Sucrose Transporter 2 Knockout Increases Sugar Content in Tomato Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Vector Construction and Tomato Transformation
2.3. Subcellular Localization
2.4. Plant Phenotyping
2.5. RNA Extraction and Gene Expression
2.6. RNA-Seq
2.7. Heterologous Expression in Yeast
2.8. Sugar Induction Experiment
3. Results
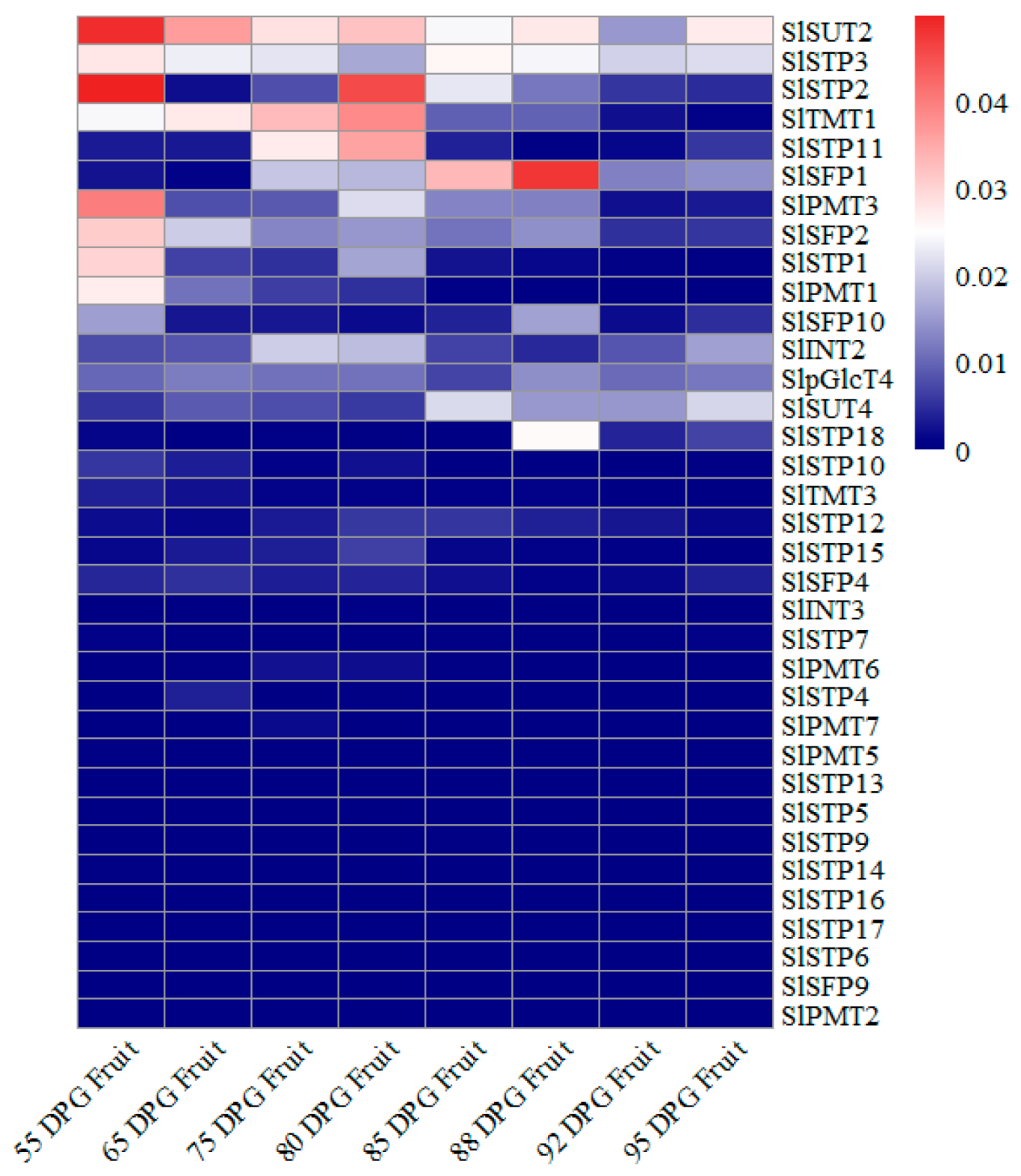
3.1. SUT2 Expression Decreased with Fruit Ripening in Tomato
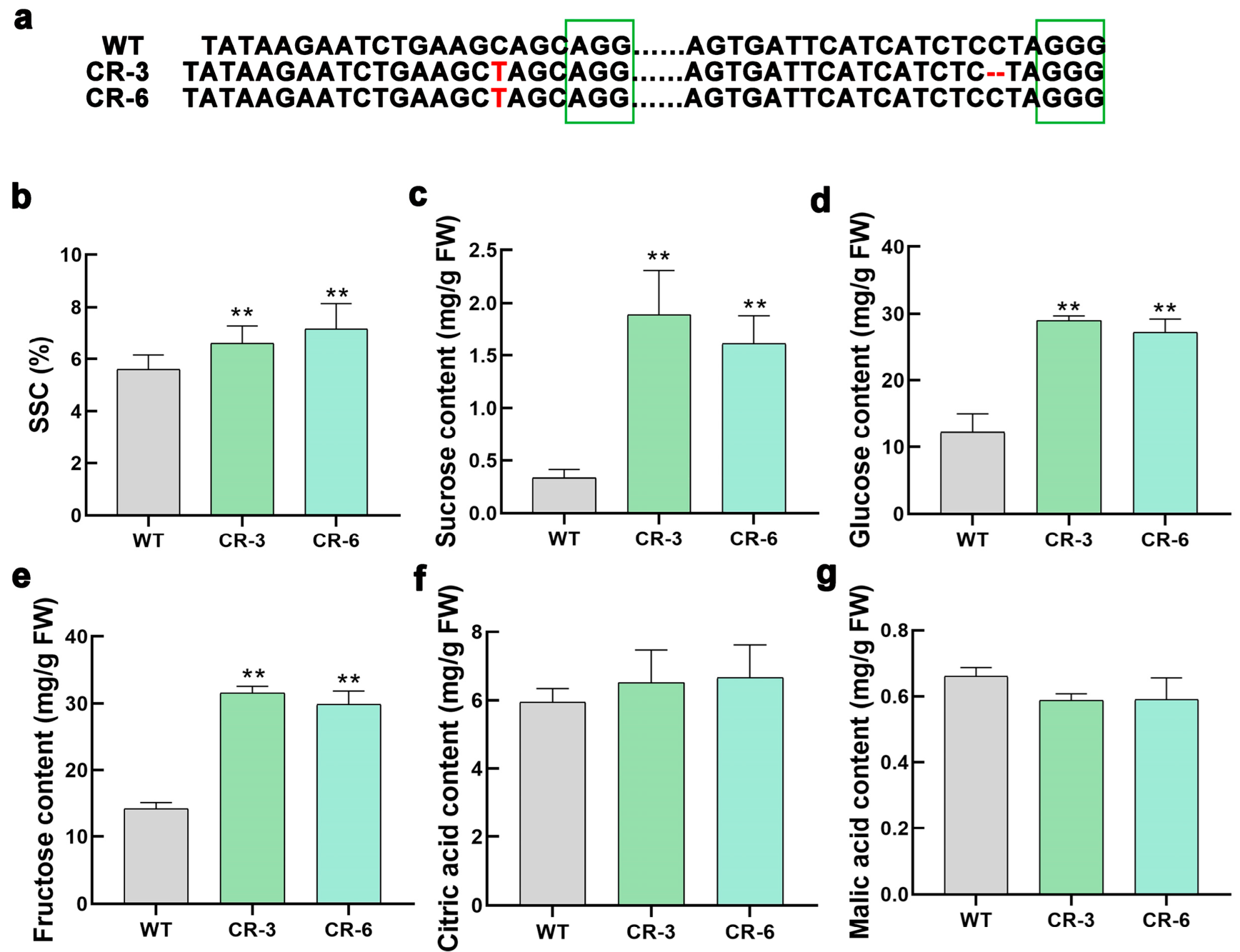
3.2. SUT2 Knockout Promotes Sugar Accumulation in Tomato Fruit
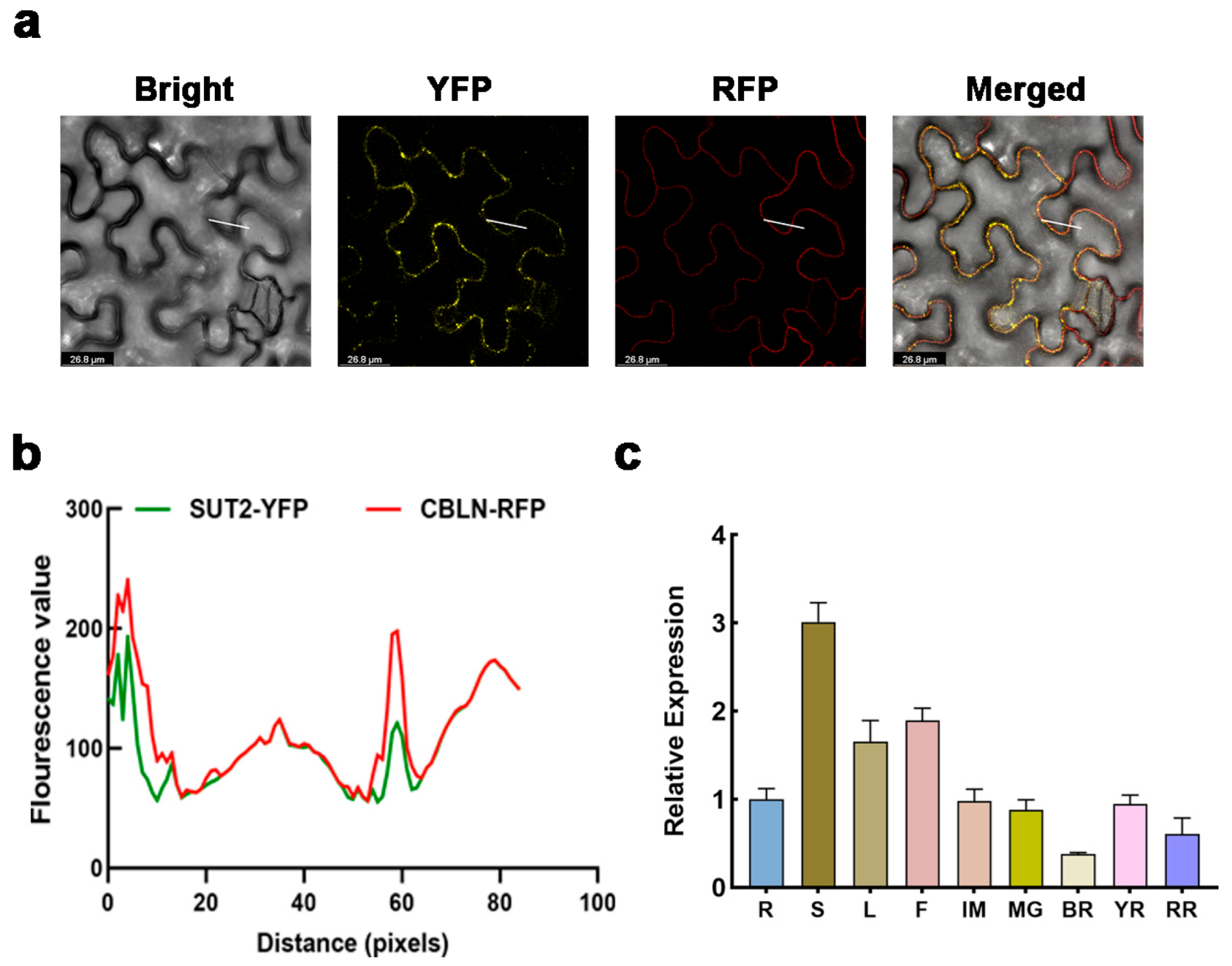
3.3. Temporal and Spatial Expression Profiles of SUT2
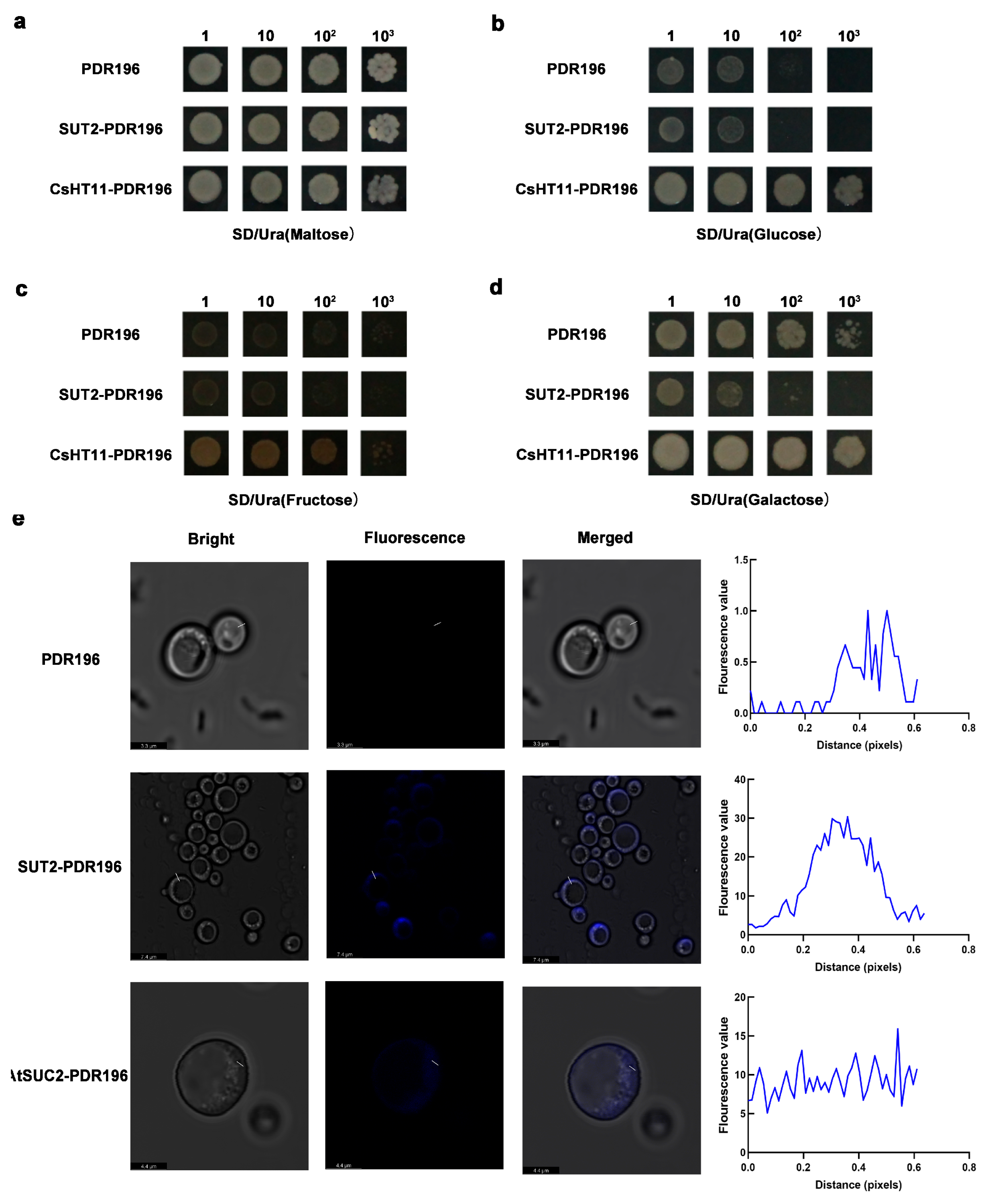
3.4. SUT2 Is a Plasma Membrane-Localized Sucrose Transporter
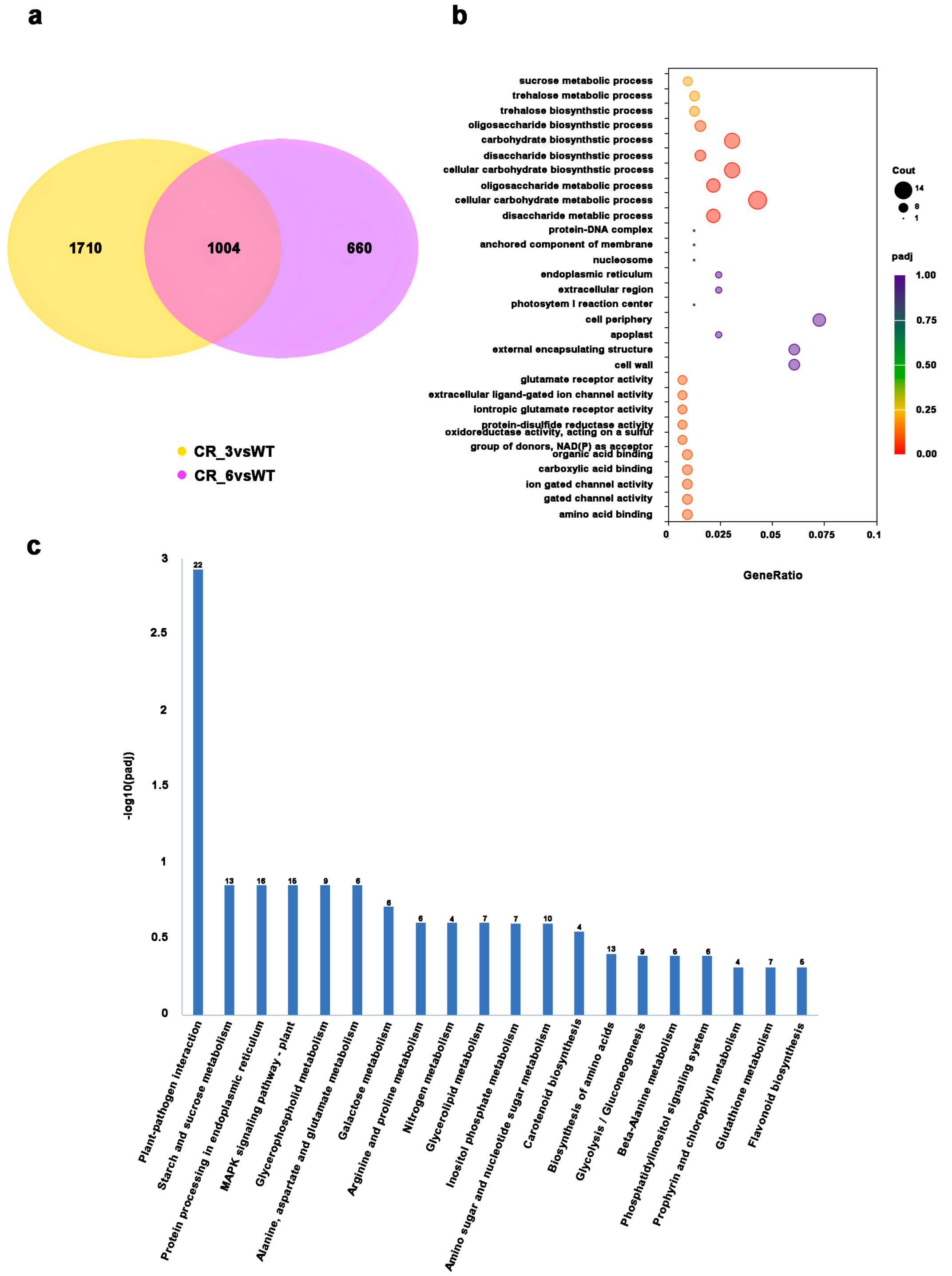
3.5. Transcriptome Profiling in Tomato Fruits of SUT2-Knockout Lines
3.6. Altered Expression of Sugar Metabolism Pathway in SUT2 Knockout Fruits
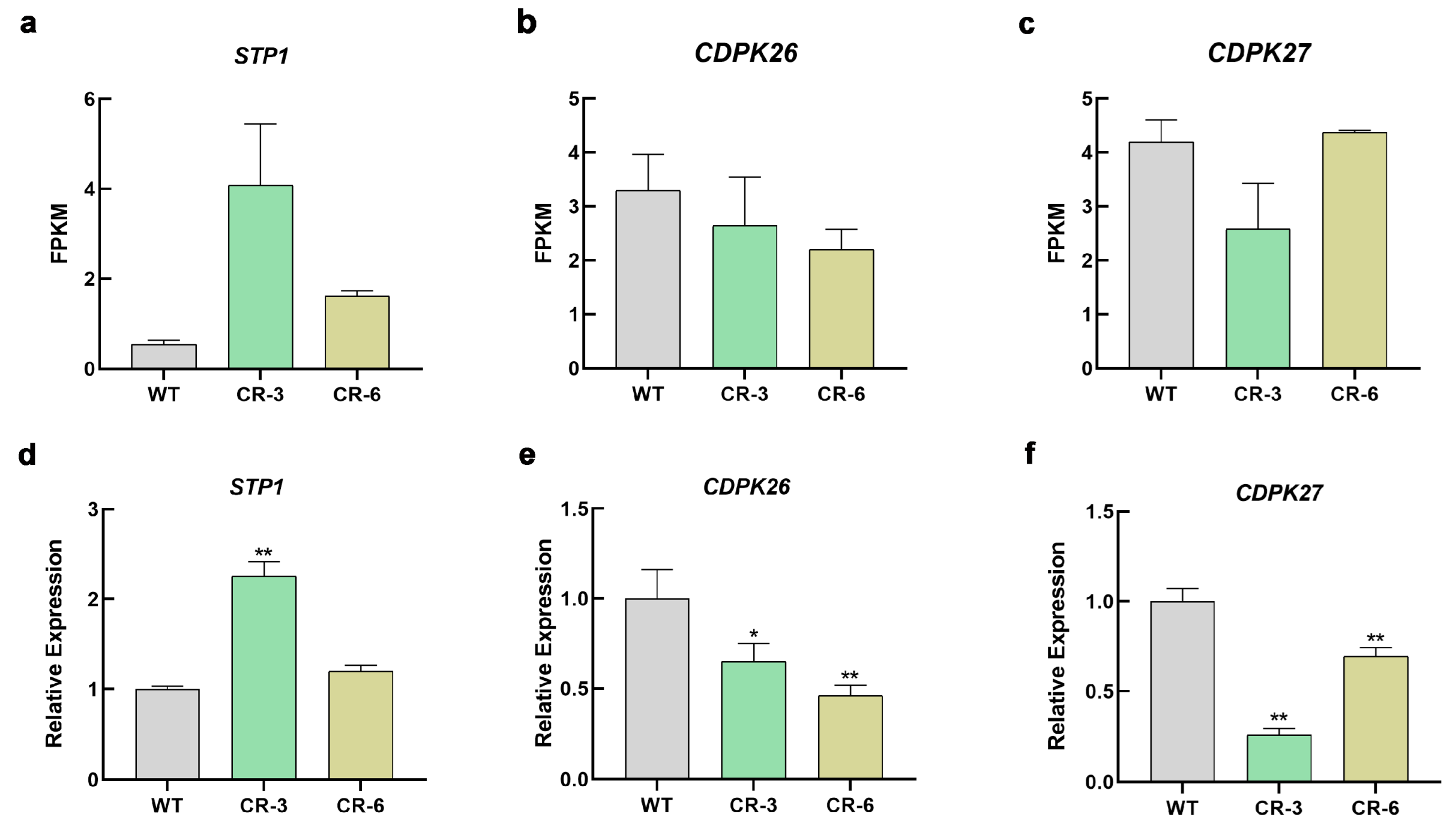
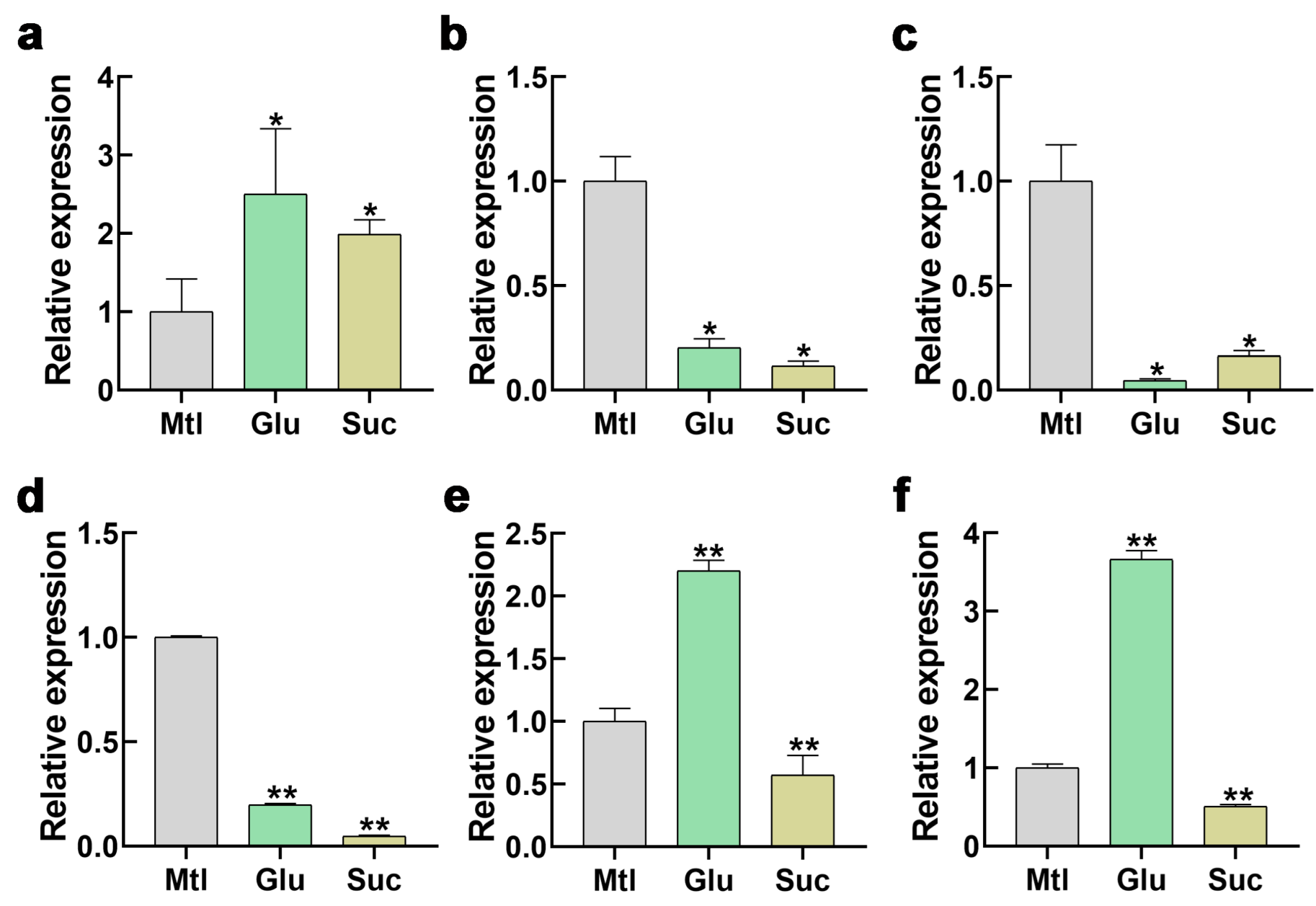
3.7. Low-Sugar Signaling Alters the Expression Levels of STP1 and CDPK26/27
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Deng, R.L.; Liu, J.W.; Lai, M.Y.; Wu, J.W.; Liu, X.D.; Shahid, M.Q. An overview of sucrose transporter (SUT) genes family in rice. Mol. Biol. Rep. 2022, 49, 5685–5695. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signalling networks. Curr. Opin. Plant Biol. 2015, 25, 130–137. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.Y.L.; Gazzarrini, S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture. Front Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef]
