerns among consumers. However, the allowed fruit OTC residue level when delivering OTC into citrus trees by trunk injection is 10 ppb or 0.01 ppm (mg/L) with a pre-harvest interval (PHI; the period between the last permitted application and harvest) of 180 days. For comparison, the residue tolerance for OTC in pome fruits is 350 ppb (0.35 ppm) with a PHI of 45 days. The residue tolerance for citrus under the 2016 section 18 emergency exemption granting foliar application of OTC was 400 ppb (0.40 ppm) (https://www.federalregister.gov/documents/2017/03/10/2017-04795/oxytetracycline-pesticide-tolerances-for-emergency-exemptions) (accessed on 8 July 2025). Moreover, based on a risk assessment study from 2011 by the United States Environmental Protection Agency, the potential dietary exposure of humans to oxytetracycline used in plant agriculture is unlikely to cause any harm compared with its pharmaceutical use [51].
Fruit OTC residues in the present study were analyzed more than 180 days after injection, in compliance with the required PHI. In both trials, the highest residues were measured when the highest OTC dose (792 mg) was injected in August or September (T6). In 2024, those residues exceeded the maximum allowed tolerance of 10 ppb despite complying with the required PHI. In contrast, residues were below 10 ppb when the same dose was injected in May (T4 and T5). Moreover, in 2024, ‘OLL-8’ fruits exceeded the tolerance limit even when lower doses of OTC (150 and 300 mg) were injected, and the treatment included a late (September) application. One reason for the substantially higher residues when performing injections late in the year could be the shorter period between injection and harvest. However, ongoing studies with ‘Hamlin’ sweet orange, a variety that is harvested several months before ‘Valencia’ or ‘OLL-8’, did not detect residues when injections were performed in June and trees were harvested in December. This suggests that the phenological stage, specifically the size of the fruits at the time of injection, is crucial for preventing higher-than-allowed residues from accumulating. Injecting in phase I appears to result in OTC dilution as fruitlets undergo cell division and expansion. This is not the case when injecting at the end of phase II, when the fruits have reached their near maximum size, consequently resulting in higher residues. It is unclear why OTC residues were higher for ‘OLL-8’ than ‘Valencia’ fruits in 2024, since both varieties mature during the same period (https://ffsp.net/varieties/citrus/oll-8/) (accessed on 8 July 2025). Environmental differences and/or slight differences in fruit physiology between the two varieties may have contributed to these findings.
Both the drill-based and the drill-free injection systems successfully delivered OTC into the trees. While the translucent wall of the Chemjets allowed easy monitoring of the injection progress, the opaque canisters used with the Trecise system had to be weighed to confirm that all OTC had been taken up by each tree. This prevented the assessment of uptake efficacy and the potential implications for the efficacy of the treatments.
Six months after initiating this study, the systemic delivery of oxytetracycline hydrochloride by trunk injection was registered for commercial use in Florida citrus production. Two formulations were registered, one in October 2022 and another in January 2023, to be administered using a drill-based method. This was followed by the rapid adoption of the technology throughout most commercial production sites [63]. The drill-free method investigated in this study was registered for use in August 2023. The drill-based method can be used for any size tree with a trunk diameter of 3 cm or larger, with doses increasing relative to the trunk diameter and a maximum dose of 1.65 g of OTC per tree per season. In contrast, the drill-free (Trecise) system is recommended for use on trees with a minimum trunk diameter of 1 cm but not exceeding 15 cm (https://invaiocitrus.com/solutions/) (accessed on 8 July 2025). The maximum dose that is to be applied with the Trecise system, based on the current label, is 0.15 g of active ingredient OTC per cropping season. The amount of OTC to be used for trees in the 10–15 cm trunk diameter range recommended for the drill-based system is 0.55–1.1 g, which is considerably more. The results from this study and a recently completed study [52] documented that OTC effects are larger with increasing amounts of active ingredient for trees in this size range. It is therefore unlikely that the drill-free system can produce the needed improvements to render citrus production profitable under Florida’s HLB-endemic conditions unless the maximum allowed dose is increased for larger trees.
