Sodium Alginate Composite Coating Inhibited Postharvest Greening and Improved Nutritional Quality of Potato Tubers by Regulating Chlorophyll Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials, Treatment, and Storage
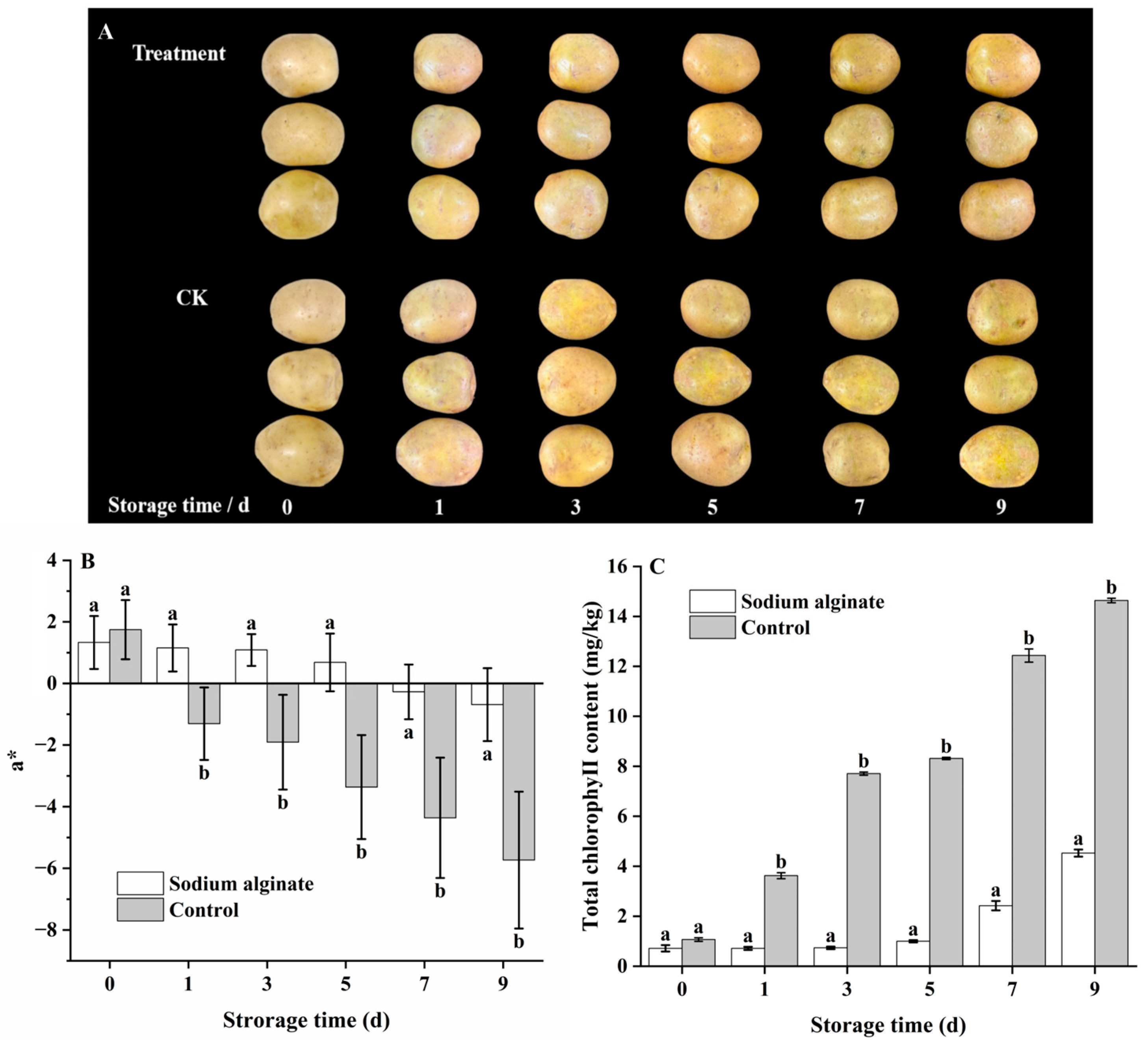
2.2. Measurement of Total Chlorophyll Content
2.3. Color Measurement
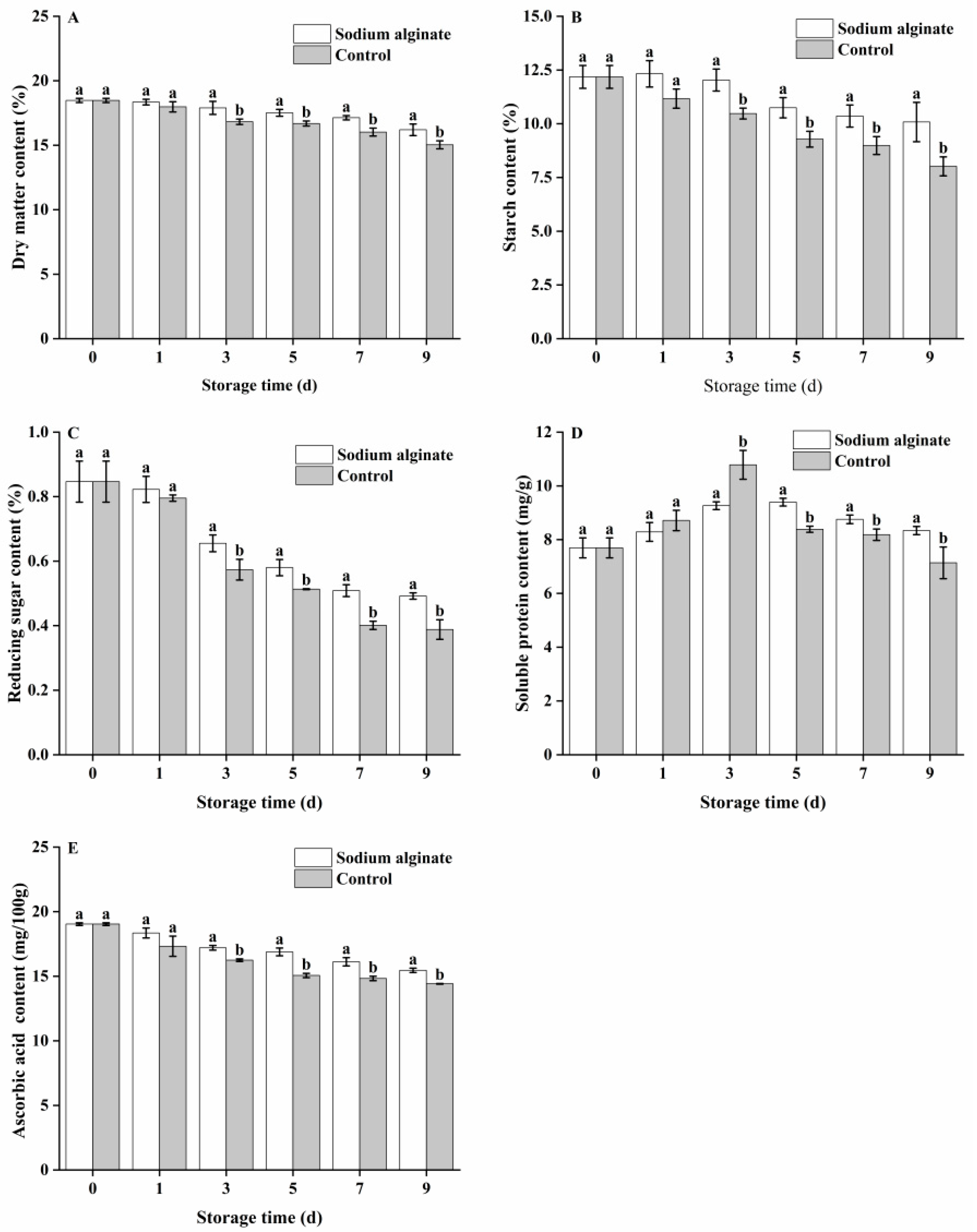
2.4. Measurement of Dry Matter Content
2.5. Measurement of Starch Content
2.6. Measurement of Reducing Sugar Content
2.7. Measurement of Soluble Protein Content
2.8. Measurement of Ascorbic Acid Content
2.9. Measurement of ALA Content
2.10. Measurement of PBG and Uro III Content
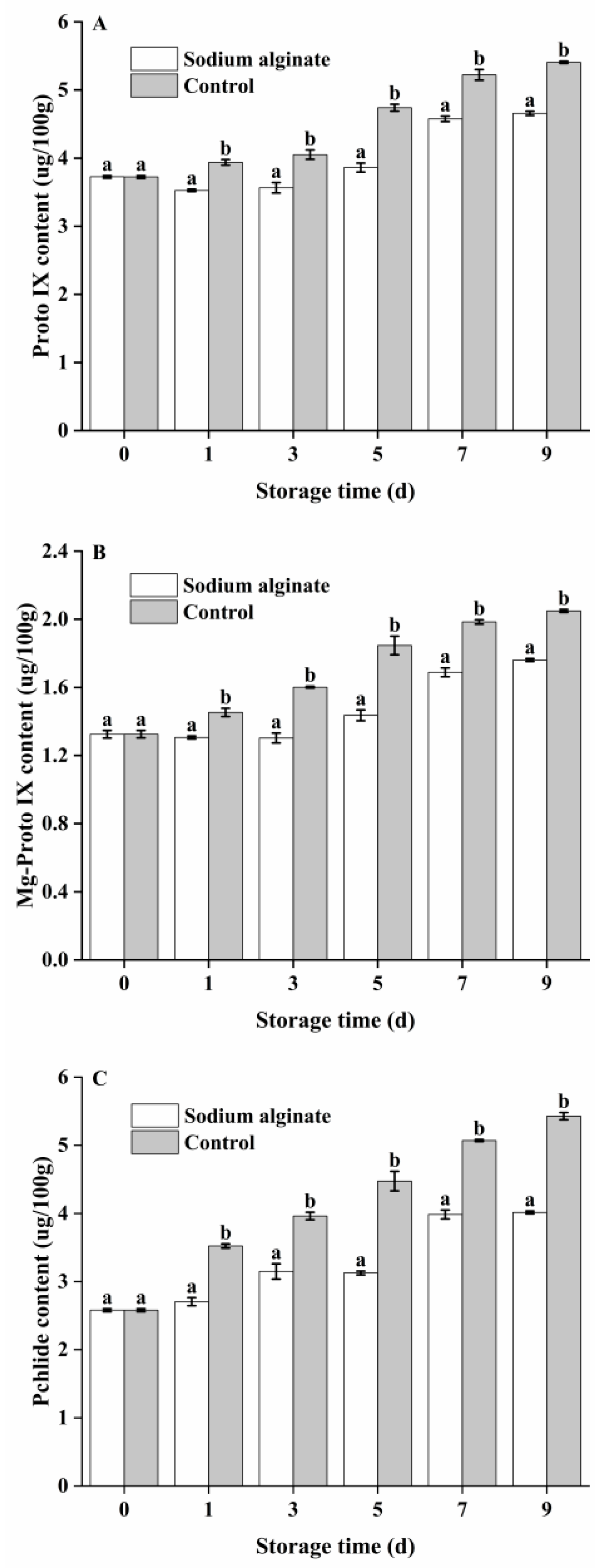
2.11. Measurement of Proto IX, Mg-Proto IX and Pchlide Content
2.12. Statistical Analysis
3. Results
3.1. Effect of Sodium Alginate Coating Treatment on Total Chlorophyll Content, a* Value and Apparent Color of Potato Tuber Epidermis
3.2. Effect of Sodium Alginate Coating Treatment on the Nutritional Quality of Potato Tubers
3.3. Effect of Sodium Alginate Coating Treatment on ALA, PBG and Uro III Contents of Potato Tuber Epidermis
3.4. Effect of Sodium Alginate Coating Treatment on Proto IX, Mg-Proto IX and Pchlide Contents of Potato Tuber Epidermis
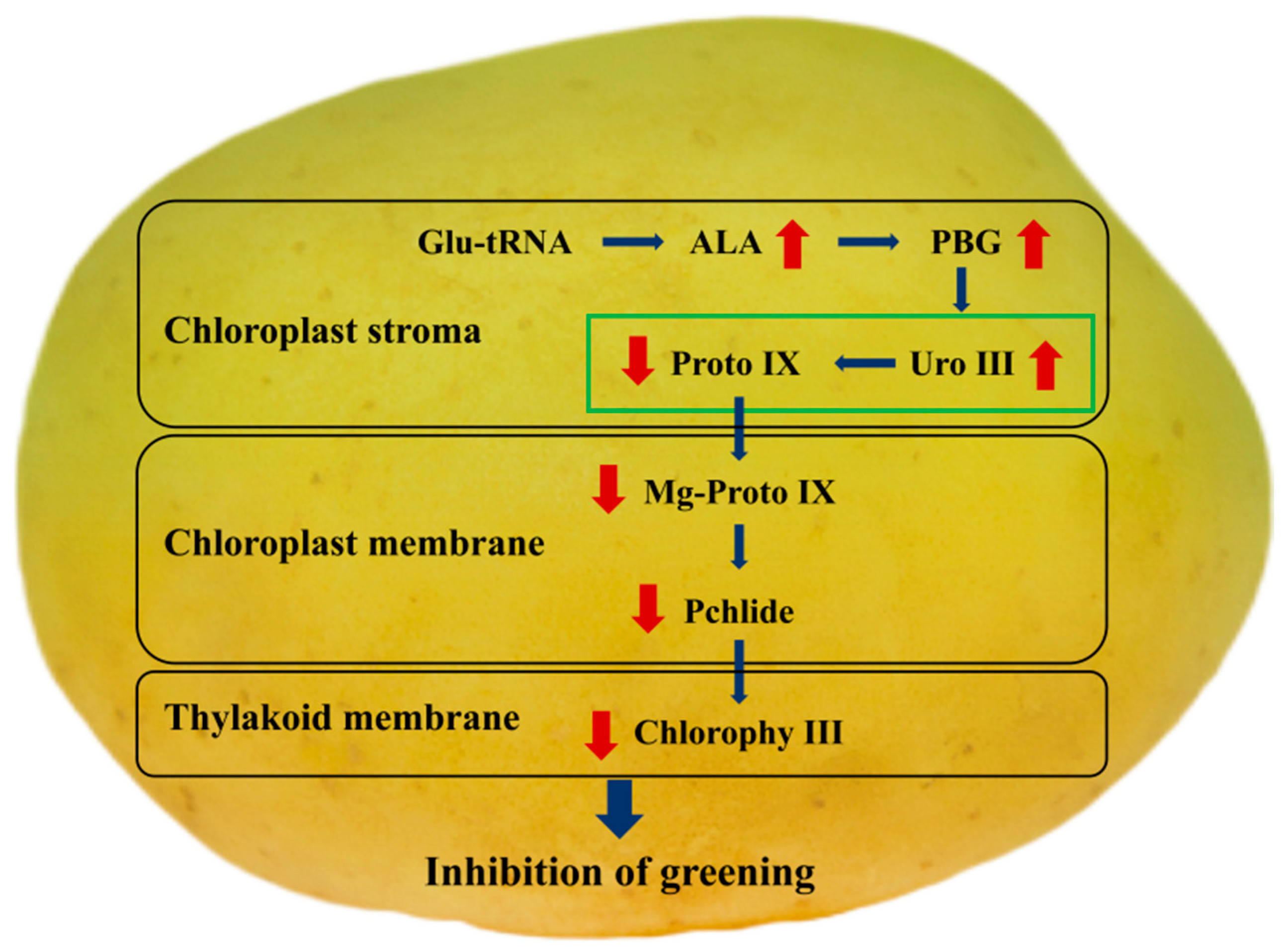
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | 5-aminolevulinic acid |
| PBG | porphobilinogen |
| Uro III | uroporphyrinogen III |
| Proto IX | protoporphyrin IX |
| Mg-Proto IX | Mg-protoporphyrin IX |
| Pchlide | protochlorophyllide |
References
- Madiwale, G.P.; Reddivari, L.; Stone, M.; Holm, D.G.; Vanamala, J. Combined effects of storage and processing on the bioactive compounds and pro-apoptotic properties of color-fleshed potatoes in human colon cancer cells. J. Agric. Food Chem. 2012, 60, 11088–11096. [Google Scholar] [CrossRef]