- De Schepper, V.; De Swaef, T.; Bauweraerts, I.; Steppe, K. Phloem transport: A review of mechanisms and controls. J. Exp. Bot. 2013, 64, 4839–4850. [Google Scholar] [CrossRef]
- Milne, R.J.; Perroux, J.M.; Rae, A.L.; Reinders, A.; Ward, J.M.; Offler, C.E.; Patrick, J.W.; Grof, C.P.L. Sucrose transporter localization and function in phloem unloading in developing stems. Plant Physiol. 2017, 173, 1330–1341. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.H.; Zong, M.; Guo, S.G.; Ren, Z.J.; Zhao, J.Y.; Li, M.Y.; Zhang, J.; Tian, S.W.; Wang, J.F.; et al. Localization shift of a sugar transporter contributes to phloem unloading in sweet watermelons. New Phytol. 2020, 227, 1858–1871. [Google Scholar] [CrossRef]
- Guo, W.J.; Pommerrenig, B.; Neuhaus, H.E.; Keller, I. Interaction between sugar transport and plant development. J. Plant Physiol. 2023, 288, 154073. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.a.W.; Neuhaus, H.E. In concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef]
- Büttner, M. The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett. 2007, 581, 2318–2324. [Google Scholar] [CrossRef]
- Cho, M.H.; Lim, H.; Shin, D.H.; Jeon, J.S.; Bhoo, S.H.; Park, Y.I.; Hahn, T.R. Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana. New Phytol. 2011, 190, 101–112. [Google Scholar] [CrossRef]