Drill-free systems are generally promoted not only as requiring less active ingredient but also as being less invasive (https://www.invaio.com/media/press-releases/2023/invaio-achieves-first-registration-for-citrus-greening-solution-featuring-trecise-technology) (accessed on 8 July 2025) [23,64]. This was not the case in this study. Aside from the lower efficacy, the drill-free system caused considerably more trunk injury, manifested by longer and wider bark cracks, than the drill-based system, despite the lower concentration of active ingredient. Using an impact hammer in combination with the orientation of the injector tip (perpendicular rather than parallel to the orientation of the xylem fibers—as per the instructions) may have been responsible for the more severe trunk damage with this delivery system. This is different from other drill-free systems, which are introduced so that the longest edge of the injector tip is oriented axially and parallel to the orientation of the xylem vessels. Axial insertion is in line with the CODIT principle as it takes advantage of the strong compartmentalization capacity of the cells of the ray parenchyma [36,37,38]. Indeed, Aćimović et al. [64] found that a drill-free, needle (blade)-type injection system inserted in the axial orientation caused less injury in apple trees than conventional drill-based systems. The damage associated with the drill-free system observed in this study may also have been exacerbated by a slower delivery of the OTC through the small openings in the microinjection tip and therefore longer exposure to the antibiotic’s damaging effects. It has previously been noted that chemicals that are not efficiently distributed cause more damage because of the resulting high local concentration [65]. Compared with the drill-free system, the damage caused by the drill-based method used in this study was moderate overall but more extensive when trees were injected in August or September than in May. This is likely associated with the lower metabolic activity of the tree at the end of the growing season than in spring and was previously demonstrated for citrus [40] and other trees [66]. Drill-based injection into the scion as opposed to the rootstock did not result in any major differences regarding wound closure and bark cracking, suggesting that both rootstock and scion may be used when performing multiple years of injection to maintain the structural integrity of the tree. Moreover, no significant differences were observed for fruit production and fruit and juice quality between scion- and rootstock-injected trees, indicating that the graft union did not play a major role in the translocation of the OTC along the vascular tissues.
5. Conclusions
The results of this study confirm that the systemic delivery of antibiotics is a promising strategy to mitigate HLB in areas where the disease is endemic, while also confirming that spray applications are not effective. Administering higher doses of OTC with the drill-based method resulted in larger effects on tree productivity and less trunk injury than administering lower doses with the drill-free system. The results further indicate that different scion varieties may respond differently to the injections and that optimization is necessary to maximize effects and weigh the risks of injections against their beneficial effects. Regardless of the variety and OTC dose used in this study, injections during the end of phase II in the fruit developmental cycle, while improving TSS, resulted in higher-than-allowed OTC fruit residues and should therefore be avoided.
Taken together, under the conditions of this study, the drill-based application of higher doses of OTC was superior to drill-free application using lower doses of OTC, not only in terms of tree productivity but also in terms of trunk injury and logistics (two vs. one annual injection using the drill-free and drill-based system, respectively). Moreover, the registered drill-free method currently does not allow injection of more than 150 mg of OTC per tree annually for the size of trees used here, which may not result in benefits large enough to justify the risks and costs of the application. Although the drill-based injection method used in this study is not the one suggested for commercial injections, both are fundamentally similar and were demonstrated to be equally effective [52].
Author Contributions
Conceptualization, U.A.; methodology, U.A., C.T., and J.d.F.; software, U.A. and C.T.; formal analysis, U.A. and C.T.; investigation, U.A., C.T., J.d.F., and G.M.; resources, U.A.; writing—original draft preparation, U.A.; writing—review and editing, C.T., G.M., and J.d.F.; visualization, U.A. and C.T.; supervision, U.A.; project administration, U.A.; funding acquisition, U.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Citrus Research and Development Foundation (22-001), USDA NIFA SCRI CDRE (2019-70016-29096), and USDA NIFA Hatch project FLA-SWF-006160.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Tiago Lelis and all UF/IFAS SWFREC Plant Physiology team members for their help in conducting the trials and collecting data, and the grower collaborator for providing the trees and tree care. The graphical abstract was created in BioRender (https://BioRender.com) (accessed on 8 August 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2015, 58, 373–387. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllid (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Florida Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G., Jr.; Li, Z.-G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci. 2008, 175, 291–306. [Google Scholar] [CrossRef]
- Kim, J.-S.; Sagaram, U.S.; Burns, J.K.; Li, J.-L.; Wang, N. Response of sweet orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ infection: Microscopy and microarray analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Achor, D.S.; Etxeberria, E.; Wang, N.; Folimonova, S.Y.; Chung, K.R.; Albrigo, L.B. Sequence of anatomical symptom observations in citrus affected with Huanglongbing disease. Plant Pathol. J. 2010, 9, 56–64. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.B. Citrus huanglongbing: The pathogen and its impact. Plant Health Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- Graham, J.H.; Bassanezi, R.B.; Dawson, W.O.; Dantzler, R. Management of huanglongbing of citrus: Lessons from São Paulo and Florida. Annu. Rev. Phytopathol. 2024, 62, 243–262. [Google Scholar] [CrossRef]
- Castro, C. Citrus Annual Report; USDA Foreign Agricultural Service: Washington, DC, USA, 2024. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Citrus%20Annual_Brasilia_Brazil_BR2023-0036.pdf (accessed on 14 May 2025).