- Emragi, E.; Jayanty, S.S. Skin Color Retention in Red Potatoes during Long-Term Storage with Edible Coatings. Foods 2021, 10, 1531. [Google Scholar] [CrossRef]
- Grunenfelder, L.A.; Knowles, L.O.; Hiller, L.K.; Knowles, N.R. Glycoalkaloid development during greening of fresh market potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2006, 54, 5847–5854. [Google Scholar] [CrossRef] [PubMed]
- Tanios, S.; Eyles, A.; Tegg, R.; Wilson, C. Potato Tuber Greening: A Review of Predisposing Factors, Management and Future Challenges. Am. J. Potato Res. 2018, 95, 248–257. [Google Scholar] [CrossRef]
- Kumar, T.A.; Charan, T.B. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef]
- Wang, H.P.; Liu, Z.C.; Luo, S.L.; Li, J.; Zhang, J.; Li, L.S.; Xie, J.M. 5-Aminolevulinic acid and hydrogen sulphide alleviate chilling stress in pepper (Capsicum annuum L.) seedlings by enhancing chlorophyll synthesis pathway. Plant Physiol. Biochem. 2021, 167, 567–576. [Google Scholar] [CrossRef]
- Xiang, L.X.; Hu, L.P.; Meng, S.; Zou, Z.R. Effects of Foliar-spraying Spermidine on Chlorophyll Synthesis Metabolism of Tomato Seedlings under Heat Stress. Acta Bot. Boreali-Occident. Sin. 2020, 40, 846–851. [Google Scholar] [CrossRef]
- Shu, H.; Wang, Y.F.; Zhang, S.K.; Liang, Y.R.; Lu, J.L.; Li, D.W.; Ou-yang, M.; Li, N.N.; Zhu, Y.J. Effect of Light-shading on Accumulation of Chlorophylls and Their Precursors in Tea Shoots. J. Tea Sci. 2012, 32, 115–121. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, S.R.; Shu, S.; Liu, F.; Liu, T.; Sun, J. Effects of Exogenous Spermidine on Chlorophyll Precursors Content of Spinach Plants under Salt Stress. Acta Bot. Boreali-Occident. Sin. 2015, 35, 2026–2034. [Google Scholar] [CrossRef]