- Klemens, P.a.W.; Patzke, K.; Trentmann, O.; Poschet, G.; Buttner, M.; Schulz, A.; Marten, I.; Hedrich, R.; Neuhaus, H.E. Overexpression of a proton-coupled vacuolar glucose exporter impairs freezing tolerance and seed germination. New Phytol. 2014, 202, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Strobl, S.M.; Kischka, D.; Heilmann, I.; Mouille, G.; Schneider, S. The tonoplastic inositol transporter INT1 from Arabidopsis thaliana impacts cell elongation in a sucrose-dependent way. Front Plant Sci 2018, 9, 1657. [Google Scholar] [CrossRef]
- Doidy, J.; Grace, E.; Kühn, C.; Simon-Plas, F.; Casieri, L.; Wipf, D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012, 17, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Kühn, C.; Grof, C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 287–298. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H. The major facilitator superfamily (MFS) revisited. FEBS J. 2013, 280, 3975. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007, 581, 2309–2317. [Google Scholar] [CrossRef]
- Yan, N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef]
- Lu, R.F.; Guyer, D.E.; Beaudry, R.M. Determination of firmness and sugar content of apples using near-infrared diffuse reflectance. J. Texture Stud. 2000, 31, 615–630. [Google Scholar] [CrossRef]
- Gong, X.; Liu, M.L.; Zhang, L.J.; Ruan, Y.Y.; Ding, R.; Ji, Y.Q.; Zhang, N.; Zhang, S.B.; Farmer, J.; Wang, C. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant. 2015, 153, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Zhang, Z.J.; Miyao, A.; Hirochika, H.; Ohsugi, R.; Terao, T. Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. J. Exp. Bot. 2010, 61, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Liu, X.L.; Hu, Z.; Bao, S.H.; Xia, H.H.; Feng, B.; Ma, L.; Zhao, G.M.; Zhang, D.C.; Hu, Y.B. Essentiality for rice fertility and alternative splicing of OsSUT1. Plant Sci. 2022, 314, 111065. [Google Scholar] [CrossRef]