- Halbert, S.E. The discovery of huanglongbing in Florida. In Proceedings of the International Citrus Canker and Huanglongbing Research Workshop, Orlando, FL, USA, 7–11 November 2005; p. H-3. [Google Scholar]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- USDA NASS. Florida Citrus Statistics 2023–2024. Available online: https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Statistics/2023-24/FCS2024.pdf (accessed on 14 May 2025).
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.W.; Singerman, A.; Ritenour, M.A.; Qureshi, J.; Alferez, F. 2024–2025 Florida Citrus Production Guide: Citrus Under Protective Screen (CUPS) Production Systems; CPG Ch. 21, HS1304/CMG19, Rev. 5 EDIS 2024 (CPG); University of Florida: Gainesville, FL, USA, 2023. [Google Scholar] [CrossRef]
- Gaire, S.; Albrecht, U.; Batuman, O.; Qureshi, J.; Zekri, M.; Alferez, F. Individual protective covers (IPCs) to prevent Asian citrus psyllid and Candidatus Liberibacter asiaticus from establishing in newly planted citrus trees. Crop Prot. 2021, 152, 105862. [Google Scholar] [CrossRef]
- Gaire, S.; Albrecht, U.; Batuman, O.; Qureshi, J.; Zekri, M.; Alferez, F. Individual protective covers improve yield and quality of citrus fruit under endemic Huanglongbing. Plants 2024, 13, 2284. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Vincent, C.I.; Hijaz, F.; Pierre, M.; Killiny, N. Systemic uptake of oxytetracycline and streptomycin in huanglongbing-affected citrus groves after foliar application and trunk injection. Antibiotics 2022, 11, 1092. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk injection as a tool to deliver plant protection materials–An overview of basic principles and special considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Esparraguera, L.B.; Queiroz, S.C.N.; Bottoli, C.B.G. Vegetative Endotherapy—Advances, Perspectives, and Challenges. Agriculture 2023, 13, 1465. [Google Scholar] [CrossRef]
- Schwarz, R.E.; Moll, J.N.; van Vuuren, S.P. Control of citrus greening and its psylla vector by trunk injections of tetracyclines and insecticides. Int. Organ. Citrus Virol. Conf. Proc. 1974, 6, 26–29. [Google Scholar] [CrossRef]
- Van Vuuren, S.P. The determination of optimal concentration and pH of tetracycline hydrochloride for trunk injection of greening-infected citrus trees. Phytophylactica 1977, 9, 77–81. [Google Scholar]
- Aubert, B.; Bové, J.M. Effect of penicillin or tetracycline injections of citrus trees affected by greening disease under field conditions in Reunion Island. Int. Organ. Citrus Virol. Conf. Proc. 1980, 8, 103–108. [Google Scholar] [CrossRef]
- Da Graça, J.V. Citrus greening disease. Annu. Rev. Phytopathol. 1991, 29, 109–136. [Google Scholar] [CrossRef]
- Zhang, M.; Powell, C.A.; Zhou, L.; He, Z.; Stover, E.; Duan, Y. Chemical compounds effective against the citrus Huanglongbing bacterium ‘Candidatus Liberibacter asiaticus’ in planta. Phytopathology 2011, 101, 1097–1103. [Google Scholar] [CrossRef]
- Zhang, M.; Powell, C.A.; Guo, Y.; Doud, M.S.; Duan, Y. A graft-based chemotherapy method for screening effective molecules and rescuing huanglongbing-affected citrus plants. Phytopathology 2012, 102, 567–574. [Google Scholar] [CrossRef]