- Yang, Q.; AI-Sha-Jiang, M.M.T.; Wang, Z.X.; Liu, G.J. Effects of DA-6 on Chlorophyll Biosythesis Pathway in Peach Leaves. Acta Hortic. Sin. 2012, 39, 621–628. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, X.J.; Liu, B.J.; Lu, L.; Zhang, X.M.; Swing, C.J.; Xia, S.Q. Effect of synergism between sodium alginate and xanthan gum on characteristics of composite film and gloss of areca nut coating. Food Biosci. 2022, 50, 102113. [Google Scholar] [CrossRef]
- Sarengaowa; Wang, L.Y.; Liu, Y.M.; Yang, C.M.; Feng, K.; Hu, W.Z. Screening of Essential Oils and Effect of a Chitosan-Based Edible Coating Containing Cinnamon Oil on the Quality and Microbial Safety of Fresh-Cut Potatoes. Coatings 2022, 12, 1492. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Shakerardekani, A.; Mirdehghan, S.H. Effect of alginate coating enriched with Shirazi thyme essential oil on quality of the fresh pistachio (Pistacia vera L.). J. Food Sci. Technol. 2020, 5, 34–43. [Google Scholar] [CrossRef]
- Erihemu; Lv, H.; Zhang, C.; Ma, H.; Shi, B.D.; Shi, K.; Wang, J.; Wu, Y.; Zhang, P.F.; Zhu, H.M. Formulation development and characterization of sodium alginate cross-linked films incorporated with polydopamine as light-blocking materials: Application on greening inhibition of whole potato tuber. Food Chem. 2025, 478, 143747. [Google Scholar] [CrossRef]
- Amir, G.; Farooq, A.M.; Lubna, M.; Adil, G.; Sabeera, M. Effect of sodium alginate coatings enriched with α-tocopherol on quality of fresh walnut kernels. Food Chem. Adv. 2023, 2, 100169. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Solís-Pacheco, J.R.; Calderón-Santoyo, M. Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT 2021, 152, 112346. [Google Scholar] [CrossRef]