- Baker, R.F.; Leach, K.A.; Boyer, N.R.; Swyers, M.J.; Benitez-Alfonso, Y.; Skopelitis, T.; Luo, A.; Sylvester, A.; Jackson, D.; Braun, D.M. Sucrose transporter ZmSUT1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiol. 2016, 172, 1876–1898. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.A.; Tran, T.M.; Slewinski, T.L.; Meeley, R.B.; Braun, D.M. Sucrose transporter 2 contributes to maize growth, development, and crop yield. J. Integr. Plant Biol 2017, 59, 390–408. [Google Scholar] [CrossRef]
- Islam, M.Z.; Jin, L.F.; Shi, C.Y.; Liu, Y.Z.; Peng, S.A. Citrus sucrose transporter genes: Genome-wide identification and transcript analysis in ripening and ABA-injected fruits. Tree Genet. Genom. 2015, 11, 97. [Google Scholar] [CrossRef]
- Wen, S.Y.; Neuhaus, H.E.; Cheng, J.T.; Bie, Z.L. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef]
- Peng, Q.; Cai, Y.M.; Lai, E.H.; Nakamura, M.; Liao, L.; Zheng, B.B.; Ogutu, C.; Cherono, S.; Han, Y.P. The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple. BMC Plant Biol. 2020, 20, 191. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; Hu, D.G.; Hao, Y.J. Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol. 2017, 174, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.J.; Sun, M.H.; Kang, H.; Lu, J.; You, C.X.; Hao, Y.J. A CIPK protein kinase targets sucrose transporter MdSUT2.2 at Ser254 for phosphorylation to enhance salt tolerance. Plant Cell Environ. 2019, 42, 918–930. [Google Scholar] [CrossRef]