- Hu, J.; Wang, N. Evaluation of the spatiotemporal dynamics of oxytetracycline and its control effect against citrus huanglongbing via trunk injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Archer, L.; Qureshi, J.; Albrecht, U. Efficacy of trunk injected imidacloprid and oxytetracycline in managing huanglongbing and Asian citrus psyllid in infected sweet orange (Citrus sinensis) trees. Agriculture 2022, 12, 1592. [Google Scholar] [CrossRef]
- Archer, L.; Kunwar, S.; Alferez, F.; Batuman, O.; Albrecht, U. Trunk injection of oxytetracycline for huanglongbing management in mature grapefruit and sweet orange trees. Phytopathology 2023, 113, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.C.; VanWoerkom, A.H.; Aćimovićc, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk injection: A discriminating delivering system for horticulture crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 126. [Google Scholar] [CrossRef]
- Shigo, A.L.; Marx, H.G. Compartmentalization of Decay in Trees; US Department of Agriculture, Forest Service: Washington, DC, USA, 1977; Volume 405, p. 73. [Google Scholar]
- Morris, H.; Brodersen, C.; Schwarze, F.W.M.R.; Jansen, S. The parenchyma of secondary xylem and its critical role in tree defense against fungal decay in relation to the CODIT model. Front. Plant Sci. 2016, 7, 1665. [Google Scholar] [CrossRef]
- Morris, H.; Hietala, A.M.; Jansen, S.; Ribera, J.; Rosner, S.; Salmeia, K.A.; Schwarze, F.W.M.R. Using the CODIT model to explain secondary metabolites of xylem in defence systems of temperate trees against decay fungi. Ann. Bot. 2020, 125, 701–720. [Google Scholar] [CrossRef]
- Archer, L.; Albrecht, U. Evaluation of trunk injection techniques for systemic delivery of huanglongbing therapies in Citrus. HortScience 2023, 58, 768–778. [Google Scholar] [CrossRef]
- Archer, L.; Albrecht, U. Wound reaction to trunk injection of oxytetracycline or water in huanglongbing-affected sweet orange (Citrus sinensis) trees. Trees 2023, 37, 1483–1497. [Google Scholar] [CrossRef]
- Girelli, C.R.; Husain, M.; Verweire, D.; Oehl, M.C.; Massana-Codina, J.; Avendano, M.S.; Migoni, D.; Scortichini, M.; Fanizzi, F.P. Agro-active endo-therapy treated Xylella fastidiosa subsp. pauca-infected olive trees assessed by the first 1H-NMR-based metabolomic study. Sci. Rep. 2022, 12, 5973. [Google Scholar] [CrossRef]
- Grandi, L.; Oehl, M.; Lombardi, T.; Rocco de Michele, V.; Schmitt, N.; Verweire, D.; Balmer, D. Innovations towards sustainable olive crop management: A new dawn by precision agriculture including endo-therapy. Front. Plant Sci. 2023, 14, 1180632. [Google Scholar] [CrossRef]
- Obreza, T.A.; Collins, M.E. Common Soils Used for Citrus Production in Florida; SL 193; University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS: Gainesville, FL, USA, 2002; Available online: https://ufdc.ufl.edu/ir00003134/00001 (accessed on 14 July 2024).
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Meth. 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wutscher, H.K.; Hill, L.L. Performance of ‘Hamlin’ orange on 16 rootstocks in East-central Florida. HortScience 1995, 30, 41–44. [Google Scholar] [CrossRef]
- John Bean Technologies. 2018. Available online: https://www.jbtc.com/foodtech/wp-content/uploads/sites/2/2021/08/Procedures-Analysis-Citrus-Products.pdf (accessed on 14 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 14 May 2025).