- Esam, E.; Diganta, K.; Sastry, S.J. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions. LWT 2022, 153, 112580. [Google Scholar] [CrossRef]
- Tilahun, S.; An, H.S.; Solomon, T.; Baek, M.W.; Choi, H.R.; Lee, H.C.; Jeong, C.S. Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content. Horticulturae 2020, 6, 107. [Google Scholar] [CrossRef]
- Das, S.; Mitra, B.; Saha, A.; Mandal, S.; Paul, P.K.; El-Sharnouby, M.; Hassan, M.M.; Maitra, S.; Hossain, A. Evaluation of Quality Parameters of Seven Processing Type Potato (Solanum tuberosum L.) Cultivars in the Eastern Sub-Himalayan Plains. Foods 2021, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Zhang, Q.; Li, Y.G.; Lu, Y.X.; Zhou, X.B.; Yin, B.F.; Zhang, Y.M. Shrubs have a greater influence on the nonstructural carbohydrates of desert mosses along precipitation decreased. Environ. Exp. Bot. 2023, 216, 105530. [Google Scholar] [CrossRef]
- Choi, I.; Chun, J.; Choi, H.-S.; Park, J.; Kim, N.-G.; Lee, S.-K.; Park, C.-H.; Jeong, K.-H.; Nam, J.-W.; Cho, J.; et al. Starch Characteristics, Sugars and Thermal Properties of Processing Potato (Solanum tuberosum L.) Cultivars Developed in Korea. Am. J. Potato Res. 2020, 97, 308–317. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Aalim, H.; Li, L.; Mao, L.C.; Luo, Z.S.; Ying, T.J. Effects of exogenous abscisic acid on bioactive components and antioxidant capacity of postharvest tomato during ripening. Molecules 2020, 25, 1346. [Google Scholar] [CrossRef]
- Harel, E.; Klein, S. Light dependent formation of δ-aminolevulinic acid in etiolated leaves of higher plants. Biochem. Biophys. Res. Commun. 1972, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, R.R.; Van-Huystee, R.B. Rapid Simultaneous Estimation of Protoporphyrin and Mg-Porphyrins in Higher Plants. J. Plant Physiol. 