- Liang, Y.F.; Bai, J.Y.; Xie, Z.L.; Lian, Z.Y.; Guo, J.; Zhao, F.Y.; Liang, Y.; Huo, H.Q.; Gong, H.J. Tomato sucrose transporter SlSUT4 participates in flowering regulation by modulating gibberellin biosynthesis. Plant Physiol. 2023, 192, 1080–1098. [Google Scholar] [CrossRef]
- Chiou, T.J.; Bush, D.R. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 1998, 95, 4784–4788. [Google Scholar] [CrossRef]
- Laurence, B.; Christina, K.; Andreas, W.; Alexander, S.; Christiane, G.; Brigitte, H.; Hanjo, H.; Waltraud, S.; John, M.W.; John, M.W. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef][Green Version]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kühn, C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Chen, Y.H.; Li, H.X.; Qian, C.J.; Huang, S.L.; Ye, Z.B. Transformation of tomatoes with osmotin and chitinase genes and their resistance to Fusarium wilt. J. Horticult. Sci. Biotechnol. 2005, 80, 517–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Ming, R.H.; Khan, M.; Wang, Y.; Dahro, B.; Xiao, W.; Li, C.L.; Liu, J.H. ERF9 of Poncirus trifoliata (L.) Raf. undergoes feedback regulation by ethylene and modulates cold tolerance via regulating a glutathione S-transferase U17 gene. Plant Biotechnol. J. 2022, 20, 183–200. [Google Scholar] [CrossRef]