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar]
- Albrecht, U.; Tardivo, C.; Nunes, L.; Pugina, G.; Moreno, G.; de Freitas, J. The Good, the Bad, and the Ugly: Optimizing OTC Injections; Citrus Industry: Newberry, FL, USA, 2024; Volume 105, pp. 6–9. Available online: http://www.mirabelsmagazinecentral.com/DigitalEdition/index.html?id=bcb2106b-a6b5-4cbe-af75-f61ecdd3c926 (accessed on 14 May 2025).
- Batuman, O.; Britt-Ugartemendia, K.; Kunwar, S.; Yilmaz, S.; Fessler, L.; Redondo, A.; Chumachenko, K.; Chakravarty, S.; Wade, T. The use and impact of antibiotics in plant agriculture: A review. Phytopathology 2024, 114, 885–909. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in agriculture. Rev. Sci. Tech. Off. Int. Epiz. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Albrecht, U.; Tardivo, C.; Moreno, G.; de Freitas, J.; Singerman, A.; Plotto, A.; Bai, J. Managing endemic huanglongbing in commercial citrus production through vascular delivery of oxytetracycline. Crop Prot. 2025, 195, 107250. [Google Scholar] [CrossRef]
- Shahzad, F.; Livingston, T.; Vashisth, T. Gibberellic acid mitigates Huanglongbing symptoms by reducing osmotic and oxidative stress in sweet orange. Sci. Hortic. 2024, 329, 112976. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of citrus huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Glusberger, P.R.; Russell, J.T.; Cohn, A.R.; Petrone, J.R.; Lai, K.-K.; Triplett, E.W. Whole genome analysis of spontaneous antimicrobial resistance in Liberibacter crescens suggests long-term efficacy for antimicrobial treatment of citrus greening disease. J. Citrus Pathol. 2023, 11, 1–5. [Google Scholar] [CrossRef]
- Irigoyen, S.; Ramasamy, M.; Pant, S.; Niraula, P.; Bedre, R.; Gurung, M.; Rossi, D.; Laughlin, C.; Gorman, Z.; Achor, D.; et al. Plant hairy roots enable high throughput identification of antimicrobials against Candidatus Liberibacter spp. Nat. Commun. 2020, 11, 5802. [Google Scholar] [CrossRef]
- Aksenov, A.; Blacutt, A.; Ginnan, N.; Rolshausen, P.E.; Melnik, A.V.; Ali, L.; Gentry, E.C.; Ramasamy, M.; Zuniga, C.; Zengler, K.; et al. Spatial chemistry of citrus reveals molecules bactericidal to Candidatus Liberibacter asiaticus. Sci. Rep. 2024, 14, 20306. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Plotto, A.; Manthey, J.A.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of Liberibacter infection (huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Rosales, R.; Burns, J.K. Phytohormone changes and carbohydrate status in sweet orange fruit from huanglongbing-infected trees. J. Plant Growth Regul. 2011, 30, 312–321. [Google Scholar] [CrossRef]
- Bain, J.M. Morphological, anatomical, and physiological changes in the developing fruit of the Valencia orange, Citrus sinensis (L.) Osbeck. Aust. J. Bot. 1958, 6, 1–23. [Google Scholar] [CrossRef]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Hinosa, A.; González-López, J.; Tardivo, C.; Monus, B.; de Freitas, J.; Strauss, S.L.; Albrecht, U. Trunk injection of oxytetracycline improves plant performance and alters the active bark and rhizosphere microbiomes in huanglongbing-affected citrus trees. Biol. Fertil. Soils 2024, 60, 563–576. [Google Scholar] [CrossRef]
- Giles, F. Florida citrus industry reflects industry optimism. Citrus Indust. 2024, 105, 20–21. [Google Scholar]
- Aćimović, S.G.; Cregg, B.M.; Sundin, G.W.; Wise, J.C. Comparison of drill- and needle-based tree injection technologies in healing of trunk injection ports on apple trees. Urban For. Urban Green. 2016, 19, 151–157. [Google Scholar] [CrossRef]
- Stennes, M.A.; French, D.W. Distribution and retention of thiabendazole hypophosphite and carbendazim phosphate injected into mature American elms. Phytopathology 1987, 77, 707–712. [Google Scholar] [CrossRef]
- Dujesiefken, D.; Liese, W. The CODIT Principle: Implications for Best Practice; International Society of Arboriculture: Champaign, IL, USA, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).