1986, 125, 311–323. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldao-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Luo, Q.J.; Zhang, J.; Xu, R.W.; Cheng, Y.J. The effect of shellac rosin coating on the shelf life quality of citrus fruits. Acta Hortic. Sin. 2023, 50, 853–863. [Google Scholar] [CrossRef]
- Yan, P.L.; Lan, W.Q.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Technol. 2023, 143, 104217. [Google Scholar] [CrossRef]
- Jasmina, M.F.; Marjana, K.R.; Mercedes, W. Plastid Transformation in Greening Potato Tuber Tissue. J. Plant Physiol. 1994, 144, 58–63. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.H.; Zhou, D.R.; Cai, W.H.; Rehman, M.; Feng, Y.H.; Kong, Y.X.; Liu, X.P.; Fahad, S.; Deng, G. Impact of dormancy periods on some physiological and biochemical indices of potato tubers. PeerJ 2023, 11, e15923. [Google Scholar] [CrossRef]
- Jakubowski, T.; Krolczyk, J.B. Method for the Reduction of Natural Losses of Potato Tubers During their Long-Term Storage. Sustainability 2020, 12, 1048. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, R.; Li, W.; Song, J.Y.; Cheng, F.S. Improved postharvest preservation effects of Pholiota nameko mushroom by sodium alginate–based edible composite coating. Food Bioprocess Technol. 2019, 12, 587–598. [Google Scholar] [CrossRef]
- Wilmer, L.; Pawelzik, E.; Naumann, M. Comparison of the Effects of Potassium Sulphate and Potassium Chloride Fertilisation on Quality Parameters, Including Volatile Compounds, of Potato Tubers After Harvest and Storage. Front. Plant Sci. 2022, 13, 920212. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yang, H.; Wang, A.J. Effect of exogenous glycine betaine on qualities of button mushrooms (Agaricus bisporus) during postharvest storage. Eur. Food Res. Technol. 2015, 240, 41–48. [Google Scholar] [CrossRef]