- Tian, Z.D.; He, Q.; Wang, H.X.; Liu, Y.; Zhang, Y.; Shao, F.; Xie, C.H. The potato ERF transcription factor StERF3 negatively regulates resistance to phytophthora infestans and salt tolerance in potato. Plant Cell Physiol. 2015, 56, 992–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Gai, W.X.; Yuan, L.D.; Shang, L.L.; Li, F.M.; Gong, Z.; Ge, P.F.; Wang, Y.R.; Tao, J.B.; Zhang, X.Y.; et al. Heat-inducible SlWRKY3 confers thermotolerance by activating the SlGRXS1 gene cluster in tomato. Hortic Plant J 2024, 10, 515–531. [Google Scholar] [CrossRef]
- Fan, R.C.; Peng, C.C.; Xu, Y.H.; Wang, X.F.; Li, Y.; Shang, Y.; Du, S.Y.; Zhao, R.; Zhang, X.Y.; Zhang, L.Y.; et al. Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol. 2009, 150, 1880–1901. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Takegawa, K. A simple and efficient procedure for transformation of schizosaccharomyces pombe. Yeast 2004, 21, 613–617. [Google Scholar] [CrossRef]
- Li, J.Y.; Sun, B.L.; Xu, Q.Q.; Jiang, L.B.; Wang, N. Transcriptome-level analysis of gene expressions in different tissues of tomato and key gene identifications during seed germination. Sci. Hortic. 2024, 337, 113565. [Google Scholar] [CrossRef]
- Kanayama, Y. Sugar Metabolism and Fruit Development in the Tomato. Horticult J. 2017, 86, 417–425. [Google Scholar] [CrossRef]
- Dong, H.Q.; Li, F.M.; Xuan, X.X.; Ahiakpa, J.K.; Tao, J.B.; Zhang, X.Y.; Ge, P.F.; Wang, Y.R.; Gai, W.X.; Zhang, Y.Y. The genetic basis and improvement of photosynthesis in tomato. Hortic Plant J 2025, 11, 69–84. [Google Scholar] [CrossRef]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.Y.; Bao, T.Y.; Zeng, X.W.; Bie, Z.L.; Cheng, J.T. CsHT11 encodes a pollen-specific hexose transporter and is induced under high level sucrose in pollen tubes of cucumber (Cucumis sativus). Plant Growth Regul. 2020, 90, 237–248. [Google Scholar] [CrossRef]