- Nkede, F.N.; Wardak, M.H.; Wardana, A.A.; Fanze, M.; Yan, X.R.; Jothi, J.S.; Phuong, N.T.H.; Tanaka, F.; Tanaka, F. Preparation and characterization of edible coating and film composed of sodium alginate/ylang-ylang oil/cellulose nanocrystals Pickering emulsion and its application to post-harvest control of mandarin (Citrus reticulata). Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133859. [Google Scholar] [CrossRef]
- Yang, J.R.; Chen, H.; Zhang, W.W.; Zhang, J.Y.; Zhang, H.; Li, K. Effect of Bleached Shellac/Sodium Alginate Composite Coating on Storage and Preservation of Chestnuts. Packag. Eng. 2024, 45, 134–141. [Google Scholar] [CrossRef]
- Chen, K.; Tian, R.M.; Xu, G.J.; Wu, K.; Liu, Y.; Jiang, F.T. Characterizations of konjac glucomannan/curdlan edible coatings and the preservation effect on cherry tomatoes. Int. J. Biol. Macromol. 2023, 232, 123359. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Kruse, E.; Mock, H.-P.; Grimm, B. Coproporphyrinogen III oxidase from barley and tobacco-sequence analysis and initial expression studies. Planta 1995, 196, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.S.; Du, J.; Xiao, Y.; Hu, X.Q.; Lin, Z.P. Vitreoscilla Hemoglobin Research and Development Prospects. Biotechnol. Bull. 2009, 11, 1–7. [Google Scholar] [CrossRef]
- Viney, J.; Davison, P.A.; Hunter, N.C.; Reid, J.D. Direct measurement of metal-ion chelation in the active site of the AAA+ ATPase magnesium chelatase. Biochemistry 2007, 46, 12788–12794. [Google Scholar] [CrossRef]