- Patzke, K.; Prananingrum, P.; Klemens, P.a.W.; Trentmann, O.; Rodrigues, C.M.; Keller, I.; Fernie, A.R.; Geigenberger, P.; Bölter, B.; Lehmann, M.; et al. The plastidic sugar transporter pSuT influences flowering and affects cold responses. Plant Physiol. 2019, 179, 569–587. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.M.; Ge, P.F.; Li, F.M.; Zhu, L.H.; Wang, Y.R.; Tao, J.B.; Zhang, X.Y.; Dong, H.Q.; Gai, W.X.; et al. A 21-bp InDel in the promoter of STP1 selected during tomato improvement accounts for soluble solid content in fruits. Hortic. Res. 2023, 10, uhad009. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Lyu, H.; Chen, J.; Cao, X.; Du, R.; Ma, L.; Wang, N.; Zhu, Z.G.; Rao, J.L.; Wang, J.; et al. Releasing a sugar brake generates sweeter tomato without yield penalty. Nature 2024, 635, 647–656. [Google Scholar] [CrossRef]
- Zou, W.J.; Yu, Q.; Ma, Y.; Sun, G.N.; Feng, X.; Ge, L. Pivotal role of heterotrimeric G protein in the crosstalk between sugar signaling and abiotic stress response in plants. Plant Physiol. Biochem. 2024, 210, 108567. [Google Scholar] [CrossRef]
- Niu, S.B.; He, Y.; Yan, S.W.; Sun, Z.L.; Cai, R.; Zhang, Y. Histological, transcriptomic, and gene functional analyses reveal the regulatory events underlying gibberellin-induced parthenocarpy in tomato. Hortic. Plant J. 2024, 10, 156–170. [Google Scholar] [CrossRef]