- Meskauskiene, R.; Nater, M.; Goslings, D.; Kessler, F.; Camp, R.O.D.; Apel, K. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 12826–12831. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.R.; Hu, X.H.; Hu, L.P.; Zou, Z.R.; Pan, X.B. Effect of Foliar-spraying Spermidine on Seedlings Growth and Contents of Chlorophyll Biosynthesis Precursors in Leaves of Tomato under Saline-alkaline Stress. Acta Bot. Boreali-Occident. Sin. 2015, 35, 125–130. [Google Scholar] [CrossRef]
- Lu, M.; Liu, H.H.; Mao, H.D.; Zhao, Q.R.; Zhao, H.X.; Hu, S.W. Changes of Chlorophyll Synthesis Metabolism in Chlorophyll-deficient Mutant in Brassica juncea. Acta Bot. Boreali-Occident. Sin. 2010, 30, 2177–2183. [Google Scholar]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, C.; Xia, X.; Zhang, D.; Zhang, Y.; Wu, Q. Sodium Alginate Composite Coating Inhibited Postharvest Greening and Improved Nutritional Quality of Potato Tubers by Regulating Chlorophyll Biosynthesis. Horticulturae 2025, 11, 950. https://doi.org/10.3390/horticulturae11080950
Kang C, Xia X, Zhang D, Zhang Y, Wu Q. Sodium Alginate Composite Coating Inhibited Postharvest Greening and Improved Nutritional Quality of Potato Tubers by Regulating Chlorophyll Biosynthesis. Horticulturae. 2025; 11(8):950. https://doi.org/10.3390/horticulturae11080950
Chicago/Turabian StyleKang, Chuhan, Xinyu Xia, Dongdong Zhang, Yurong Zhang, and Qiong Wu. 2025. "Sodium Alginate Composite Coating Inhibited Postharvest Greening and Improved Nutritional Quality of Potato Tubers by Regulating Chlorophyll Biosynthesis" Horticulturae 11, no. 8: 950. https://doi.org/10.3390/horticulturae11080950
APA StyleKang, C., Xia, X., Zhang, D., Zhang, Y., & Wu, Q. (2025). Sodium Alginate Composite Coating Inhibited Postharvest Greening and Improved Nutritional Quality of Potato Tubers by Regulating Chlorophyll Biosynthesis. Horticulturae, 11(8), 950. https://doi.org/10.3390/horticulturae11080950