- Guan, H.L.; Yang, X.L.; Lin, Y.X.; Xie, B.X.; Zhang, X.Y.; Ma, C.J.; Xia, R.; Chen, R.Y.; Hao, Y.W. The hormone regulatory mechanism underlying parthenocarpic fruit formation in tomato. Front Plant Sci. 2024, 15, 1404980. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liberatore, K.L.; Macalister, C.A.; Huang, Z.J.; Chu, Y.H.; Jiang, K.; Brooks, C.; Ogawa-Ohnishi, M.; Xiong, G.Y.; Pauly, M.; et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 2015, 47, 784–792. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, P.; Wang, Y.; Cao, Y.; Li, F.; Zhang, X.; Xu, H.; Yang, Y.; Wang, Z.; Lin, J.; Zhu, P.; et al. Sucrose Transporter 2 Knockout Increases Sugar Content in Tomato Fruits. Horticulturae 2025, 11, 956. https://doi.org/10.3390/horticulturae11080956
Ge P, Wang Y, Cao Y, Li F, Zhang X, Xu H, Yang Y, Wang Z, Lin J, Zhu P, et al. Sucrose Transporter 2 Knockout Increases Sugar Content in Tomato Fruits. Horticulturae. 2025; 11(8):956. https://doi.org/10.3390/horticulturae11080956
Chicago/Turabian StyleGe, Pingfei, Ying Wang, Yuyang Cao, Fangman Li, Xingyu Zhang, Haobo Xu, Yang Yang, Ziyuan Wang, Junshen Lin, Pengyu Zhu, and et al. 2025. "Sucrose Transporter 2 Knockout Increases Sugar Content in Tomato Fruits" Horticulturae 11, no. 8: 956. https://doi.org/10.3390/horticulturae11080956
APA StyleGe, P., Wang, Y., Cao, Y., Li, F., Zhang, X., Xu, H., Yang, Y., Wang, Z., Lin, J., Zhu, P., & Zhang, Y. (2025). Sucrose Transporter 2 Knockout Increases Sugar Content in Tomato Fruits. Horticulturae, 11(8), 956. https://doi.org/10.3390/horticulturae11080956





